Abstract
Anti-tumor vaccines have demonstrated efficacy in patients with castration-resistant metastatic prostate cancer. One vaccine, Prostvac-VF®, using a heterologous prime-boost strategy with vaccinia and fowlpox viral vectors encoding PSA, is currently being evaluated in a registration phase III multinational clinical trial. The current trial was planned to assess the clinical efficacy of this vaccine in patients with castration-resistant metastatic prostate cancer receiving subsequent docetaxel chemotherapy. 10 patients with metastatic castration-resistant prostate cancer, with a predicted survival of at least 18 months, were enrolled out of a planned 144 patients. Eight of 10 patients were treated and were randomized to receive docetaxel chemotherapy alone (Arm B, n = 2) versus treatment with Prostvac-VF (days 1, 15, 29, 43, 57) followed by docetaxel (Arm A, n = 6) chemotherapy beginning at month 3. The primary endpoint of the trial was overall survival, and secondary endpoints included time to radiographic progression and immunological response. The trial was opened within the Eastern Cooperative Oncology Group, but due to slow accrual was closed by CTEP after only 10 patients were enrolled within 13 months. Results: Presented here are the safety, clinical, and immunological results from 8 eligible patients who underwent treatment. Two of 6 patients treated on Arm A, with vaccine followed by docetaxel, had a >50% PSA response, with one of these patients experiencing a PSA decline during treatment with vaccine. Significant PSA-specific CD4+ and CD8+ T-cell responses and IgG antibody responses specific for PSA were not detected. The primary endpoint of overall survival cannot be assessed due to limited accrual. The lack of T-cell responses, even in this small cohort, suggests that further validation and development of immune biomarkers will be important for future studies. Other trials remain ongoing to evaluate the role of anti-tumor vaccination in sequence with other traditional anti-tumor therapies.
Introduction
The treatment of metastatic, castration-resistant prostate cancer (mCRPC) has changed dramatically in the last several years given the FDA approval of docetaxel, sipuleucel-T, cabazitaxel, abiraterone, enzalutamide, and radium-223.Citation1-7 Treatment with each of these agents was demonstrated to provide a median survival benefit, compared to control, by a few months. Consequently, despite these successes, there remains a large need to identify agents, alone or in combination, which can further extend this survival benefit. The possibility that combination therapy, in particular, can provide even more substantial benefit was recently demonstrated with results from the CHAARTED trial in which the combination of docetaxel with androgen deprivation as initial treatment for extensive metastatic prostate cancer was demonstrated to provide a median survival benefit over 13 months longer than androgen deprivation therapy alone.Citation8
Anti-tumor vaccines have demonstrated benefit in patients with advanced prostate cancer. This has most clearly been demonstrated by sipuleucel-T, treatment with which demonstrated a survival benefit in independent randomized double-blind phase III clinical trials.Citation3,9 Given the complexity of autologous cellular vaccines, other antigen-specific vaccine approaches have also been evaluated. In particular, poxviral-based vaccines encoding PSA have been investigated in numerous clinical trials, all of which have established the safety of this approach.Citation10-16 These types of vaccines are able to deliver transgenes for tumor-associated antigens directly to antigen presenting cells (APCs), where they are then processed and expressed within the major histocompatibility complexes (MHC), leading to T-cell activation.Citation17-20 In a phase II trial, 125 patients with mCRPC, with Gleason scores <7, were prospectively randomized 2:1 in favor of vaccine vs. an empty fowlpox vector as control. Specifically, patients randomized to receive vaccine were given an rV-PSA-TRICOM prime with monthly boosts of rF-PSA-TRICOM, while control patients were given subcutaneous injections of fowlpox. While there was no significant difference observed in the primary endpoint (progression-free survival (PFS) at 6 months), those patients treated with PSA-TRICOM had a median overall survival over 8 months longer than those treated with control vector (25.1 months versus 16.6 months, p = 0.0061).Citation21 In a similar study conducted in the same population of 32 patients, the survival benefit observed was greatest in patients with a predicted survival >18 months using the Halabi predictive nomogram.Citation22 Specifically, patients with a predicted survival <18 months had an actual median survival of 14.6 months (compared with 12.3 months predicted), whereas patients with a predicted survival >18 months had an actual median survival of >37.Citation23 The survival benefit observed in these studies, greater than what was observed in the randomized phase III trials leading to the approval of other agents described above, has led to a confirmatory registration phase III multinational clinical trial with a similar trial design, but evaluating overall survival as its primary endpoint, that is currently underway.
Results from several clinical trials have suggested that anti-tumor vaccines might modulate the clinical effect from subsequent chemotherapy treatments. For example, in an early phase trial of patients with non-small cell lung cancer, 29 patients were treated with an adenovirus-based vaccine targeting P53. A proportion of patients (61.9%), higher than expected, had objective response to salvage chemotherapy initiated after vaccine treatments.Citation24 Similar findings have been observed in patients with mCRPC. In a survival analysis of the randomized phase III IMPACT trial, patients who received sipuleucel-T prior to docetaxel demonstrated a longer survival compared to patients receiving placebo prior to docetaxel (p = 0.023).Citation25 A similar outcome was also observed in 34 patients with mCRPC treated with the whole tumor-cell vaccine GVAX. 13 patients who went on to receive a taxane-based chemotherapy after receiving vaccine had a mean overall survival of 35.2 months vs. 17.2 months for those who did not receive chemotherapy.Citation26 Finally, a possible improved time to progression following chemotherapy was also observed in patients who had prior treatment with an earlier pox-viral vaccine containing PSA.Citation27 In that study, the median time to progression (predominantly based on PSA progression) on chemotherapy following vaccine was 6.1 months whereas the median time to progression on the same chemotherapy regimen at the same institution with the same progression criteria was 3.7 months.Citation27 Taken together, while these studies suggest a benefit for sequential vaccine followed by chemotherapy, randomized studies designed to evaluate these treatments in sequence are needed.
The current trial was designed as a prospectively randomized phase II trial to specifically assess the effect of the PSA-TRICOM vaccine in patients with mCRPC who subsequently received docetaxel chemotherapy. 144 patients with mCRPC, with a predicted survival of at least 18 months based on the Halabi nomogram, were the planned subject population.Citation23 Patients were randomized to receive docetaxel chemotherapy alone, vs. treatment with Prostvac-VF (days 1, 15, 29, 43, 57) followed by docetaxel chemotherapy at month 3. The primary endpoint of the trial was overall survival. Secondary endpoints included time to radiographic progression, including time to progression as a function of when they started chemotherapy, and immunological response, specifically whether subsequent chemotherapy affected immune responses that might have been elicited with vaccination.
Materials and Methods
Study agent
The PSA-TRICOM vaccine has been previously described.Citation28 In brief, this consists of 2 poxviral vectors (vaccinia and fowlpox), delivered in a heterologous prime-boost strategy, and each encoding PSA with a single amino acid modification to increase affinity for an HLA-A2-specific epitope (L155), and each encoding 3 costimulatory molecules (B7–1, ICAM-1, and LFA-3). In patients receiving this vaccine, 2 × 108 pfu of recombinant vaccinia-PSA was delivered subcutaneously on day 1, and 1 × 109 pfu of recombinant fowlpox-PSA was delivered subcutaneously on days 15, 29, 43, and 57.
Patient population
Eligible patients were those with histologically confirmed adenocarcinoma of the prostate, with evidence of metastatic disease confirmed by CT and/or bone scintigraphy, and with evidence of disease progression despite androgen depriving therapy. Patients were required to have a predicted survival time >18 months as predicted by the Halabi nomogram,Citation22 an Eastern Cooperative Oncology Group (ECOG) performance score of <3, and normal bone marrow, liver and renal function by peripheral blood laboratory analysis. Patients were excluded if they had been treated with immunosuppressive therapy (corticosteroids, or extensive radiation therapy) within 4 weeks of study entry, chemotherapy within 6 months of randomization, prior anti-cancer vaccination, or were on concurrent medications with possible anti-cancer effects. Patients were also excluded if they had potential risks for vaccinia immunization or a recent history of stroke, myocardial infarction, unstable angina, or significant heart failure or cardiomyopathy. There were no restrictions to enrollment if patients had symptomatic or asymptomatic disease.
Study design
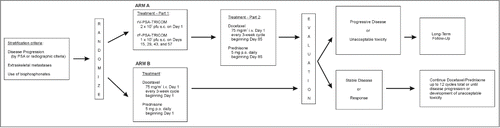
The primary objective was to evaluate the overall survival in patients treated with PSA-TRICOM and docetaxel chemotherapy versus docetaxel chemotherapy alone. Patients randomized to Arm A received the 12-week vaccination course followed by up to 12 3-week cycles of docetaxel (75 mg/m2 on day 1 of each cycle) and prednisone (5 mg twice daily), while patients randomized to Arm B were treated with docetaxel and prednisone alone (). Staging studies (CT of abdomen and pelvis and bone scintigraphy) were performed at baseline, and at 3-month intervals while patients remained on study. Patients came off active treatment with evidence of radiographic progression in Arm B. For patients treated in Arm A, however, radiographic progression was defined using the scan obtained at 3 months as the basis for comparison, since patients on that arm went on to receive docetaxel chemotherapy irrespective of disease progression. Rise in serum PSA only was not used as criteria for disease progression. The description and severity of adverse events was collected using the NCI Common Terminology Criteria for Adverse Events version 4.0. Overall survival was defined as the time from randomization to death. Secondary endpoints included time to radiographic progression after beginning docetaxel chemotherapy, objective response per RECIST version 1.1, PSA response, and immune response. This study was powered to distinguish a 36-month median overall survival in Arm A from a 21-month median overall survival in Arm B with 90% power, while maintaining a one-sided type I error rate of 0.10 using the stratified log-rank test and assuming exponential survival. A total of 135 eligible patients were to be randomized to either Arm A or Arm B, using a 2:1 randomization scheme, over a period of 16 months with 46 months of follow-up. To account for potential dropouts and ineligible patients, an additional 9 patients were to be accrued, for a total of 144 patients.
Immunological analysis
Peripheral blood was collected from all patients (n = 8 who underwent treatment) at baseline, 12 weeks (C1D1 of docetaxel chemotherapy) and 24 weeks (C5D1 of docetaxel chemotherapy) for those subjects treated in Arm A, and at baseline and 12 weeks (C5D1 of docetaxel chemotherapy) for those subjects treated in Arm B. Given that this was a multi-center trial, samples were shipped to a central lab and cryopreserved for subsequent batched analysis. Peripheral blood mononuclear cells (PBMC) were evaluated for T-cell responses specific for PSA or prostatic acid phosphatase (PAP, negative control) by IFNγ ELISPOT and intracellular cytokine staining, evaluating for antigen-specific production of IFNγ, TNFα, granzyme B, IL-2, IL-4, IL-10, and IL-17 using methods previously described.Citation29 Sera obtained at these same time points were assessed for IgG responses specific or PSA by ELISA, as previously described.Citation29
Results
Study course
This study was activated by ECOG as a multicenter trial in December 2010 and opened at the lead institution (University of Wisconsin Carbone Cancer Center) in February 2011. 12 other institutions opened the trial over the following year. The study was terminated in March 2012 by the NCI Clinical Trials Evaluation Program (CTEP) sponsor due to slow accrual. The final accrual was 10 patients (7 patients on Arm A and 3 patients on Arm B), of whom 8 patients received assigned therapy, while 2 patients never started protocol therapy due to medical decision (18007, Arm A) and registration error (18009, Arm B). Demographic and disease characteristics of the patients at study entry are shown in .
Table 1. Patient demographics and disease characteristics at study entry
Clinical evaluation
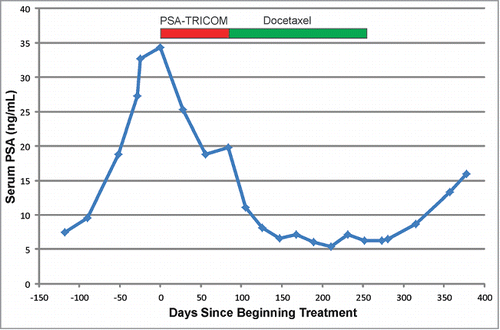
Treatment-related adverse events, of grade 3 or higher, are shown in . Of note, one lethal adverse event (treatment-related sepsis) was observed in a patient on Arm A after receiving 1 cycle of docetaxel. No unexpected adverse events, or events grade 3 or higher, specifically associated with the PSA-TRICOM vaccine were observed; adverse events grade 3 or higher observed in patients treated in Arm A were observed during treatment with docetaxel. As of this report, 6 patients have experienced radiographic progression (4 on Arm A, 2 on Arm B). One of 6 patients on Arm A had evidence of radiographic progression at month 3 following vaccination, and no patients were removed from study at that point without going on to receive docetaxel and prednisone. One patient on Arm B achieved a partial response per RECIST criteria, and 3 patients experienced a PSA response (decline by >50%), 2 on Arm A, and one on Arm B. Of note, one of these patients on Arm A had a decline in serum PSA while treated with the PSA-TRICOM vaccine prior to receiving docetaxel (). The median number of cycles of docetaxel received was 9.5 (9.5 for Arm A, and 10 for Arm B). As of the last analysis in December 2013, the median follow-up is 20.5 months. Three patients have died, with a median survival of 20.8 months (95% CI: 3.4, –) for Arm A, and not reached (95% CI: 14.8 months, –) for Arm B. While overall survival was the primary endpoint of the study, given the small sample size, a proper evaluation of this endpoint could not be achieved.
Table 2. Adverse events. Shown are adverse events that were deemed treatment-related, and grade 3 or higher in severity, for the 8 subjects who received assigned treatment
Immunological evaluation
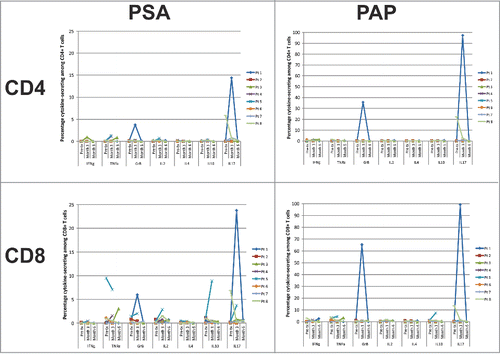
Blood samples were obtained from all treated subject prior to treatment, after completing the immunization series (Arm A), and after 4 cycles of docetaxel chemotherapy. The intent was to determine whether T-cells specific for PSA were elicited, and whether this was affected by subsequent treatment with docetaxel and prednisone. T-cell responses specific for PSA were evaluated by IFNγ ELISPOT () and intracellular cytokine staining (). Significant PSA-specific CD4+ and CD8+ T-cell responses were not detected by these methods using criteria as previously established.Citation29 IgG antibody responses specific for PSA were not detected in any individual (data not shown).
Figure 3. T-cell response evaluation by IFNγ ELISPOT. Cyropreserved PBMC obtained from treated subjects were assessed for IFNγ release following culture with PSA (test antigen, left panel) or phytohemagglutinin (PHA, positive control, right panel) at the individual time points for each subject. Shown are the spot-forming units (sfu) per million PBMC for the antigen-specific conditions subtracting the sfu from media alone.
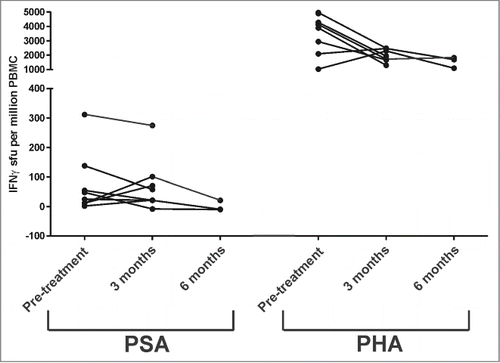
Figure 4. T-cell response evaluation by intracellular cytokine staining. Cryopreserved PBMC obtained from treated subjects were assessed for cytokine (IFNγ, TNFα, granzyme B (GrB), IL-2, IL-4, IL-10, and IL-17) production by CD4+ (top panels) and CD8+ (bottom panels) T cells following stimulation with PSA (test antigen, left panels) or prostatic acid phosphatase (PAP, control antigen, right panels). Shown is the percentage of CD4+ or CD8+ T cells expressing each cytokine or enzyme at the individual time points for each subject.
Discussion
This trial was designed to distinguish a 36-month median overall survival in Arm A from a 21-month median survival in Arm B with 90% power, and maintaining a one-sided type I error rate of 0.10. A total of 144 patients with favorable prognosis were to be randomized. Regrettably, due to slow accrual, the trial was closed early after 13 months. While the trial did not meet its accrual goal, and hence the small number of patients makes it impossible to answer the primary endpoint or draw other significant conclusions, a few lessons can be learned from this trial related to trial design and competing therapies.
First, the activation of this trial was delayed at several institutions, and only opened at a few institutions within the ECOG, due to biosafety level 2 (BL2) requirements. While not insurmountable, the extra administrative (institutional biosafety and hospital safety) review processes led to delays in study activation and hence delays in study accrual. This is a cautionary point for future studies using recombinant materials as one may underestimate the time required for this longer review process. In addition, after 6 months we conducted a study-wide assessment of potential barriers to accrual. One key observation was that in an attempt to identify patients with favorable prognosis, many of these patients were not keen to begin chemotherapy for their disease. Contrarily, some treating physicians did not feel comfortable introducing a possible 3-month delay if they believed patients required chemotherapy for treatment. Hence, otherwise potentially eligible subjects were not presented with this trial as a treatment option. While our study numbers were small, it should be noted that there were no patients removed from trial during the 3-month period of vaccination in Arm A. And as highlighted in , one patient experienced an immediate treatment effect on vaccine alone with a decline in PSA. Hence, while a small population, we found no evidence that delaying chemotherapy by 12 weeks put patients at significant risk. Finally, another barrier to accrual was the concurrent conduct of several phase III competing clinical trials. During the period of time that this trial was conducted (2011–2012), other trials open at these institutions included trials of oral androgen synthesis inhibitors or androgen receptor antagonists, some of which were subsequently FDA-approved.
Immune analyses were planned to assess whether immune responses elicited to PSA by vaccination were affected by subsequent treatment with docetaxel and prednisone. Unfortunately, given the small number of subjects enrolled (6 patients on Arm A), no general conclusions can be reached. The assessment of subsequent chemotherapy, and chemotherapy regimens using corticosteroids, remains an important issue for the development and detection of immune responses, although results from a previous phase II trial suggested that concurrent docetaxel, administered weekly with this same vaccine approach, did not inhibit the generation of PSA-specific immunity.Citation27 In our analysis of the current trial, however, no substantial T-cell immune responses to PSA were detected, despite likely sample error with one patient sample (). T cells specific for PSA may still have been elicited, but may not have been at sufficient frequencies in the peripheral blood for detection directly without in vitro stimulation in this small number of patients. In a larger review of 104 patients treated on multiple clinical trials, PSA-specific T cells were detectable by IFNγ ELISPOT in 57% (59/104) patients.Citation30 The development and validation of standardized immune measures, and in particular those that are associated with long-term clinical outcome, remains critically important for the evaluation of vaccines alone and in combination therapies.
Immune therapies have demonstrated prolonged benefit, even after discontinuation of the agent.Citation31 The survival benefits observed, with manageable or minimal adverse effects, present great opportunities for combining immune therapies with other immune therapies and other conventional therapies, including radiation therapy and chemotherapies. While this study did not answer the specific question of whether PSA-TRICOM improved overall survival when given prior to docetaxel chemotherapy, or prolonged the period of disease response following docetaxel chemotherapy, these will remain open questions for the future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
This study was conducted by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs).
Funding
This study was supported in part by Public Health Service Grants CA180820, CA180794, CA180799, CA180802, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
References
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351:1513-20; PMID:15470214; http://dx.doi.org/10.1056/NEJMoa041318
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351:1502-12; PMID:15470213; http://dx.doi.org/10.1056/NEJMoa040720
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411-22; PMID:20818862; http://dx.doi.org/10.1056/NEJMoa1001294
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364:1995-2005; PMID:21612468; http://dx.doi.org/10.1056/NEJMoa1014618
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367:1187-97; PMID:22894553; http://dx.doi.org/10.1056/NEJMoa1207506
- Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fossa SD, Chodacki A, Wiechno P, Logue J, Seke M, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369:213-23; PMID:23863050; http://dx.doi.org/10.1056/NEJMoa1213755
- de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376:1147-54; PMID:20888992; http://dx.doi.org/10.1016/S0140-6736(10)61389-X
- Sweeney CJ, Chen YH, Carducci MA, Liu G, Jarrard DF, Eisenberger MA, Wong Y-N, Hahn NM, Kohli M, Vogelzang NJ, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): an ECOG-led phase III randomized trial. J Clin Oncol 2014; 32:5s (suppl; abstr LBA2); http://dx.doi.org/10.1200/JCO.2013.49.4757
- Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009; 115:3670-9; PMID:19536890; http://dx.doi.org/10.1002/cncr.24429
- Gulley J, Chen AP, Dahut W, Arlen PM, Bastian A, Steinberg SM, Tsang K, Panicali D, Poole D, Schlom J, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate 2002; 53:109-17; PMID:12242725; http://dx.doi.org/10.1002/pros.10130
- Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, Gritz L, Mazzara G, Oh WK, Arlen P, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res 2000; 6:1632-8; PMID:10815880
- Sanda MG, Smith DC, Charles LG, Hwang C, Pienta KJ, Schlom J, Milenic D, Panicali D, Montie JE. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology 1999; 53:260-6; PMID:9933036; http://dx.doi.org/10.1016/S0090-4295(98)00539-1
- Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Ménard C, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res 2005; 11:3353-62; PMID:15867235; http://dx.doi.org/10.1158/1078-0432.CCR-04-2062
- Schuetz T, Marshall J, Kaufman H, Safran H, Panicali D. Two phase I studies of prime-boost vaccinations with vaccinia-fowlpox vaccines expressing CEA, MUC-1, and TRICOM costimulatory molecules (B7.1/ICAM-1/LFA-3) in patients with advanced pancreatic cancer. J Clin Oncol 2004; 22(14S):2564
- Arlen PM, Gulley J, Dahut W, Skarupa L, Morin S, Pazdur M, Todd N, Panicali D, Tsang KY, Schlom J. A phase I study of sequential vaccinations with recombinant Fowlpox-PSA (L155)-TRICOM (rF) alone, or in combination with recombinant vaccinia-PSA (L155)-TRICOM (rV), and the role of GM-CSF, in patients (Pts) with prostate cancer. J Clin Oncol 2004; 22(14S):2522; PMID:15173215
- Arlen PM, Skarupa L, Pazdur M, Seetharam M, Tsang KY, Grosenbach DW, Feldman J, Poole DJ, Litzinger M, Steinberg SM, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol 2007; 178:1515-20; PMID:17707059; http://dx.doi.org/10.1016/j.juro.2007.05.117
- Essajee S, Kaufman HL. Poxvirus vaccines for cancer and HIV therapy. Expert Opin Biol Ther 2004; 4:575-88; PMID:15102606; http://dx.doi.org/10.1517/14712598.4.4.575
- Bernards R, Destree A, McKenzie S, Gordon E, Weinberg RA, Panicali D. Effective tumor immunotherapy directed against an oncogene-encoded product using a vaccinia virus vector. Proc Natl Acad Sci U S A 1987; 84:6854-8; PMID:3477812; http://dx.doi.org/10.1073/pnas.84.19.6854
- Irvine K, Kantor J, Schlom J. Comparison of a CEA-recombinant vaccinia virus, purified CEA, and an anti-idiotypic antibody bearing the image of a CEA epitope in the treatment and prevention of CEA-expressing tumors. Vaccine Res 1993; 2:79-94
- Kass E, Schlom J, Thompson J, Guadagni F, Graziano P, Greiner JW. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Res 1999; 59:676-83; PMID:9973217
- Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Onc 2010; 28(7): 1099-1105.
- Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, Levine EG, Blumenstein BA, Vogelzang NJ. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol 2003; 21:1232-7; PMID:12663709; http://dx.doi.org/10.1200/JCO.2003.06.100
- Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, Jones JL, Poole DJ, Higgins JP, Hodge JW, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 2010; 59:663-74; PMID:19890632; http://dx.doi.org/10.1007/s00262-009-0782-8
- Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, Bepler G, Simon G, Janssen W, Lee JH, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res 2006; 12:878-87; PMID:16467102; http://dx.doi.org/10.1158/1078-0432.CCR-05-2013
- Petrylak D. Defiing the optimal role of immunotherapy and chemotherapy: advanced prostate cancer patients who receive Sipuleucel-T (PROVENGE) followed by docetaxel derive the greatest survival benefit. 14th Annual Meeting of the Chemotherapy Foundation Symposium. New York, NY, 2006.
- Small EJ, Sacks N, Nemunaitis J, Urba WJ, Dula E, Centeno AS, Nelson WG, Ando D, Howard C, Borellini F, et al. Granulocyte macrophage colony-stimulating factor–secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res 2007; 13:3883-91; PMID:17606721; http://dx.doi.org/10.1158/1078-0432.CCR-06-2937
- Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res 2006; 12:1260-9; PMID:16489082; http://dx.doi.org/10.1158/1078-0432.CCR-05-2059
- Dipaola R, Plante M, Kaufman H, Petrylak D, Israeli R, Lattime E, Manson K, Schuetz T. A Phase I Trial of Pox PSA vaccines (PROSTVAC(R)-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOMtrade mark) in Patients with Prostate Cancer. J Trans Med 2006; 4:1; PMID:16390546; http://dx.doi.org/10.1186/1479-5876-4-1
- McNeel DG, Becker JT, Eickhoff JC, Johnson LE, Bradley E, Pohlkamp I, Staab MJ, Liu G, Wilding G, Olson BM. Real-time immune monitoring to guide plasmid DNA vaccination schedule targeting prostatic acid phosphatase in patients with castration-resistant prostate cancer. Clin Cancer Res 2014; 20:3692-704; PMID:24850844; http://dx.doi.org/10.1158/1078-0432.CCR-14-0169
- Gulley JL, Madan RA, Tsang KY, Jochems C, Marte JL, Farsaci B, Tucker JA, Hodge JW, Liewehr DJ, Steinberg SM, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res 2014; 2:133-41; PMID:24778277; http://dx.doi.org/10.1158/2326-6066.CIR-13-0108
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33; PMID:23724867; http://dx.doi.org/10.1056/NEJMoa1302369