Abstract
We studied the persistence of serum bactericidal antibody using rabbit and human complement (rSBA/hSBA, cut-offs 1:8) 5 y after a single dose of meningococcal serogroups A, C, W, Y tetanus toxoid conjugate vaccine (MenACWY-TT) compared with age-appropriate control vaccines in toddlers and children (NCT00427908). Children were previously randomized (3:1) to receive either MenACWY-TT or control vaccine (MenC-CRM197 in 1-<2 y olds; MenACWY-polysaccharide vaccine [Men-PS] in 2-<11 y olds). Subjects with rSBA-MenC titers <1:8 at any time point were revaccinated with MenC conjugate vaccine and discontinued from the study. A repeated measurement statistical model assessed potential selection effects due to drop-outs. At year 5 in MenACWY-TT-vaccinated-toddlers for serogroups A, C, W, and Y respectively, percentages with rSBA titers ≥1:8 were 73.5%, 77.6%, 34.7%, and 42.9%, hSBA ≥1:8 were 35.6%, 91.7%, 82.6% and 80.0%. For MenC-CRM197 recipients, 63.6% had persisting rSBA-MenC titers ≥1:8 and 90.9% had hSBA-MenC ≥1:8 (not significantly different versus MenACWY-TT for either assay: exploratory analyses). In 2-<11 y olds rSBA titers ≥1:8 in MenACWY-TT-vaccinees were 90.8%, 90.8%, 78.6%, and 78.6% and 15.4%, 100%, 0.0%, 7.7% in Men-PS-vaccinees (significantly different for serogroups A, W and Y, exploratory analyses). Serogroups A, W and Y rSBA GMTs were ≥ 26-fold higher in MenACWY-TT-vaccinees. As expected, GMTs modeled at year 5 to assess the impact of subject drop out (mainly for revaccination), appeared lower for serogroup C. No vaccine-related SAEs were reported. Antibody persistence was observed for all serogroups up to 5 y after MenACWY-TT vaccination.
Abbreviations
| ATP | = | according to protocol |
| CI | = | confidence interval |
| CRM197 | = | mutant diphtheria toxoid |
| DT | = | diphtheria toxoid |
| GMT | = | geometric mean titer |
| hSBA | = | serum bactericidal activity assay using human complement |
| IMD | = | invasive meningococcal disease |
| MenACWY | = | quadrivalent meningococcal vaccine containing serogroups A, C, W and Y polysaccharide |
| MenC | = | meningococcus serogroup C |
| PHE | = | Public Health England |
| rSBA | = | serum bactericidal activity assay using baby rabbit complement |
| TT | = | tetanus toxoid |
Introduction
Invasive infection and meningitis caused by Neisseria meningitidis remains an important cause of childhood morbidity and mortality globally.Citation1,2 The introduction of meningococcal conjugate vaccination to childhood immunization schedules is estimated to have decreased invasive meningococcal disease (IMD) incidence rates in Europe by approximately 50%.Citation3 Nevertheless, the highest incidence of IMD continues to be in infants and young children, and mortality in infected individuals remains unchanged at approximately 10%.Citation1,3,4
Monovalent meningococcal serogroup C (MenC) vaccines have been available for use in infants and young children since 1999.Citation5 However, in Europe, serogroup B as well as serogroups W and Y are also important causes of IMD.Citation6 In Finland, meningococcal serogroup B causes the majority of IMD, followed by serogroup Y, C and W (2012 data).Citation7 Finland is one of several northern European countries, such as Sweden, in which serogroup Y has emerged as an important cause of IMD. The highest incidence of IMD occurs in children aged <1 y (4.99/100,000 in 2012), which is lower than the rate for Europe of 11.4/100,000 in this age group.Citation7 While a meningococcal vaccine targeting serogroup B has been licensed for use in Europe,Citation8 broadly protective quadrivalent meningococcal conjugate vaccines that target 4 serogroups (MenACWY conjugate vaccines) and that are immunogenic in young children, could provide added benefit in European countries.
IMD is thought to develop soon after acquisition of a new meningococcal strain to which the individual has not been previously exposed.Citation9,10 This, coupled with evidence from children in the United Kingdom who experienced vaccine failure after MenC immunization,Citation11 suggests that circulating antibody is necessary for protection against meningococcal disease.
Worldwide, 3 MenACWY-TT conjugate vaccines are currently licensed for use in children. Of these, 2 are available in Europe: MenACWY-TT (Nimenrix™ Pfizer, formerly Wyeth) has all 4 serogroup polysaccharides conjugated to tetanus toxoid (TT) and is licensed for use as a single dose in children from 12 months of age. MenACWY-CRM197 (Menveo™ GSK Vaccines) uses a mutated diphtheria toxoid as conjugate protein and is licensed as of 2 y of age in Europe. MenACWY-DT (Menactra™ Sanofi Pasteur) uses diphtheria toxoid as conjugate protein and is licensed for use in the United States, but not currently in Europe.
To date, the need for booster doses after vaccination with meningococcal conjugate vaccines during childhood is not known. Available persistence studies in infant, toddler and adolescent populations for up to 5 y after immunization with MenACWY conjugate vaccines or monovalent MenC-CRM197 vaccines in various schedules all point to waning immunity over the first 5 y after vaccination.Citation12-18 Vaccine effectiveness studies in US adolescents show that protection also decreases with time since vaccination.Citation12 Together the data suggest that the duration of protection after meningococcal conjugate vaccination is limited.
We previously demonstrated that a single dose of MenACWY-TT administered to children between 12 months and 10 y of age induced high levels of bactericidal antibodies and was non-inferior to age-appropriate licensed control vaccines: MenC-CRM197 vaccine (Meningitec™, Pfizer) in toddlers between 12 and 23 months of age and MenACWY-polysaccharide vaccine (Men-PS; Mencevax™ ACWY Pfizer, formerly Wyeth) in children between 2 and 10 y of age.Citation17,19 Assessment 3 y after vaccination showed good antibody persistence for all 4 serogroups using serum bactericidal assay with rabbit complement source (rSBA).Citation17,19 In toddlers in whom antibody persistence was also assessed using SBA with human complement source (hSBA), good persistence of antibodies for serogroups C, W and Y was also observed.Citation17 We subsequently studied long term antibody persistence for up to 5 y after primary vaccination with MenACWY-TT or control vaccines. All subjects who had participated in the primary vaccination studyCitation17 were invited to return 4 y (year 4) and 5 y (year 5) after vaccination for assessment of antibody persistence. As per protocol, individual subjects noted to have rSBA-MenC titers <1:8 at any time point were revaccinated with a licensed MenC conjugate vaccine and discontinued from subsequent persistence analyses.
Results
There were 423 subjects who returned at year 4 and 176 who returned at year 5 (). Subjects who returned at year 5 included 64 in the 1-<2 y subgroup (49 MenACWY-TT recipients and 11 MenC-CRM recipients) and 112 in the 2-<11 y subgroup (98 MenACWY-TT recipients and 13 Men-PS recipients). The main reason for attrition of subjects between year 4 and year 5 was due to re-vaccination, which was offered to subjects with MenC rSBA titer <1:8 at year 4. In 1-<2 y olds, 113 (49.3%) children in the MenACWY-TT group and 42 (56.0%) in the MenC-CRM group had received re-vaccination with monovalent MenC vaccine by year 5. Among 2-<11 y olds, 86 (37.2%) subjects in the MenACWY-TT group and 56 (71.8%) in the Men-PS group had received re-vaccination by year 5 (). No subject declined to participate due to an adverse event or serious adverse event (). At each time-point the treatment groups were similar in terms of age ().
Figure 1. Study flow. ATP = According-to-protocol persistence cohort, LTF = lost to follow-up, TVC = Total vaccinated cohort.
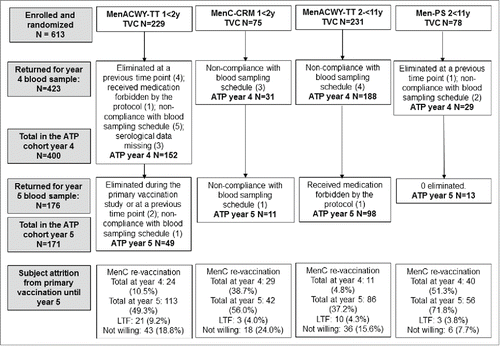
Table 1. Summary of demographic characteristics at follow up time points (According to protocol cohorts for persistence at year 4 and year 5)
Antibody persistence in children vaccinated as 1-<2 y olds
rSBA
Five years after primary vaccination of toddlers, the percentage of subjects in the MenACWY-TT group with rSBA antibody titers ≥1:8 was 73.5% for serogroup A, 77.6% for serogroup C, 34.7% for serogroup W and 42.9% for serogroup Y (). In the group that received MenC-CRM197, the percentage with rSBA-MenC antibody titers ≥1:8 was 63.6%. Exploratory evaluations did not suggest any difference between groups at year 4 or year 5 in terms of the percentage of subjects retaining rSBA titers ≥1:8 or ≥1:128, and the geometric mean antibody titer (GMT) for serogroup C. Between 4 and 5 y after vaccination, the observed MenC GMT increased artificially in both groups when considering the patients from the cohorts at Year4 and at Year5, due to drop out of MenC revaccinated subjects (see ). Indeed, when considering at Year 4 and Year 5 only the patients included in the cohort at Y5, the observed MenC GMT decreased between 4 and 5 y (109.7 vs 48.9 in the MenACWY-TT group and 137.2 vs 26.5 in MenC-CRM197 group).
Table 2. rSBA antibody persistence tested 4 and 5 y after vaccination of toddlers 1-<2 y of age with MenACWY-TT or MenC-CRM, and 2-<11 y olds with MenACWY-TT or Men-PS (According to protocol cohorts for persistence year 4 and year 5)
hSBA
Five years after primary vaccination, the percentage of MenACWY-TT-vaccinated toddlers with hSBA antibody titers ≥1:8 was 35.6% for serogroup A, 91.7% for serogroup C, 82.6% for serogroup W and 80.0% for serogroup Y (). In the group that received MenC-CRM197, the percentage with hSBA-MenC antibody titers ≥1:8 was 90.9%. As previously described for rSBA-MenC data, an artificial increase in hSBA-MenC GMT was observed between year 4 and year 5 (). Exploratory analyses detected no differences in hSBA antibody titers ≥1:4 or ≥1:8 and GMTs for serogroup C between the treatment groups at year 4 or year 5.
Figure 2. Percentage of toddlers with observed hSBA titers ≥1:8 prior to through 5 y after vaccination of toddlers with MenACWY-TT or MenC-CRM197.Footnote ATP cohorts for immunogenicity (primary vaccination) and ATP cohort for persistence at each time point. Vertical lines indicate 95% CIs.
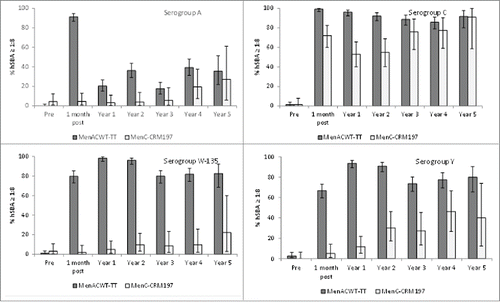
Figure 3. hSBA geometric mean titers (GMT) in toddlers prior to through 5 y after vaccination of toddlers with MenACWY-TT or MenC-CRM197. Footnote ATP cohorts for immunogenicity (primary vaccination) and ATP cohort for persistence at each time point. Vertical lines indicate 95% CIs.
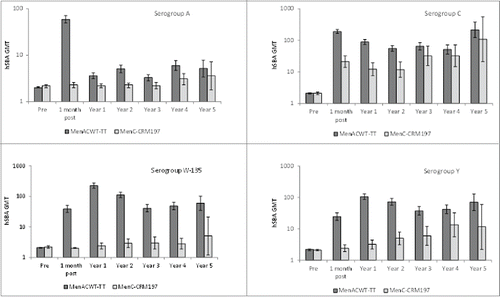
The MenC-CRM group did not receive vaccination against serogroups A, W and Y and the percentage with hSBA titers ≥1:8 increased gradually over time. At year 5 after primary vaccination, the percentages of subjects with hSBA titers ≥1:4, ≥1:8 and GMT remained statistically significantly lower in the MenC-CRM group than in the MenACWY-TT group for serogroup W only.
Antibody persistence in children vaccinated as 2-<11 y olds
rSBA
Five years after primary vaccination of children, the percentage of subjects in the MenACWY-TT group with rSBA antibody titers ≥1:8 was 90.8% for serogroups A and C, and 78.6% for serogroups W and Y (). In the group that received Men-PS, the percentages with rSBA antibody titers ≥1:8 were 15.4%, 100%, 0.0% and 7.7%, respectively. Exploratory evaluations indicated that at year 4 and year 5 there were higher percentages of subjects in the MenACWY-TT group with rSBA titers ≥1:8 and ≥1:128, and higher GMTs for serogroups A, W and Y compared with the Men-PS group. As previously mentioned, between 4 and 5 y after vaccination, the observed MenC GMT increased artificially in both groups. We also observed an increase of the serogroup A GMT in the MenACWY-TT group between 4 and 5 y.
Subject attrition and modeling results
By year 5, 48.5% of all subjects dropped out because of MenC re-vaccination, with a larger proportion of re-vaccinated subjects in both of the control groups, particularly for children 2-<11 y of age, than in the groups that received MenACWY-TT (). A repeated measurement model, done to assess the impact of the high subject drop-out on the results at year 5, confirmed that the rSBA-MenC values observed at year 5 were overestimated in both groups and both age strata (). However, the GMTs estimated by the model confirm the conclusions based on observed data: in toddlers persistence of rSBA-MenC titers was similar among MenACWY-TT and MenC-CRM197 recipients, while in children 2-<11 y of age, rSBA antibody persistence after MenACWY-TT was at least as good as for MenC, but higher for A, W and Y compared to Men-PS.
Figure 4. rSBA geometric mean titers (GMT) observed in the clinical trial or predicted via mathematical modeling in toddlers (serogroup C) and children (serogroups A, C, W and Y) at year 5. Footnote Observed = GMTs from subjects who returned to the year 5 visit (ATP cohort for persistence year 5). Estimated = GMTs estimated from the observed values in each group from post-vaccination (month 1) to year 5. For each time point, the model took into account the values of the previous time points in order to correct for possible selection effect due to drop out. Vertical lines indicate 95% CIs.
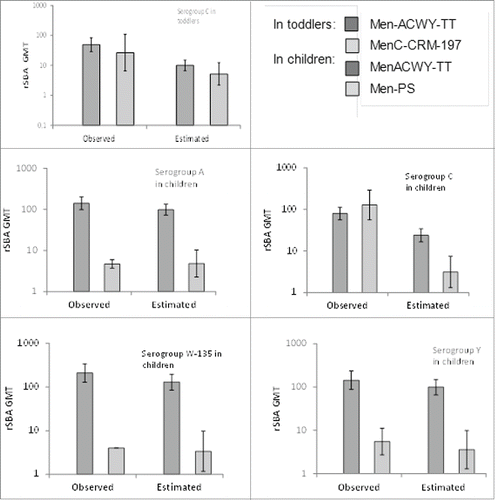
This is supported by the observed rSBA-MenC GMT at year 4 (where the same rSBA assay was used) for toddlers who returned at year 5, which was 109.7 in the MenACWY-TT group and 137.2 in the MenC-CRM group, contrasting with GMTs of 11.2 and 11.4, respectively among all subjects tested at year 4, of whom a percentage subsequently received MenC re-vaccination and were withdrawn from the study. In children, the observed year 4 rSBA-MenC GMT in those who returned at year 5 was 118.3 and 206.8 in the MenACWY-TT and MenPS groups, respectively (vs. 21.7 and 23.5, respectively, in the cohort who returned for the year 4 persistence visit). This suggests that subject drop-out due to revaccination between year 4 and year 5 influenced the results for MenC at year 5.
Safety
No related serious adverse events were reported from 6 months after primary vaccination study up until the visit at Year 5.
Discussion
Five years after vaccination of toddlers with MenACWY-TT, rSBA titers ≥1:8 persisted in the majority of subjects for serogroups A and C, but declined to 34.7% for serogroup W and 42.9% for serogroups Y. In 2-<11 y olds vaccinated with MenACWY-TT, rSBA antibodies ≥1:8 persisted in at least 78% of subjects for all serogroups at year 5. Higher persistence for serogroups A, W and Y in the MenACWY-TT group than in Men-PS vaccinees is consistent with results at year 3.Citation19
Persistence of MenC antibodies in both age groups appeared to be similar to licensed vaccines. However these data should be interpreted cautiously given that subjects whose MenC titers had decreased to below the assay cut-off were discontinued from the study. Longitudinal modeling suggested that MenC titers in both age groups and in both vaccine groups were likely to be overestimated, but also suggested that persistence of rSBA-MenC may have been higher after MenACWY-TT than after control vaccines in both groups. The modeling exercise also helps explain the apparent observed increase in rSBA antibody titers between year 4 and year 5 for all treatment groups and age strata.
Assessment of hSBA in toddlers showed a gradual increase in the percentage of subjects who were not originally vaccinated with A, W, and Y conjugates with hSBA titers ≥1:8 for serogroups A, W and Y. This is suggestive of the acquisition of natural immunity in these children due to exposure to meningococci or to cross-reacting bacteria. It is noteworthy that at all time points after vaccination both hSBA GMTs and the percentage with hSBA titers ≥1:8 were observed to be higher in the MenACWY-TT group than in the control group for serogroups A, W and Y, suggesting a benefit of vaccination during the second year of life over acquisition of natural immunity.
Although the high drop-out rate limits the utility of these data in informing on booster policy, the observation that around 50% of toddlers in both groups required re-vaccination before year 5 suggests that a booster dose earlier than 5 y after priming may be warranted. This is supported by results of a recent study (NCT00955682) in which MenACWY-TT-primed toddlers received a booster dose of the same vaccine 4 y after priming. Robust booster responses (rSBA and hSBA) to all serogroups were observed, with more than 95% of children maintaining rSBA and hSBA titers ≥1:8 one year after the booster dose.Citation20 In our study, fewer children vaccinated with MenACWY-TT as 2-<11 y olds required re-vaccination by year 5, suggesting that a longer interval between boosters may be possible when priming occurs beyond infancy and a benefit in terms of the durability of the immune response after conjugate versus Men-PS vaccination.
To our knowledge this is one of only a few studies that have assessed antibody persistence extending 5 y after vaccination with a quadrivalent MenACWY vaccine during childhood or adolescence. Citation12,15,20,21 Our study adds to a growing body of persistence data following MenACWY-TT vaccination in toddlers and children. Comparisons with other studies are hampered when different assays (rSBA or hSBA) have been used and because of inter-laboratory variation within the same assay. Within these limitations, rSBA (and hSBA where available) persistence for serogroup C following MenACWY-TT appears to be comparable to that following vaccination with other meningococcal conjugate vaccines licensed for use in children (MenC-CRM197 and HibMenC-TT).Citation22,23 Moreover, our results in toddlers appear to be in line with rSBA observed 2-to-3 y after a single dose of MenACWY-DT in 2-to-3 y olds (% rSBA ≥ 1:128: 75% for serogroup A, 52% for C, 61% for W and 90% for Y).Citation24 While the percentage with hSBA serogroup C ≥1:4 reduced markedly 2-to-3 y after MenACWY-DT vaccination at 2 y of age (14.6%),Citation16 we observed good hSBA-MenC persistence until year 5 (hSBA serogroup C ≥1:8: 91.7%), although the high drop-out rate means that our estimate is likely to be overestimated. Five year persistence after a single dose of MenACWY-TT administered in the second year of life appears to compare favorably with 3 y persistence after 2 doses of MenACWY-DT administered between 9 and 15 months of age (% hSBA ≥1:8 [approximate]: 45% for serogroup A, 11% for C, 14% for W and 21% for Y).Citation25 MenACWY-CRM197 persistence data using comparable ages/schedules are not available, but 5 y after 2 or 3 priming doses in infancy with a booster dose at 12 months of age hSBA titers ≥1:8 were present in ≤11% of subjects for serogroup A, ≤ 45% for C, ≤85% for W and ≤71% for Y.Citation15
This study is potentially limited by the lack of an ACWY conjugate control group in both age groups, because no quadrivalent meningococcal conjugate vaccines were licensed for toddlers in any country at the time of the primary vaccination study. The change in the rSBA assay during the persistence phase limits interpretation of the results with respect to previous time points. The available data indicate that rSBA assay conducted at GSK Vaccines' laboratories were more sensitive to naturally acquired antibodies than the PHE rSBA assay,Citation26,27 suggesting persistence of antibodies might be expected to be higher if the test was not changed. Finally, attrition of subjects due to the re-vaccination policy meant that low responders were discontinued from further participation. This also led to a low number of subjects in control groups at year 5, which implies an imprecision in the point estimates, and potentially limited applicability to the wider population. Nevertheless, correction of the selection effect using a repeated measurement statistical model confirmed that MenC antibody persistence in toddlers was similar 5 y after MenACWY-TT or after MenC-CRM197 vaccination; and that in children, antibody persistence to all 4 serogroups appeared better 5 y after MenACWY-TT vaccination than after Men-PS.
In conclusion, antibody persistence was observed for all serogroups up to 5 y after MenACWY-TT vaccination in 1–10 year-old children. Additional serogroups in MenACWY-TT compared to MenCCRM197 did not affect MenC persistence in 1-<2 year-olds. Functional antibodies against serogroups A, W and Y persisted longer in 2–10 year-olds vaccinated with MenACWY-TT than with Men-PS. Evaluation of this cohort in terms of antibody persistence is ongoing.
Methods
Study design and objectives
The previous phase 2 vaccination study (NCT00427908) was conducted in 11 study centers in Finland.Citation17,19 Healthy children were randomized (3:1) to receive MenACWY-TT or MenC-CRM197 vaccine (toddlers between 12 and 23 months of age) or Men-PS (children between 2 and 10 y of age).
Subjects were excluded from participating in the follow up studies if they had developed meningococcal disease or had received any meningococcal vaccination since the primary vaccination study. Additional follow-up is planned yearly until year 10.
The study objectives at each persistence time point were to assess persistence of antibodies in both study groups, and to describe serious adverse events related to vaccination and any event related to the lack of vaccine efficacy (i.e. meningococcal disease) from 6 months after vaccination until each follow-up time point.
The study protocol and associated documents were reviewed and approved by the Ethics Committee of Pirkanmaa Hospital District. Written informed consent was obtained from parent(s)/guardian(s) at the time of entry to the primary vaccination study. The study was conducted in accordance with Good Clinical Practice, all applicable regulatory requirements and the Declaration of Helsinki.
Immunogenicity assessment
A change in the rSBA assay took place during the study and was implemented from year 4, precluding direct comparison with results available from year 3 and earlier that were performed with the historical assay. Prior to year 4 samples were tested at GSK Vaccines' laboratories, whereas samples collected from year 4 were tested at Public Health England (PHE) in the UK.
Blood samples were collected from all subjects who returned at the year 4 and year 5 follow up time points. Samples from all subjects were tested for serum bactericidal antibodies using rSBA. Samples from toddlers were also tested using hSBA measured at GSK Vaccines' laboratories. In contrast to the rSBA data, hSBA results were available from the beginning of the study. All assays were based on the Centers for Disease Control protocol.Citation28 The assay cut-offs were a 1:8 dilution for rSBA and a 1:4 dilution for hSBA. Seroprotection for MenC has been determined as an antibody titer ≥1:8 for rSBA,Citation29 and ≥1:4 for hSBA.Citation30 These cut-offs were also applied to the other serogroups.Citation31 In addition, data were analyzed using higher thresholds of 1:128 (rSBA) and 1:8 (hSBA).
Safety assessment
Serious adverse events considered by the investigator to be causally related to study procedures or GSK concomitant medication were captured retrospectively at each study visit at year 4 and year 5.
Statistical analyses
The primary analysis of persistence was done on the According-to-Protocol persistence cohorts at each time point, which included subjects who had complied with all protocol-defined procedures and who had data available for at least one immunogenicity endpoint. Analyses were performed for the 1<2 y and the 2-<11 y age strata.
Statistical analyses were performed using SAS® software version 9.2 (SAS Institute Inc., Cary, NC, United States) and ProcStatXact 8.1 (Cytel Software Corp., Cambridge, MA). At each time point and for each vaccine serogroup, the percentages of subjects with titers above the planned cut-offs were calculated with exact 95% confidence interval (CIs). GMTs with 95% CIs were tabulated.
For each of the serogroups, an exploratory evaluation of the differences in the immune response at each time point was performed in terms of differences in the percentage of subjects with rSBA antibody titers ≥1:8 and ≥1:128 (and hSBA titers ≥1:4 and ≥1:8 in toddlers) with standardized asymptotic 95% CIs and the ratio of the GMTs with 95% CIs between the MenACWY-TT and control groups. The hSBA GMT ratio was calculated using an Analysis of Covariance (ANCOVA) model on the logarithm10 transformation of the titers using the pre-vaccination logarithm10 transformation of the titers, the age strata and the vaccine group as covariates. For rSBA data an Analysis of Variance (ANOVA) model was used. A significant difference was indicated if the value ‘1’ was excluded from the 95% CI on the GMT ratios or if the value ‘0’ was excluded from the 95% CI on differences between groups. Note that potential differences should be interpreted with caution considering that there was no adjustment for multiplicity for these comparisons, and that statistically significant findings may have occurred by chance alone.
To investigate potential selection bias in the persistence analysis due to the drop-out of subjects due to any reason, including re-vaccination with MenC conjugate vaccine, a longitudinal repeated measurement statistical model (mixed model, SAS Proc mixed) was fitted, taking into account the group and the rSBA titer values (using the GSK rSBA or PHE rSBA assay, as appropriate) for subjects until they dropped out, to correct for the estimation of the GMTs at each time point.
NIMENRIX, MENITORIX and MENVEO are registered trademarks of the GSK Group of Companies. NIMENRIX trademark has been licensed to Pfizer, formerly Wyeth. MENINGITEC, MENCEVAX are registered trademarks of Pfizer, formerly Wyeth.
Disclosure of Potential Conflicts of Interest
Pr Vesikari received personal consulting fees, support for meetings, travel or accommodation expenses for the study and fees for participation in review activities from GSK group of companies. Pr Vesikari received also personal consulting fees from MedImmune, Merck, Sanofi Pasteur-MSD, and Novartis. Ms Bianco and Drs Van der Wielen and Miller are employees of GSK Vaccines. Drs Van der Wielen and Miller declare restricted stock shares in the GSK group of companies. Dr Miller is also inventor on a patent owned by the GSK group of companies. Dr Forsten has no conflict to disclose.
Authors' Contributions
Pr Vesikari and Dr Forsten were investigators involved in the supervision of the study, administrative, logistic and technical supports, the recruitment and the medical evaluation of subjects, the evaluation of any reported AEs/ SAEs for severity and causality, the collection and interpretation of the data, and the drafting and approval of the manuscript. Dr JM. Miller, Dr M. Van der Wielen (clinical development scientists) and V. Bianco (biostatistician) are employed by GSK Vaccines and were involved in all stages of the study (study design, data analyses and interpretations, drafting and approval of the manuscript).
Acknowledgments
The authors thank the families and children who participated in the study, and the study investigators, nurses and other staff members without whom this study would not have been possible. The authors thank Public Health England for coordinating and performing the rSBA immunogenicity testing. We are also grateful to teams in GSK Vaccines for their contribution to this study, including Markuu Pulkkinen and Taneli Puumalainen for their assistance in coordination of the study, Emmanuel Aris and Kolhe Devayani for input into statistical analysis, Leentje Moerman for protocol writing and Pascal R Lestrate for conducting laboratory assays. The authors also thank Joanne Wolter (Independent medical writer on behalf of GSK Vaccines) for preparing the first draft of the manuscript and Virginie Durbecq (XPE Pharma & Science on behalf of GSK Vaccines) for providing editorial support.
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript. The corresponding author had full access to the data and was responsible for submission of the publication.
References
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med 2001; 344:1378-88; PMID:11333996; http://dx.doi.org/10.1056/NEJM200105033441807
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27 (Suppl 2):B51-63; PMID:19477562; http://dx.doi.org/10.1016/j.vaccine.2009.04.063
- European Centre for Disease Prevention and Control. Annual epidemiological report Reporting on 2009 surveillance data and 2010 epidemic intelligence data 2011 [Internet]. 2011 [cited 2013 Feb 4]. Available from: http://ecdc.europa.eu/en/publications/Publications/1111_SUR_Annual_Epidemiological_Report_on_Communicable_Diseases_in_Europe.pdf
- Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, Pondo T, Arnold KE, Baumbach J, Bennett N, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis 2010; 50:184-91; PMID:20001736; http://dx.doi.org/10.1086/649209
- Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine 2001; 20 (Suppl 1):S58-67; PMID:11587814; http://dx.doi.org/10.1016/S0264-410X(01)00299-7
- Trotter CL, Chandra M, Cano R, Larrauri A, Ramsay ME, Brehony C, Jolley KA, Maiden MCJ, Heuberger S, Frosch M. A surveillance network for meningococcal disease in Europe. FEMS Microbiol Rev 2007; 31:27-36; PMID:17168995; http://dx.doi.org/10.1111/j.1574-6976.2006.00060.x
- European Centre for Disease Prevention and Control. Surveillance of invasive bacterial diseases in Europe [Internet]. [cited 2015 Feb 19]. Available from: http://www.ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx?List=4f55ad51-4aed-4d32-b960-af70113dbb90&ID=1261
- Bexsero. meningococcal group-B vaccine (rDNA, component, adsorbed) [Internet]. European Medicines Agency 2013 [cited 2013 Dec 28]; Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002333/human_med_001614.jsp&mid=WC0b01ac058001d124
- Edwards EA, Devine LF, Sengbusch GH, Ward HW. Immunological investigations of meningococcal disease. III. Brevity of group C acquisition prior to disease occurrence. Scand J Infect Dis 1977; 9:105-10; PMID:408918; http://dx.doi.org/10.3109/inf.1977.9.issue-2.09
- Andersen J, Berthelsen L, Jensen BB, Lind I. Surveillance of cases of meningococcal disease associated with military recruits studied for meningococcal carriage. Scand J Infect Dis 2000; 32:527-31; PMID:11055659; http://dx.doi.org/10.1080/003655400458820
- Auckland C, Gray S, Borrow R, Andrews N, Goldblatt D, Ramsay M, Miller E. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. J Infect Dis 2006; 194:1745-52; PMID:17109348; http://dx.doi.org/10.1086/509619
- Meningococcal conjugate vaccines policy update: booster dose recommendations. Pediatrics 2011; 128:1213-8; PMID:22123893; http://dx.doi.org/10.1542/peds.2011–2380
- Gill CJ, Baxter R, Anemona A, Ciavarro G, Dull P. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin 2010; 6:881-7; PMID:21339701; http://dx.doi.org/10.4161/hv.6.11.12849
- Vu DM, Welsch JA, Zuno-Mitchell P, Dela Cruz JV, Granoff DM. Antibody persistence 3 years after immunization of adolescents with quadrivalent meningococcal conjugate vaccine. J Infect Dis 2006; 193:821-8; PMID:16479517; http://dx.doi.org/10.1086/500512
- Khatami A, Snape MD, Davis E, Layton H, John T, Yu L-M, Dull PM, Gill CJ, Odrjlin T, Dobson S, et al. Persistence of the immune response at 5 years of age following infant immunisation with investigational quadrivalent MenACWY conjugate vaccine formulations. Vaccine 2012; 30:2831-8; PMID:22394992; http://dx.doi.org/10.1016/j.vaccine.2012.02.046
- Granoff DM, Morgan A, Welsch JA. Persistence of group C anticapsular antibodies two to three years after immunization with an investigational quadrivalent Neisseria meningitidis-diphtheria toxoid conjugate vaccine. Pediatr Infect Dis J 2005; 24:132-6; PMID:15702041
- Vesikari T, Forstén A, Boutriau D, Bianco V, Van der Wielen M, Miller JM. Randomized trial to assess the immunogenicity, safety and antibody persistence up to three years after a single dose of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in toddlers. Hum Vaccin Immunother 2012; 8:1892-903; PMID:23032159; http://dx.doi.org/10.4161/hv.22166
- Vesikari T, Forstén A, Bianco V, Van der Wielen M, Miller J. Antibody persistence 4 years after vaccination with a quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy toddlers. Milan, Italy: 2013.
- Vesikari T, Forstén A, Boutriau D, Bianco V, Van der Wielen M, Miller JM. A randomized study to assess the immunogenicity, antibody persistence and safety of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in children aged 2–10 y. Hum Vaccin Immunother 2012; 8:1882-91; PMID:23032168
- Vesikari T, Forstén A, Bianco V, Van der Wielen M, Miller J. Immunogenicity and safety after booster vaccination with a quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy children. Milan, Italy: 2013.
- Jacobson RM, Jackson LA, Reisinger K, Izu A, Odrljin T, Dull PM. Antibody persistence and response to a booster dose of a quadrivalent conjugate vaccine for meningococcal disease in adolescents. Pediatr Infect Dis J 2013; 32(4):e170–7; http://dx.doi.org/10.1097/INF.0b013e318279ac38
- Knuf M, Baine Y, Bianco V, Boutriau D, Miller JM. Antibody persistence and immune memory 15 mo after priming with an investigational tetravalent meningococcal tetanus toxoid conjugate vaccine (MenACWY-TT) in toddlers and young children. Hum Vaccin Immunother 2012; 8:866-72; PMID:22485049; http://dx.doi.org/10.4161/hv.20229
- Booy R, Richmond P, Nolan T, McVernon J, Marshall H, Nissen M, Reynolds G, Ziegler JB, Stoney T, Heron L, et al. Three-year antibody persistence and safety after a single dose of combined Haemophilus influenzae type b (Hib)-Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine in Hib-primed toddlers. Pediatr Infect Dis J 2013; 32:169-74; PMID:23080288
- Pichichero M, Papa T, Blatter M, Mitchell D, Kratz R, Sneed J, Bassily E, Casey J, Gilmet G. Immune memory in children previously vaccinated with an experimental quadrivalent meningococcal polysaccharide diphtheria toxoid conjugate vaccine. Pediatr Infect Dis J 2006; 25:995-1000; PMID:17072120; http://dx.doi.org/10.1097/01.inf.0000243215.46312.4a
- Johnson DR. Menactra®Infant Indication [Internet]. 2011 [cited 2012 Apr 13]; Available from: http://www.cdc.gov/vaccines/recs/acip/meetings.htm
- Lechevin I, Le Bras V, Lestrate P, Wauters D. Identification of key parameters that impact the sensitivity of the MenC-rSBA assay to natural antibodies [internet]. 18th International Pathogenic Neisseria Conference, Würzburg, Germany: 2012 [cited 2015 Apr 30]. Available from: http://neisseria.org/ipnc/2012/IPNC_2012_abstracts.pdf
- Lechevin I, Wauters D, Durant N, Bernard A, De Schrevel N, Henckaerts I, Bai X, Findlow J, Borrow R, Lestrate P. Bridging of two serum bactericidal antibody assays using rabbit complement performed at the Health Protection Agency and at GlaxoSmithKline Biologicals [Internet]. 18th International Pathogenic Neisseria Conference, Würzburg, Germany: 2012 [cited 2015 Apr 30]. Available from: http://neisseria.org/ipnc/2012/IPNC_2012_abstracts.pdf
- Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, Arhin FF, Devi SJ, Frasch CE, Huang JC, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol 1997; 4:156-67; PMID:9067649
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection–serum bactericidal antibody activity. Vaccine 2005; 23:2222-7; PMID:15755600; http://dx.doi.org/10.1016/j.vaccine.2005.01.051
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med 1969; 129:1307-26; PMID:4977280; http://dx.doi.org/10.1084/jem.129.6.1307
- Centers for Disease Control and Prevention. Inadvertent misadministration of meningococcal conjugate vaccine–United States, June-August 2005. MMWR Morb Mortal Wkly Rep 2006; 55:1016-7; PMID:16988640
