Abstract
The cost of HPV vaccines and the need for 3 doses remains a barrier for their inclusion in routine vaccination schedules for girls in low and middle income countries. In a non-inferiority study, we aimed to compare the immunogenicity of a standard 3 doses and a 2 doses schedule. We enrolled 450 participants in an open-label non-randomized clinical trial to evaluate the immunogenicity induced at different ages by the licensed HPV6/11/16/18 quadrivalent vaccine in a 2 doses schedule (0–6 months, n = 150 girls aged 9–10 y) and 3 doses schedule (0, 2, and 6 months; n = 150 girls aged 9–10 y and n=150 women aged 18 to 24 years). To assess the antibody response, blood samples were obtained at Month 7 and 21 after the first vaccination from participants in all study groups. cLIA testing was performed at Merck Research Laboratories. Antibody levels were expressed as milli-Merck units (mMU) per ml. Primary outcome was non-inferiority (95% CI, lower bound >0.5) of the geometric mean titers (GMT) ratios for HPV6, HPV11, HPV16 and HPV18 antibodies 7 and 21 months after the first dose among girls receiving 2 doses compared with young women and girls receiving 3 doses. All vaccinees were seropositive for both HPV16 and HPV18 antibodies at month 7. At month 21, 98.5 and 56.6% of women 18–24 y old were seropositive for HPV16 and 18, respectively. For girls in the three doses group, seropositivity rates were 99.3 and 86.3% for HPV16 and 18, respectively. For girls in the two doses group rates were 99.3 and 70.2% for HPV16 and 18, respectively. The two doses schedule was non-inferior compared to the 3 doses schedule in same-age girls and to the group of adult women after 21 months of the first vaccine dose. Our results are in agreement with similar trials evaluating the immune response of a 2 doses schedule of both HPV vaccines, supporting the recent WHO recommendation as well as the Mexican policy to incorporate the 2 doses schedule for girls aged 9–11 y.
Introduction
It is estimated that HPV genotypes 16 and 18, which are found in bivalent and quadrivalent HPV vaccines, contribute to at least 70% of invasive cervical cancer cases in Latin America.Citation1 These vaccines are highly effective,Citation2 providing high levels of immunogenicityCitation3 with an acceptable safety profile.Citation4 The maximum cost-benefit ratio has been estimated to occur when the vaccine is administered before exposure to HPV,Citation5 and universal HPV vaccination schemes should therefore focus on adolescent girls who have not yet become sexually active.Citation6 These vaccines have been incorporated into public vaccination policies with 3 doses schemes administered over a period of 6 months.Citation7
High effectiveness of the tetravalent vaccine in preventing genital warts has been observed in SwedenCitation8 and Denmark,Citation9 and a herd immunity effect has been documented in Australia.Citation10 In the United States, 4 y after the introduction of a vaccination program, 56% reductions in HPV prevalence have been observed in women between 14 and 19 y of age despite the low coverage rates achieved for the second and third doses.Citation11 Reduction in the incidence of high grade cytological abnormality has also been observed in AustraliaCitation12 and in Denmark.Citation13
Studies of antibody responses and duration following HPV vaccination have shown a maximum peak in antibody titers 7 months after beginning the vaccination scheme.Citation14,15 Subsequently, a gradual decline in antibody levels is observed, and by the 24th month these levels stabilize and remain constant until at least the 60th month.Citation16 To date, the minimum level of antibodies correlated with clinical protection is not yet known,Citation17 but a correlation has been established between the presence of antibodies and a protective effect. However, it has been observed that in women between 10 and 17 years of age, the geometric mean titers (GMTs) of IgG are at least twice as high as those in women aged from 18 to 25 y.Citation18 The immune response of 9–11 y old girls after 2 doses of quadrivalent HPV (qHPV) vaccine is similar to or greater than that the one obtained after 3 doses in women 16–26 y of age, the age group for which the efficacy of the vaccine has been demonstrated.Citation19 In subjects vaccinated under the traditional scheme, the application of an extra (fourth) dose of the vaccine 60 months after the first vaccination resulted in a rapid and vigorous memory immune response against all 4 HPV genotypes included in the qHPV vaccine, which exceeded the levels of GMTs observed one month after the third dose.Citation20 This response was similar to the response observed with the Hepatitis B vaccine.Citation21
In 2008 Mexico implemented an extended vaccination schedule of 0–6–60 months in girls younger than 16 y.Citation22 There is now evidence that alternative vaccination schemes are not inferior to the traditional scheme in terms of immunogenicity.Citation23 In April 2014 the World Health Organization Strategic Advisory Group of Experts in Immunization recommended a 2 doses schedule (0–6 Months) for girls under 15 y of age, taking into consideration evidence from non-inferiority immunogenicity trials.Citation24 Currently, the European Union, in addition to some other countries worldwide approved the administration of 2 doses of HPV vaccine.Citation25 Furthermore, since April 2014, the regulatory authority in Mexico has approved the use of 2 doses of the HPV vaccine in girls aged 9–14 y.Citation26
In this article we present the immunogenicity achieved using a qHPV vaccine at Month 21 in 3 comparison groups of Mexican women. These results are useful for monitoring the effect of the vaccine in terms of the immune response generated by a 2 doses schedule compared to the standard scheme.
Results
Four hundred fifty participants were enrolled in this trial. All 150 girls assigned to the standard schedule received complete doses. Of the 150 girls assigned to the 2 doses schedule, 98% (148/150) received the complete vaccine series. One hundred forty three (95.3%) women aged 18–24 y received the qHPV vaccine on the standard 3 doses schedule (). Regardless of their group, participants received the vaccine within the 6-month interval between the first (month 0) and last dose (month 6).
Figure 1. CONSORT diagram. Figure legend. Flowchart of participants disposition throughout the study.
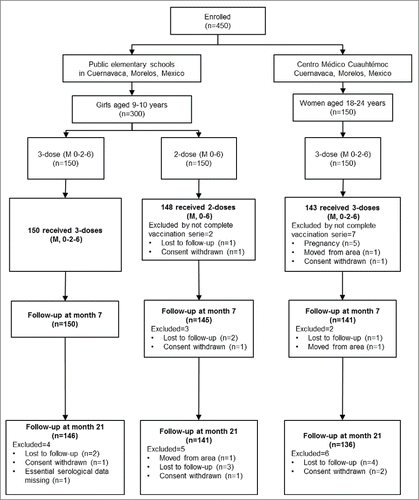
The time range between the last dose of the vaccine and the blood sample collection for the first serological evaluation (Month 7) ranged from 0.7–1.6 months for all girls (independently of the vaccine schedule group), and 0.7–1.7 months for the adult women. All of the groups complied with the predefined second serologic assessment between Months 19 and 23 after the first dose.
All of the vaccinees were seropositive for both HPV16 and HPV18 antibodies at Month 7 (). However, only 56.6% of the women aged 18–24 y were seropositive for HPV18 at Month 21.
Table 1. Seropositivity prevalence
The distribution of serum antibody titers for HPV6/11/16/18 of girls with 2 doses regime and adult women with 3 doses according to the quartiles categories of antibody titers defined in the reference group of girls receiving the 3 doses regime is shown in . We observed the 2 doses group tended to have a greater distribution of HPV16 antibodies across the top quartiles values defined by the reference group distribution: 19.1% were in the lowest quartile and 28.4% were in the highest quartile. In contrast, a large proportion of girls (29.8%) in the 2 doses group were seronegative and 24.8% in the lowest quartile of anti-HPV18 of the reference group. Regarding women aged 18–24 y we should emphasize the large proportion (43.4%) of this group who were seronegative for HPV18 at month 21, while only 5.1% of this group had comparable levels to those in the top quartile.
Table 2. Distribution in quartiles of titers of HPV16/18/6/11 antibodies, as measured in milli-Merck units per mL (mMU/mL), for the cohort of 3 study groups at Months 7 and 21 according to protocol cohort analysis
The GMTs for both HPV-type antibodies at Months 7 and 21 for all groups are shown in . The GMTs for HPV6, 11, 16 and 18 antibodies at 21 months after the first vaccine dose in the 2 doses group of girls were higher and statistically non-inferior to those in the group of adult women. Non-inferiority was demonstrated because the lower limit of the 95% CI of the GMT ratios for HPV6, HPV11, HPV16 and HPV18 for girls (2 doses) compared with young women (3 doses) as well as girls (3 doses) 21 months after the first dose was above the predefined limit of 0.5 (). When comparing the 2 doses vs. the 3 doses group among girls, antibodies were higher in the 3 dose group, but the levels for those receiving 2 doses were statistically non-inferior ().
Table 3. Comparisons of the geometric mean titers (GMTs) of HPV16, 18, 6 and 11 antibodies, as measured in milli-Merck units per mL (mMU/mL), for the cohort of 3 study groups at Months 7 and 21 according to protocol cohort analysis
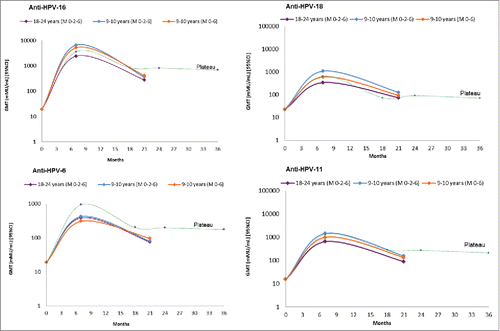
The kinetics of antibodies against HPV6/11/16/18 in the 2 doses group of girls aged 9–10 y followed a similar pattern to those observed in both other groups; after a peak response one month after the third dose (month 7), a decline in antibody titers was observed at Month 21 (). However, the levels remained above those reported in women with natural infection with the exception of HPV 11, whose titers dropped to the level of natural infection.Citation27
Figure 2. Kinetics of antibody responses to HPV-16/18/6/11 from quadrivalent vaccine. *GMTs, geometric mean titers; milli-Merck units per mL (mMU/mL); CI, confidence interval; M 0,2,6, HPV-16/18/6/11 vaccine at standard schedule at 0,2,6 months; M 0–6–60, HPV-16/18/6/11 vaccine at extended schedule at 0–6–60 months. Plateau (dotted line), GMT values for Canadian women ages 16–26 y who were vaccinated with 3 doses of the quadrivalent vaccine.Citation28
Safety was evaluated in all 450 of the participants who received at least one dose and who attended the subsequent post-vaccination appointment. Among the solicited reports of adverse events following any vaccination, we had very infrequent reports in all 3 comparison groups (). Pain at the injection site was the most common local symptom across groups. No participants withdrew from the study because of an adverse event, and there were no serious or fatal events.
Table 4. Symptoms reported during the next vaccination period by schedule of vaccine administration and age (total vaccinated cohort)
Discussion
Our findings are consistent with previous studies documenting that in terms of immunogenicity 2 doses of the HPV6/11/16/18 vaccine in girls 9 to 10 years-old are not inferior to 3 doses among girls at the same age group as well as women 18 to 24 y.
In 2008 the Mexican government adopted an extended HPV vaccination scheme (0–6–60 month) focusing on 9 to 11 years-old girls. This schedule was adopted trying to achieve maximum antibody levels just before their sexual activity begins, under the hypothesis that the delay in application of the third dose would lead to an increase in antibody levels. This was a controversial decision, since in the absence of efficacy studies for a 2 doses HPV vaccination schedule it assumed plausibility of protection from HPV infection as a function of the immune response at an early age.
The principal basis for alternative 2 doses vaccination schedules is that the magnitude of the immune response is inversely proportional to the age at which the vaccine is applied,Citation4 and the application of 2 doses of HPV vaccines at a minimum 6-month interval appears to provide a level of protection similar to 3 doses, during the initial years after vaccination.Citation28 The serum levels of HPV antibodies observed after vaccination last longer the younger the age at vaccination as long as there is a 6 to 12 month interval between the first and second doses.Citation23 The principal public health implications of this policy are to lower costs and flexibility in the interval between doses, which could improve operational efficiency and allow increased coverage in low- and middle-income countries, as well as eliminate the observed low adherence to the third dose.Citation29
In relation to persistence of immune response to the HPV vaccine, a number of studies have reported that the magnitude of response could increase when the interval between the first and second dose is between 6 and 12 months.Citation4 In fact, a study on immunogenicity after the application of the HPV6/11/16/18 vaccine in a cohort of adolescents which included Mexican young men and women recently documented persistence of a high level of antibody levels for HPV types 6, 11, 16 and 18.Citation30 The HPV6, 11, 16 and 18 antibodies levels we observed in the present study are consistent with the non-inferiority of 2 doses in young girls as compared with 3 doses in adult women, in accordance to a similar previous study.Citation28 We recognize that the efficacy of HPV vaccine depends not only on the amount of antibodies induced by vaccines, but it is also relying on some other factors like the quality of these antibodies, or the cellular immunity such as immune memory.Citation31 However, the evaluation of those immune factors is out of the scope for the present analysis. We strongly recommend further evaluations in this area.
We should emphasize that a large proportion of women aged 18–24 y (43.4%) were seronegative for HPV 18 at month 21, while among the girls with 3 doses and 2 doses, only 13.7 or 29.8% of them were seronegative, respectively. This reduction of seropositivity for HPV18 by cLIA is consistent with findings in aprevious study in Colombia, which reported a 45% of seropositivity for HPV18 at month 72, nevertheless a sustained absence of disease due to HPV18, as well as due to the other HPV vaccine types was observed.Citation32 However, when the total IgG assay is used, the proportion of women who are seropositive for HPV18 at month 72 is substantially higher (81.5% overall). The high seronegativity rate for HPV18 deserves further evaluation to better understand the long term protection of less than 3 doses schedule. In addition, it should be kept in mind that the cLIA assay is a competitive assay that measures only the subset of neutralizing antibodies that compete with the specific monoclonal antibody used in the assay for VLP surface binding. Thus, the assay can underrepresent the potentially protective antibody response induced by the vaccine.Citation33
Alternative two doses HPV vaccination schedules are emerging as a new global strategy, including in high-income countries. Between the months of December, 2013 and February, 2014 the European Medicines Agency, which is responsible for scientific evaluation and supervision of medicines, approved the use of 2 doses for the bivalent and tetravalent vaccine for girls under 15-years-old. After this, Great Britain,Citation34 the Provinces of Quebec and British Columbia in CanadaCitation35 Switzerland,Citation36 South AfricaCitation37 and AustriaCitation37 adopted policies with 2 doses for HPV vaccination in girls under 15-years-old. A two-dose system has also been approved in some other countries like Bangladesh, Pakistan, the Philippines, Ghana, Nigeria, South Africa, El Salvador, Guyana, Haiti, Panama, Brazil, Colombia, Chile, Guatemala among others.Citation38
In Mexico, the medicine regulation agency (COFEPRIS) approved a 2 doses (0–6 months) schedule for the bivalent and HPV6/11/16/18 vaccines in 11-year-old girls on April 28th, 2014.Citation26 Similarly, Chile is currently preparing for implementation of a 2 doses scheme (0–12 months) and 52 countries have begun processes of approval of 2 doses schemes within their regulating agencies. In this context, October 24th, 2014 the World Health Organization made public its recommendation of 2 vaccine doses for girls 14-years-old or under, derived from the recommendations of their Strategic Advisory Group of Experts (SAGE).Citation39
Although the HPV vaccines are highly efficacious and immunogenic, to date there is no immune correlate of protection against cervical disease. Duration of protection of a 2 doses scheme requires further evaluation. To add to our knowledge and understanding of the protection against HPV infection induced by HPV vaccination, future studies addressing effectiveness of alternative schedules should evaluate virological endpoints such as 6-month persistent HPV 16/18. These virogical endpoints have been recommend by IARC as surrogate of clinical end-points in HPV trials.Citation40,41
We recognize some limitations of our trial. The lack of randomization among our population may not guarantee a homogenous distribution of socio-demographic or other relevant population characteristics, and we did not collect individual socio-demographic data to confirm potential differences. However, girls are from schools located in the urban area of Cuernavaca, Mexico, and they are both public schools that receive students from lower to middle-class socioeconomic backgrounds living close to those schools.
Vaccination should be one element in efforts to strengthen cervical cancer prevention and control efforts, in addition to early detection and treatment with guaranteed quality control. An epidemiological surveillance system should be incorporated using a centralized laboratory that quantifies HPV16 and 18 antibodies in the vaccinated population to evaluate the immune response to 2 doses and to estimate the possible protective effect, as this study among Mexican women did. We currently face the situation that most anti-HPV vaccine immunogenicity studies have used assays developed by the companies that develop the vaccines, which are not easily available to research groups, unless they participate in studies funded by the pharmaceutical industry. This means that there is a need to develop, validate and standardize serological studies that are easily available for the benefit of epidemiological surveillance systems. Given this, we should implement mechanisms for monitoring antibodies in alternative HPV vaccination schedules as a central part of an epidemiological surveillance system that could provide alerts of a decrease in the potential impact on cervical cancer prevention.
Material and Methods
This study is an open-label, nonrandomized clinical trial (NCT01717118) to evaluate the immunogenicity induced by the HPV6/11/16/18 vaccine administered using an alternative (0–6 months) and the standard (0–2–6 months) vaccination schedules.
Our objective was to conduct a preliminary non-inferiority analysis among 9 to 10 y girls having received 2 doses of qHPV vaccine compared with girls aged 9 to 10 y and women aged 18 to 24 y 21 months after the administration of a standard 3 doses scheme.
The trial was conducted in accordance with the International Conference on Harmonization of Good Clinical Practice Guidelines. The protocol was approved by the Institutional Review Board of the National Institute of Public Health (# 833), and all of the subjects provided written, informed consent; for underage participants, parents provided the written consent.
Study population
The study was conducted in the metropolitan area of Cuernavaca in Mexico. The study population of girls consisted of female students who were recruited at 18 public primary schools. In the state of Cuernavaca there are 97 primary and public schools that offer the morning shift. From those, 18 were selected by logistical convenience; they were the primary schools that were not already recruited by the study that made simultaneously in the same area. Each school has an average of 200 girls distributed along 6 y of primary education, and approximately 30 girls (from 18 to 42 per class) in the fifth year, those are the girls aged 9 and 10 y This age section is currently the target of the national vaccination program. All the girl's parents of 5th grade were invited to an informative session where they were told about the study and were invited to participate. This was supported by the school Direction. The attendance was of 87% of parents (470/540). From these attending parents only 75% of them (352) gave their consent for the inclusion of their daughter's in the study; the participation rate across schools varied from 45 to 82%. The intervention made inside the schools was allocated by clusters, the first one was the intervention of a 2-dose vaccination schedule (7 schools) and the second one (11 schools) was the control cluster. From these groups we formally included 150 participants to reach the pre-defined total sample size per arm. A third group was created with adult women aged 18–24 y recruited at a center for clinical studies of the Instituto Nacional de Salud Pública named Centro Médico Cuauhtémoc (). Most of the recruited adult women were those who responded to the promotion of the study made in universities of the metropolitan area of Cuernavaca, where the invitation was made to adult women. Some additional women attended on their own following recommendation of other participants, and because they were teachers or related to the study girls. We formally recruited the first 150 women that accepted and complied with all the inclusion criteria. We did not document refusal rates. Exclusion criteria included fever at the time of vaccination, previous HPV vaccination, and history of allergy to vaccine components, history of thrombocytopenia, administration of any other vaccine during the 15 d prior to study enrollment, immunosuppression, diarrhea, vomiting, and blood disease. Likewise, the presence of diarrhea or vomiting at the time of administering the HPV vaccine was considered for exclusion.
Antibody assays
We used Gardasil®, a quadrivalent human papillomavirus (Types 6, 11, 16, and 18) vaccine (Merck, Whitehouse Station, NJ, USA) provided by the Mexican Ministry of Health. To assess antibody response, blood samples were obtained at 7 and 21 months after the first vaccination from the participants in all study groups. Merck cLIA testing was performed at Merck Research Laboratories as previously described,Citation42 and antibody levels were expressed as milli-Merck units (mMU) per ml. The PsV NAb assay was performed as previously described.Citation43 Briefly, HPV16 and HPV18 PsV containing a reporter plasmid encoding red fluorescent protein (RFP) were prepared and titrated in 293TT cells. The sera were serially diluted, mixed with 100 infectious units of the respective HPV PsV, and inoculated onto 293TT cells in microtiter plates. The cultures were read by fluorescence microscopy after 4 to 6 d The endpoint was the highest dilution of serum that completely blocked the expression of RFP (100% neutralization). Seven and 21 month samples were tested in duplicate in the same assay run, and GMTs were calculated. Subjects were considered to be neutralizing antibodies seropositive for the respective HPV type if the GMT was 20, 16, 20, and 24 mMU/ml for HPV6, 11, 16, and 18, respectively, according to previous validation studies.Citation44 The testing laboratories were blinded to the dosing regimens.
Safety
The study staff recorded safety profile assessments of local symptoms (pain and redness at the injection site) and general symptoms (fever, headache, fatigue, and gastrointestinal symptoms that included nausea, vomiting, diarrhea and/or abdominal pain, arthralgia, myalgia, and rash) at the next scheduled appointment after the administration of each vaccine. The staff also inquired about and recorded any serious adverse events at each contact and among women withdrawing from the study.
Statistical analysis
The immunogenicity analysis was conducted on study participants who complied with protocol procedures and had data available for at least one vaccine antibody result. For the immunogenicity objectives, immunogenicity data (GMTs) with a 95% confidence interval (CI) were computed for HPV6/11/16/18 antibodies at Months 7 and 21 for the 3 comparison groups.
To compare the distributions of antibody response across the study groups, the serum antibody titers for the HPV6/11/16/18 antigens of the girls in the 3 doses group were categorized into quartiles and used as a reference, because we considered that the optimal immune response expected by HPV vaccine may be observed in this particular group, as has been documented in previous studies. Then, the HPV antibody distributions of the other 2 groups were compared to the reference group distribution.
Primary outcome was non-inferiority (95% CI, lower bound >0.5) of geometric mean titer (GMT) ratios for HPV6, HPV11, HPV16 and HPV18 for girls (2 doses) compared with women aged 18–24 y (3 doses) as well as girls (3 doses) at months 7 and 21 after the first dose. Our non-inferiority definition was based on criteria previously used in immunogenicity clinical trials of the qHPV vaccineCitation19 that included results from Mexican women.Citation4 The seronegative participants at 7 and 21 months were excluded from the GMT's estimation.
The natural infection GMTs that we used to examine the kinetic immune response correspond to the GMTs previously reported in subjects who had cleared a natural infection.Citation45 To evaluate the kinetics of the immune response across groups, we used the GMT obtained from serologic measurements at Months 7 and 21 after the first vaccine was administered. The plateau curve was adapted from the GMTs achieved until Month 36 in a non-inferiority immunogenicity trial of the qHPV vaccine in girls aged 16–26 y from Canada who received the vaccine on the standard schedule (M, 0–2–6). Citation28
Statistical analyses were performed with STATA Version 12.0 (Stata Corp. College Station, TX, USA).
Disclosure of Potential Conflicts of Interest
Stanley M has acted as consultant for SPMSD Lyon France, MSD Merck and GSK Biologicals Wavre Belgium. Muñoz N receives honoraria from Merck MSD for attending meetings of the HPV Global Advisory Board. JS and EL have received funding through their institutions for research projects from Qiagen, GSK, Merck, Roche, Becton Dickinson, Gen-Probe, DICIPA, Silanes, and Arbor Vita. None of the other authors have potential conflict of interest to report.
Funding
Funding was provided by the Ministry of Health (Mexico) and the National Institute of Public Health (Mexico). Immunogenicity testing was provided by Merck, which had no role in the study design and conduct, data collection, management, analysis, or interpretation or in the preparation, review, or approval of the manuscript.
References
- de Sanjose S, Quint WGV, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11(11):1048-56; PMID:20952254; http://dx.doi.org/10.1016/S1470-2045(10)70230-8
- Brisson M, Laprise JF, Drolet M, Van de Velde N, Franco EL, Kliewer EV, Ogilvie G, Deeks SL, Boily MC. Comparative cost-effectiveness of the quadrivalent and bivalent human papillomavirus vaccines: a transmission-dynamic modeling study. Vaccine 2013; 31(37):3863-71; PMID:23830974; http://dx.doi.org/10.1016/j.vaccine.2013.06.064
- Stanley M, Pinto LA, Trimble C. Human papillomavirus vaccines–immune responses. Vaccine 2012; 30 Suppl 5:F83-7; PMID:23199968; http://dx.doi.org/10.1016/j.vaccine.2012.04.106
- Reisinger KS, Block SL, Lazcano-Ponce E, Samakoses R, Esser MT, Erick J, Puchalski D, Giacoletti KE, Sings HL, Lukac S, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26(3):201-9; PMID:17484215; http://dx.doi.org/10.1097/01.inf.0000253970.29190.5a
- Demarteau N, Van Kriekinge G, Simon P. Incremental cost-effectiveness evaluation of vaccinating girls against cervical cancer pre- and post-sexual debut in Belgium. Vaccine 2013; 31(37):3962-71; PMID:23777952; http://dx.doi.org/10.1016/j.vaccine.2013.06.008
- Canfell K, Chesson H, Kulasingam SL, Berkhof J, Diaz M, Kim JJ. Modeling preventative strategies against human papillomavirus-related disease in developed countries. Vaccine 2012; 30 Suppl 5:F157-67; PMID:23199959; http://dx.doi.org/10.1016/j.vaccine.2012.06.091
- Kane MA, Serrano B, de Sanjose S, Wittet S. Implementation of human papillomavirus immunization in the developing world. Vaccine 2012; 30 Suppl 5:F192-200; PMID:23199963; http://dx.doi.org/10.1016/j.vaccine.2012.06.075
- Leval A, Herweijer E, Ploner A, Eloranta S, Fridman Simard J, Dillner J, Young C, Netterlid E, Sparén P, Arnheim-Dahlström L. Quadrivalent human papillomavirus vaccine effectiveness: a Swedish national cohort study. J Natl Cancer Inst 2013; 105(7):469-74; PMID:23486550; http://dx.doi.org/10.1093/jnci/djt032
- Baandrup L, Blomberg M, Dehlendorff C, Sand C, Andersen KK, Kjaer SK. Significant decrease in the incidence of genital warts in young Danish women after implementation of a national human papillomavirus vaccination program. Sex Transm Dis 2013; 40(2):130-5; PMID:23324976
- Korostil IA, Peters GW, Law MG, Regan DG. Herd immunity effect of the HPV vaccination program in Australia under different assumptions regarding natural immunity against re-infection. Vaccine 2013; 31(15):1931-6; PMID:23434388; http://dx.doi.org/10.1016/j.vaccine.2013.02.018
- Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, Unger ER. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010. J Infect Dis. 2013; 208(3):385-93; PMID:23785124; http://dx.doi.org/10.1093/infdis/jit192
- Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011; 377(9783):2085-92; PMID:21684381; http://dx.doi.org/10.1016/S0140-6736(11)60551-5
- Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia–nationwide follow-up of young Danish women. J Natl Cancer Inst 2014; 106(3):djt460; PMID:24552678; http://dx.doi.org/10.1093/jnci/djt460
- Schwarz TF, Flamaing J, Rumke HC, Penzes J, Juergens C, Wenz A, Jayawardene D, Giardina P, Emini EA, Gruber WC, et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged >/=65 years. Vaccine 2011; 29(32):5195-202; PMID:21619909; http://dx.doi.org/10.1016/j.vaccine.2011.05.031
- Moreira ED, Jr., Palefsky JM, Giuliano AR, Goldstone S, Aranda C, Jessen H, Hillman RJ, Ferris D, Coutlee F, Vardas E, et al. Safety and reactogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 viral-like-particle vaccine in older adolescents and young adults. Hum Vaccin 2011; 7(7):768-75; PMID:21712645; http://dx.doi.org/10.4161/hv.7.7.15579
- Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine 2008; 26(52):6844-51; PMID:18930097; http://dx.doi.org/10.1016/j.vaccine.2008.09.073
- Wang JW, Roden RB. Virus-like particles for the prevention of human papillomavirus-associated malignancies. Expert Rev Vaccines 2013; 12(2):129-41; PMID:23414405; http://dx.doi.org/10.1586/erv.12.151
- Schwarz TF, Leo O. Immune response to human papillomavirus after prophylactic vaccination with AS04-adjuvanted HPV-16/18 vaccine: improving upon nature. Gynecol Oncol 2008; 110(3 Suppl 1):S1-10; PMID:18653222; http://dx.doi.org/10.1016/j.ygyno.2008.05.036
- Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, Castellsagué X, Rusche SA, Lukac S, Bryan JT, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics 2006; 118(5):2135-45; PMID:17079588; http://dx.doi.org/10.1542/peds.2006-0461
- Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, Iversen OE, Høye J, Steinwall M, Riis-Johannessen G, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 2007; 25(26):4931-9; PMID:17499406; http://dx.doi.org/10.1016/j.vaccine.2007.03.049
- Stanley M. Prospects for new human papillomavirus vaccines. Curr Opin Infect Dis 2010; 23(1):70-5; PMID:19926987; http://dx.doi.org/10.1097/QCO.0b013e328334c0e1
- Lazcano-Ponce E, Salmeron-Castro J, Garcia-Carranca A, Aranda-Flores C, Madrid-Marina V, Gomez-Altamirano CM, Martínez-Montañez OG. [Recommendations for the definition of a policy on vaccination against papillomavirus in Mexico. Strategic Advisory Group of Experts of the World Health Organization]. Salud Publica Mex 2009; 51(4):336-41; PMID:19668929; http://dx.doi.org/10.1590/S0036-36342009000400011
- Neuzil KM, Canh do G, Thiem VD, Janmohamed A, Huong VM, Tang Y, Diep NT, Tsu V, LaMontagne DS. Immunogenicity and reactogenicity of alternative schedules of HPV vaccine in Vietnam: a cluster randomized noninferiority trial. JAMA 2011; 305(14):1424-31; PMID:21486975; http://dx.doi.org/10.1001/jama.2011.407
- Meeting of the Strategic Advisory Group of Experts on immunization, April 2014 – conclusions and recommendations. Wkly Epidemiol Rec 2014; 89(21):221-36; PMID:24864348
- Stanley MA, Sudenga SL, Giuliano AR. Alternative dosage schedules with HPV virus-like particle vaccines. Expert Rev Vaccines 2014; 13(8):1027-38; PMID:25001893; http://dx.doi.org/10.1586/14760584.2014.935767
- Proyecto de Norma Oficial Mexicana PROY-NOM-036-SSA2-2014, Prevención y control de enfermades. Aplicación de vacunas, toxoides, faboterápicos (sueros) e inmmunoglobulinas en el humano.
- Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson SE, Høye J, Steinwall M, Riis-Johannessen G, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95(11):1459-66; PMID:17117182; http://dx.doi.org/10.1038/sj.bjc.6603469
- Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309(17):1793-802; PMID:23632723; http://dx.doi.org/10.1001/jama.2013.1625
- Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, Curtis RC, Markowitz L; Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014–United States. MMWR Morb Mortal Weekly Rep 2014; 63(29):620-4; PMID:25055185
- Ferris D, Samakoses R, Block SL, Lazcano-Ponce E, Restrepo JA, Reisinger KS, Mehlsen J, Chatterjee A, Iversen OE, Sings HL, et al. Long-term study of a quadrivalent human papillomavirus vaccine. Pediatrics 2014; 134(3):e657-65; PMID:25136050; http://dx.doi.org/10.1542/peds.2013-4144
- Bachmann MF, Kalinke U, Althage A, Freer G, Burkhart C, Roost H, Aguet M, Hengartner H, Zinkernagel RM. The role of antibody concentration and avidity in antiviral protection. Science 1997; 276(5321):2024-7; PMID:9197261; http://dx.doi.org/10.1126/science.276.5321.2024
- Luna J, Plata M, Gonzalez M, Correa A, Maldonado I, Nossa C, Radley D, Vuocolo S, Haupt RM, Saah A. Long-term follow-up observation of the safety, immunogenicity, and effectiveness of Gardasil in adult women. PloS One 2013; 8(12):e83431; PMID:24391768; http://dx.doi.org/10.1371/journal.pone.0083431
- Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J Infect Dis 2009; 200(2):166-71; PMID:19519255; http://dx.doi.org/10.1086/599988
- Public Health England DoHaNE. HPV vaccination programme: change from 3 to 2 doses. In: Health Do, editor. England: PHE Publications Gateway; 2014.
- http://www.msss.gouv.qc.ca/sujets/santepub/vaccination/index.php?aid=136 : Gouvernement du Québec; [cited 2015 November 2014]
- OFSP Ofdlsp. Vaccination contre les HPV : passage du schema a 2 doses chez les adolescentes de moins de 15 ans. Bull OFSP 2012; 6:711-7
- Available from:http://www.klusemann.at/wir/Schularzt/Mail%20im%20Mai/impfplan2014.pdf.
- Mapes D. The HPV cancer vaccine: Are two doses as good as three? 2014. Available from: https://www.fredhutch.org/en/news/center-news/2014/08/hpv-cancer-vaccine-are-two-doses-as-good-as-three.html
- Human papillomavirus vaccines: WHO position paper, October 2014. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2014; 89(43):465-91.
- Report IAfRoCIWG. Primary End-Points for Prophylactic HPV vaccine trials. IARC, 2014.
- Lowy DR, Herrero R, Hildesheim A, Participants in the INCIwoPEfPHPVVT. Primary endpoints for future prophylactic human papillomavirus vaccine trials: towards infection and immunobridging. Lancet Oncol 2015; 16(5):e226-e33; PMID:25943067; http://dx.doi.org/10.1016/S1470-2045(15)70075-6
- Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagno Lab Immunol 2003; 10(1):108-15; PMID:12522048
- Krajden M, Cook D, Yu A, Chow R, Mei W, McNeil S, Money D, Dionne M, Karunakaran KP, Palefsky JM, et al. Human papillomavirus 16 (HPV 16) and HPV 18 antibody responses measured by pseudovirus neutralization and competitive Luminex assays in a two- versus three-dose HPV vaccine trial. Clin Vaccine Immunol 2011; 18(3):418-23; PMID:21248158; http://dx.doi.org/10.1128/CVI.00489-10
- Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol 2005; 12(8):959-69; PMID:16085914
- Perez G, Lazcano-Ponce E, Hernandez-Avila M, Garcia PJ, Munoz N, Villa LL, Bryan J, Taddeo FJ, Lu S, Esser MT, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like-particle vaccine in Latin American women. Int J Cancer 2008; 122(6):1311-8; PMID:18000825; http://dx.doi.org/10.1002/ijc.23260
