Abstract
Since Edward Jenner's discovery that intentional exposure to cowpox could provide lifelong protection from smallpox, vaccinations have been a major focus of medical research. However, while the protective benefits of many vaccines have been successfully translated into the clinic, the cellular and molecular mechanisms that differentiate effective vaccines from sub-optimal ones are not well understood. Dendritic cells (DCs) are the gatekeepers of the immune system, and are ultimately responsible for the generation of adaptive immunity and lifelong protective memory through interactions with T cells. In addition to lymph node and spleen resident DCs, a number of tissue resident DC populations have been identified at barrier tissues, such as the skin, which migrate to the local lymph node (migDC). These populations have unique characteristics, and play a key role in the function of cutaneous vaccinations by shuttling antigen from the vaccination site to the draining lymph node, rapidly capturing freely draining antigens in the lymph node, and providing key stimuli to T cells. However, while migDCs are responsible for the generation of immunity following exposure to certain pathogens and vaccines, recent work has identified a tolerogenic role for migDCs in the steady state as well as during protein immunization. Here, we examine the roles and functions of skin DC populations in the generation of protective immunity, as well as their role as regulators of the immune system.
Abbreviations
| DCs | = | Dendritic Cells |
| APCs | = | Antigen Presenting Cells |
| LCs | = | Langerhans Cells |
| migDC | = | migratory DCs |
| FLT-3 | = | Fms-like Tyrosine Kinase 3 |
| STAT3 | = | Signal Transducer and Activator of Transcription 3 |
| LN | = | Lymph Node. |
Introduction
The immune system is responsible for defending its host against a wide variety of pathogens on a daily basis. Two key characteristics of this protection are the generation of specific, sterilizing immunity, and the formation of long lived immunological memory, both of which require an adaptive immune response. Clinically, the protective benefits of the immune system have been successfully harnessed through the development of vaccines. However, the cellular mechanisms underlying long-term protective immunity remain incompletely characterized.
The Dendritic Cell (DC), was discovered in the 1970s by Ralph Steinman and Zanvil Cohn.Citation1 As the major antigen presenting cells (APC) responsible for driving the generation of the adaptive immune response, DCs are central to establishing long-term protective immunity. Classically, DCs are dependent on the transcription factors ID2Citation2 and PU.1,Citation3,4 as well as FMS-like Tyrosine Kinase 3 (Flt3)Citation5,6 and Signal Transducer and Activator of Transcription 3 (STAT3)Citation7 signaling. In vivo, DCs generate potent, cytotoxic CD8+ T cells,Citation8 as well as regulate the polarization of helper CD4+ T cells toward the appropriate subtype (TH1, TH2, TH17, etc).Citation9 Two specific populations of DC, which will not be discussed further but are mentioned here briefly, are plasmacytoid DCs and monocyte-derived DCs. Specialized for innate anti-viral immunity, plasmacytoid DCs express TLR7 and TLR9, and are the primary producers of type I IFNs in response to viral infections.Citation10,11 DCs may also arise from monocytes in the setting of inflammation, taking on antigen presentation function to both CD4+ and CD8+ T cells,Citation12 as well as an anti-microbial role through the production of TNFα and iNOS.Citation13
In addition to plasmacytoid and monocyte derived DCs, there are several unique populations of DCs,Citation14 which can be broadly grouped into 2 categories based on their anatomical location in the steady-state: lymphoid resident DCs ( also know as classical DC), or migratory DCs (migDC). Lymphoid resident DCs are found in the spleen and LNs of both mice and humans, originate from blood precursors, and include CD8α+ and CD11b+ subsets.Citation15 In contrast, migDCs are comprised of several different populations (as discussed in detailCitation16), which constitutively traffic to the local draining LN from a non-lymphoid organ, such as the skin, lungs, or gut, while being notably absent from the spleen.Citation17 MigDC in the skin are perhaps the best characterized of these tissue DC populations, though their role in the generation of protective immunity is not fully understood.
The skin is a unique immune environment, hosting both a diverse network of immune cells,Citation18 as well as a significant microbiome made up of both fungalCitation19 and bacterialCitation20 populations. Furthermore, when directly compared to other routes of vaccine administration, such as intraperitoneal or intramuscular, cutaneous immunization offers superior cellular and humoral immune responses to both influenzaCitation21 and vaccinia,Citation22 suggesting that the skin might be a specialized site for immune priming. As one such example, intradermal influenza is required at just one fifth of the dose needed via intramuscular injection, to achieve comparable vaccine titers and serum conversion.Citation21 However the cellular mechanisms underlying enhanced immunization via the skin are incompletely understood and afford a key opportunity to improve rational vaccine design.
In addition to hosting numerous commensal species, the skin is a site of high cellular turnover, between hair follicles and regular keratinocyte replacement. These features contribute to the unique nature of the skin, and play a significant role in the regulation of the immune system, as it must discriminate between normal cellular turnover and infection induced cell death. Hair follicles are considered to be an immune privileged site,Citation23 yet when mechanically stimulated, produce CCL2 and CCL20, recruiting Langerhans Cell precursors and allowing the entry of immune cells into the epidermis.Citation24 However, despite high cellular turnover, healthy skin is not constantly inflamed, as the cell death of keratinocytes is closely regulated. Indeed, in mice with keratinocytes deficient in either FADDCitation25 or caspase-8,Citation26 skin lesions and constitutive inflammation develop. The skin must maintain tolerance to self, as well as to commensal bacteria, while responding appropriately to dangerous pathogens. This delicate balance is maintained through a specialized network of dendritic cells.
In the skin of both humans and mice, there are a number of individual DC populations (4 or 5 respectively) as defined by both extracellular markersCitation27 as well as dependence upon specific transcription factors.Citation14,28-31 Langerhans Cells (LCs) are the only DC located in the epidermis, where they maintain surveillance of tight junctions,Citation32-34 while binding broadly with keratinocytes through E-cadherin binding.Citation35 In the dermis, an interstitial network of DCs is comprised of at least 3 individual populations, in addition to LC migrating through the dermis en route to the skin draining LN.Citation31,36-39 These populations include CD103+ DC (BDCA3+ in humans), CD11b+ DC (BDCA1+ in humans), and CD11b− Langerin− DC (no human counterpart identified as of yet, although a BDCA3+BDCA1+ human DC subset exists which has yet to be correlated to a mouse counterpart). Together with LCs, the dermal skin DCs form a complex system responsible for maintaining the equilibrium between barrier immunity and self-tolerance.
Unique Properties of Skin DC
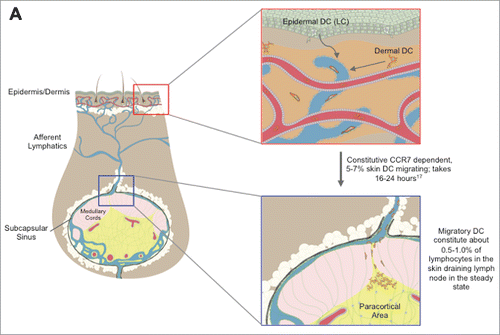
Skin DCs survey the skin for pathogens and are capable of migrating to skin draining LNs bearing antigens to present to T cells (). This migration is CCR7 dependent, as mice genetically deficient for CCR7 mice do not have any migDCs in their cutaneous LNs.Citation17 Unexpectedly, skin DC constitutively migrate in both germ free and MyD88/TRIF knockout mice,Citation40 but fail to migrate when NF-κB signaling is inhibited in DCs,Citation41 suggesting that the migratory program occurs independently of TLR signaling, but requires NF-κB. However, irritant induced inflammation,Citation42 TLR ligation,Citation43 and infectionCitation44 result in increased trafficking, demonstrating that skin DC migration is responsive to immune stimuli. In addition, recent studies have demonstrated that skin DC will also increase their migration in response to low molecular weight hyaluronan through a TLR4 dependent mechanism, suggesting that skin DC may respond to a wide variety of stimuli.Citation45
Figure 1. Graphical Schematic of Skin DC Migration to the Cutaneous Lymph Nodes. Epidermal (Langerhans Cells) and dermal skin DC colonize their respective layers of the skin. In both the inflamed and steady state setting, LC and dermal DC enter the lymphatic vessels, before migrating in a CCR7 dependent manner to the local cutaneous draining lymph node bearing either pathogen derived or self antigens. Upon arrival at the lymph node, skin migratory DC enter the paracortical area and present skin derived antigens to T cells. Additionally, post migration, migratory DC can capture and present antigens that have drained freely, such as DEC205 targeted vaccinations. Images were provided by Matthew Woodruff (Wikimedia commons).
We and others have observed that skin DCs (in the steady state) are transcriptionally far more closely related to each other than to their LN resident counterparts.Citation28,46,47 This finding was initially surprising, as some skin and LN populations share both common precursors, as well as a capacity for functionally similar properties, such as cross-presentation. Furthermore, even LCs are more closely related to the other skin DC populations,Citation28 despite arising from a unique developmental pathway, more akin to monocytes and microglia than the remaining skin resident DCs.Citation48,49 Moreover, skin DC in the steady-state and during Flt3L treatment maintain a transcriptional signature that is both mature as well as tolerogenic,Citation28,46 while lymphoid resident DC tend to selectively express these markers during maturation, highlighting broad programming differences between these 2 groups.
Phenotypically, skin (and other tissue-derived) migDCs can be distinguished from LN resident DCs based on the expression level of classical DC identifiers; MHC II (or HLA-DR in human) and CD11c. While migDCs are identified as MHCIIhiCD11cint, LN resident DCs can be distinguished as MCHIIintCD11chi cells in the steady state. However, during an immune response to an infection, this distinction blurs- LN resident DCs upregulate MHC II and therefore resemble migDCs. Interestingly, Flt3L treatment can expand the numbers of both LN resident DCs, as well as migDCs in the skin and skin draining LN, with the notable exception of Langerhans cells.Citation6,39 However, in contrast to LN resident DCs, migDCs constitutively express high levels of the classic DC activation markers CD80 and CD86, despite having an immune dampening effect in some models, as will be discussed later. These findings, among others, have stimulated a discussion concerning the definition of a “mature” or even “semi mature” DC based on phenotypic markers, instead opting for functional studies about the licensing state of the DCs in question.Citation50 Nevertheless, skin migDCs are a unique network of several DC populations with distinct functional and phenotypic characteristics.
Approaches to Studying Skin DC
A number of different approaches have been utilized to study the function of skin DC. Among these, preclinical models allowing for the restriction or ablation of individual DC populations have been indispensable for understanding how these populations function both individually and as a network.Citation51 These models drive the human diphtheria toxin receptor under a promoter of specific marker genes, such as Langerin,Citation52,53 CD11c,Citation8 or Zbtb46.Citation54 Each of these models results in the ablation of a unique set of cells, sometimes with overlapping results (). For example, use of the CD11c-DTR model results in ablation of classical DCs preferentially but also ablates some macrophages, monocytes, and pDC,Citation8 while the Zbtb46 DTR model ablates only classical Flt3 dependent DC in both lymphoid and non-lymphoid organs.Citation54 The use of a Langerin-DTR can deplete both LCs and a dermal DC population that expresses both Langerin and CD103 (but is quite functionally and developmentally distinct from LC).Citation52,53 However, when bone marrow from Langerin-DTR mice is used to reconstitute a wildtype animal, only the dermal Langerin+ CD103+ DC population is sensitive to the administration of diphtheria toxin, as LCs are radioresistant, and host LCs (no DTR expression) are left intact from the chimerization.Citation55 Another method to selectively delete LCs is the use of Langerin-DTA model, where diphtheria toxin is driven under the human Langerin promoter, resulting in constitutive deletion of LCs.Citation56 However, only the LCs, both in the epidermis and migrating through the dermis, are affected in this model, leaving the other dermal resident Langerin+ CD103+ DCs intact.Citation55
Table 1. Approaches to study skin DC
While several models of inducible ablation have been established, there is no known model that can specifically ablate all skin migDCs while leaving cDCs intact. One model that has been used to study the effects of migDCs as a whole is the CCR7 knockout mouse. However, while migration of migDCs to the skin draining LN is blocked in these mice (thereby removing them from LN during immune priming), CCR7 also plays a critical role in central memory T cells, T regulatory cells, and B cells, and its loss can alter LN architecture, while naïve and effector T cell are left largely intact. This model may be used in selective contexts (such as excluding a role for CCR7 dependence when immune responses are not lost), but cannot pinpoint a role for migDC exclusive of other CCR7-dependent populations. Thus it can be very useful for broad definitions, but is suboptimal for more detailed study of migDC function.Citation57,58
In addition to diphtheria toxin driven ablation strategies, several models have been established where DC subsets fail to develop due to the loss of requisite transcription factors. Batf3 knockout mice are deficient in LN resident CD8α+ DCs as well as CD103+ dermal DC (discussed in detail below), while leaving other cell populations intact.Citation59 In Batf3 deficient mice, exogenous or inflammation derived IL-12 results in the recovery of the CD8α+ DC subset, but not the CD103+ DCs.Citation60,61 Another model, CD11c-Cre IRF4 fl/fl, has been found to prevent the accumulation of CD11b+ dermal DC in the skin draining LN, despite their continued presence in the skin.Citation62
The application of topical agents may help define the role of skin DC in inflammatory contexts. “FITC painting,” involves the application of the fluorescent molecule FITC along with an irritant, such as dibutyl phthalate acetone.Citation63 After painting, migDC rapidly transport FITC from the skin to the skin draining LN. Similarly, application of DNCB results in contact hypersensitivity, though unlike the FITC model, inflammation from this model seems to by driven by TH1 cells.Citation64 Repeated application of TLR 7/8 agonists (Imiquimod or Resiqiumod) can model human psoriasis in mice, including increased keratinocyte proliferation, recruitment of multiple immune subsets, and dependence on increased IL-17 signaling.Citation65 Mild injury and wounding is modeled through “tape stripping,” which removes part of the stratum corneum while leaving the hair follicle intact.Citation66 This mechanical disruption of the epidermis induces inflammation and increases migration of LCs to the skin draining LN, while increasing recruitment of new LC precursors to the affected area.Citation66 This model has often been used in conjunction with the topical administration of a target antigen,Citation34 such as ovalbumin, to study T cell responses to topical treatments. In addition to these topical treatments, a number of skin-tropic infections have been studied in the context of skin DC, including Candida Albicans,Citation67-70 HSV,Citation71-73 vaccinia virus,Citation74-76 Staphlycoccus,Citation34 and Leishmania.Citation77-79 As these pathogens are predisposed to infect the skin, skin DCs have a unique role in the generation of the immune response to them, as will be discussed later on.
Imaging studies of the skin have shed light on the anatomical structure and location of skin DCs, highlighting their location both in the skin as well as in the LN (). In addition to conventional immunofluorescence, imaging studies may utilize mouse models where fluorescent reporters are expressed under DC specific promoters, such as CD11cCitation80 and Langerin,Citation52 allowing for either live, in vivo imaging or immunoflourescent staining. Reporter mice have been invaluable in demonstrating the increased motion of LCs following tape stripping,Citation52 localization of DCs at the tumor border,Citation81 or visualizing the interactions between T cells and DCs during the immune response.Citation80 In addition to visualizing skin DC, specialized isolation protocols have been developed to isolate skin DC, either by directly digesting the skinCitation29 or skin draining LNs,Citation42 or by allowing DC to “crawlout” in culture.Citation39,82 Furthermore, enzymatic digestion allows for a physical separation of epidermis from dermis, in both murineCitation83 and humanCitation84 skin, thereby isolating anatomically distinct cell populations prior to further processing.
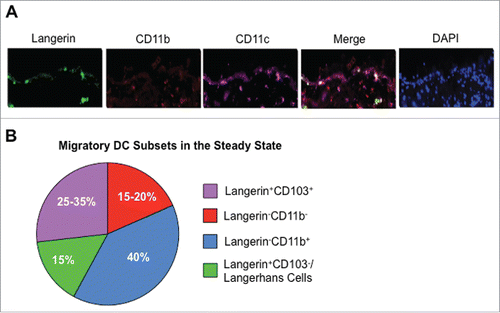
Figure 2. Migratory DC in the Skin and Cutaneous Draining Lymph Node. (A) Murine skin was stained for CD11c (purple), Langerin (green), CD11b (red), and DAPI (blue) in the steady-state. Images were taken at 20 × and are courtesy of Sze-Wah Tse. (B) Proportions of individual migratory DC populations among the total migratory DC subset in the cutaneous draining lymph node in the steady state. Data are adapted from Henri et al.Citation106 and Mollah et al.Citation39
In addition to in vitro studies, a number of methods have been explored for targeting DCs in vivo, specifically utilizing antigens fused to DC specific antibodies,Citation85 such as DEC205 (CD205),Citation86 Langerin (CD207),Citation87 DC-SIGN (CD209)Citation88 or Clec9a (DNGR).Citation89 Of these potential DC targets, DEC205 targeting is the most widely used and first established strategy. By fusing antigens to DEC205, the vaccine is targeted to DEC205+ DCs and specifically delivered into the cross-presentation pathway of antigen processing- the major pathway by which viral and tumor antigens are presented. This approach facilitates increased activation of CD4+ and CD8+ T cells compared to untargeted protein vaccines,Citation86,90,Citation91 and increased anti-tumor immunity in the preclinical setting of metastatic melanoma.Citation92 Recently, we demonstrated that combining DEC205-targeted antigen with adjuvant and Flt3L treatment (to expand DC) can further amplify the resulting immune response.Citation46 However, in the absence of an adjuvant to mature or license DC, DEC205 targeting generates immune tolerance. In a murine model of multiple sclerosis (Experimental Autoimmune Encephalitis), DEC205 fused to Myelin Oligodendrocyte Gylcoprotein (MOG) was able to prevent and ameliorate the disease phenotype.Citation93-95
Clinically, DEC205 targeting has been found to be safe and effective in generating cellular and humoral responses in healthy volunteers.Citation96 Additionally, the results of a phase I trial using a DEC205 targeted NY-ESO-1 vaccine (given in combination with resiquimod or Hiltonol) were recently reported.Citation97 In this study, patients with NY-ESO-1 expressing tumors saw an increase in both cellular and humoral immunity to the target antigen following vaccination, despite the immune tolerance generated by the tumor. Intriguingly, 75% of patients receiving this vaccine who subsequently enrolled in checkpoint blockade studies had objective tumor regression, suggesting the remarkable potential to combine this vaccine with combinatorial immune checkpoint blockade therapies in the future.Citation98
DEC205 targeting has been largely successful in the generation of adaptive immunity in both preclinical as well as clinical studies. However, we and others have observed high DEC205 expression on CD8α+ lymphoid resident and all subsets of migDCs.Citation39 As such, DEC205-targeted antigens are directed to both populations.Citation46 The identification of a series of individual DC subsets with unique functional properties offers an opportunity to fine-tune this vaccination strategy by directing targeted vaccination to a specific DC population based on the desired cellular or humoral outcome.
Skin Dendritic Cells in Immune Activation
Skin based responses to cytolytic viral infection are highly dependent on cross-presentation of vaccine antigens, suggesting that cell death of locally infected cells may be a licensing cue for optimal skin DC subset activation. In a series of elegant murine experiments, Shen et. al. added the human CMV gene product US11 to the vaccinia virus genome, generating a virus that prevents direct presentation by interfering with MHCI:peptide expression.Citation99 In this model, CD8+ T cell responses were severely impaired when vaccinia was administered by intravenous, intraperitonial, or intramuscular injection, indicating a role for direct presentation in these vaccination routes. In contrast, when vaccinia was administered by either intradermal injection or skin scarification, the resultant CD8+ T cell response was unaffected by the loss of direct presentation, highlighting the unique importance of cross-presentation for these routes of vaccination. In agreement with these findings, the loss of DNGR-1 (a DC receptor that recognizes dead cells and mediates cross-presentation of scavenged antigens) results in significantly decreased CD8+ T cell immunity to vaccinia virus (when given by skin scarification).Citation75 This requirement for cross-presentation may also translate into superior immunity, as shown by Liu and Kupper et al., who found that skin based vaccinations with vaccinia were superior to other routes in terms of generating prophylactic protective immunity to either viral or tumor challenge through the generation of tissue resident memory T cells.Citation22 However, within skin based vaccination routes, skin scarification is superior to intradermal vaccination in the generation of protection against either viral rechallengeCitation22 or tumor growth,Citation74 though the molecular and cellular mechanisms defining these differences in vaccine efficacy are not well understood. Yet, the presence of a unique combination of DC populations () suggests that the interaction between the skin and its resident DC network are of major significance for the generation of protective immunity.
Langerhans Cells
In both the murine and human epidermis, Langerhans Cells (LCs) are the only migDC population.Citation14 Although long considered the prototype DC, LCs have unique characteristics that align them more closely with monocytes than other DC populations, including other skin DC. For example, during neonatal development, LC precursor cells are originally recruited to the skin in a CCR2 and CCR6 dependent mannerCitation24 from the yolk sac, but are then replaced by precursors from the fetal liver,Citation84 while the other skin DC populations are derived from the bone marrow. LCs require TGFβ, but not Flt3L signaling for their development, while Langerin+ CD103+ dermal DC are independent of TGFβ, but, like other classical DC, are Flt3L dependent.Citation39,100 Additionally, LCs demonstrate radio-resistance when compared to dermal skin DC, which are sensitive to radiation.Citation55 LCs are largely reconstituted from skin resident, rather than blood derived progenitors in the steady-state,Citation14 relying on IL-34 signaling through the CSF-1 receptor.Citation101 However, during inflammatory situations, both skin resident precursorsCitation102 as well as blood derived monocytes contribute to the repopulation of LCs in skin.Citation103,104 Indeed, following infection with Herpes Simplex Virus, LCs are rapidly depleted from the infected area. Once the infection has been cleared, the subsequent LC repopulation of the previously infected skin is derived about half from monocyte derived precursors and half from long lived skin resident progenitor cells.Citation73
One of the major functions of LCs is to monitor the integrity of the skin as a barrier tissue. In order to thoroughly survey this unique environment, LCs extend dendrites through keratinocyte tight junctions to monitor the external barrier of the skin,Citation33,105 and respond to mechanical injury to the epidermis (in the form of tape stripping) by increasing motility and projection of dendrites.Citation24,52 By extending dendrites through the tight junctions without compromising the epithelial barrier, LCs are able to generate immunity to antigens that have not breached the barrier yet, thereby generating protection without true infection. This was demonstrated in a model where patch immunization against the Staphylococcus aureus derived exfoliative toxin (ET) protected against blistering during toxin rechallenge, despite the ET not penetrating the epithelial barrier.Citation34 This is advantageous to the host because it allows the immune system to generate adaptive immunity prior to a true pathogenic challenge, similar to the ideology behind prophylactic vaccination.
In the LN, LCs can be distinguished from dermal migDCs by their co-expression of Langerin and EpCam,Citation83 while lacking expression of CD103.Citation106 In the steady state, LCs in the LN express high levels of CD80, CD86, CD40, PDL1, and PDL2, though this expression can be further upregulated during exposure to a contact allergen, 2,4,6-trinitro-1-chlorobenzene (TNCB).Citation42 In addition to these markers, LCs express high levels of IL-15Citation107 as well as the IL-15 receptor, IL-15R-α, allowing them to present IL-15 to responding T cells. This co-stimulatory cytokine results in increased expansion of the T cell response through STAT5 signaling, even when presenting a normally tolerizing antigen, such as the tumor antigen Wilm's Tumor-1.Citation108 LCs may also be uniquely prone to tailoring the humoral response to antigens toward an IgG1 response, as the loss of LCs resulted specifically in the decrease of this antibody isotype in a gene gun vaccination model.Citation83
In the setting of infectious disease, LCs have a demonstrated role in adaptive immunity to C. Albicans. In response to skin infection with this pathogen, LCs increase production of IL-1β and IL-6 while presenting pathogen derived antigens to CD4+ T cells, resulting in TH17 differentiation.Citation69,70 Interestingly, this production of IL-6 is dependent upon the engagement of a specific C type lectin receptor, Dectin-1, whose ligand is only expressed by the yeast form of C. Albicans, when it is located on the epidermis.Citation67 Surprisingly, MyD88 signaling is not involved in LC migration in response to C. Albicans, and the generation of both the CD4+ TH1 and CD8+ T cell response is instead dependent on a separate, dermal resident DC population, CD103+ migDCs, which will be discussed later. However, the TH17 cells generated by LC are important in generating protective immune memory in the skin, while TH1 cells provide systemic protectionCitation67 in this model, thereby highlighting the role of LCs in maintaining and protecting the skin as a barrier tissue.
Langerin−CD11b− DCs
In addition to the epidermal resident LC, several populations of migDCs coexist in the dermis of both mice and humans. The first of these populations was uncharacterized until very recently, and is identified as lacking either Langerin or CD11b expression.Citation39,106 This population shares a common progenitor with other classical DC,Citation106 is dependent upon FLT3 signaling, and requires the classical DC transcription factor ZBTB46 for development.Citation39 We demonstrated that these cells are skin resident and actively transport FITC from the skin to the draining LN like other migratory DC subsets, present antigen with equal efficiency to other skin and LN DC subsets and, by hierarchy transcriptome analysis, we observed that Langerin− CD11b− DCs likely derive from a precursor (pre-DC) that also gives rise to Flt3L dependent CD11b+ migDC, to which they are most closely related.Citation39 These cells are equally capable of presenting antigens with an efficiency that parallels both other migDC populations and lymphoid resident DCs ex vivo.Citation39 Quite recently this population has gained increasing recognition and was found to depend on KLF4 (Kruppel-like factor 4 transcription factor) by an independent group (though KLF4 loss impacted both this subset and CD11b+ migratory DC). This study demonstrated a shared role for these populations in response to house dust mite allergen and Schistosoma mansoni infection.Citation109 However, it is currently unknown if this population has a human counterpart, if phenotypic labeling of BDCA3+ dermal DC in humans incorporates its counterpart, or if this population is related to in BDCA1+BDCA3+ intermediary cells. Further research will explore the in vivo relevance of this population in vaccination and infection, and determine whether these cells have a unique function when compared to other dermal DCs, or functional redundancy with other skin DC subsets. This may also represent a subset that can be further specialized, differentiated, or licensed in an appropriate infectious context.
Langerin−CD11b+ DCs
In addition to the CD11b− DCs, a separate, Langerin−CD11b+ population of DCs was identified in mice. The human counterpart of this subset is identified based on expression of BDCA-1.Citation110,111 Up regulation of CCR7 and the subsequent migration of Langerin−CD11b+ DCs depends on IRF4 signaling, as tested by modeling contact hypersensitivity in mice lacking IRF4.Citation112 In terms of antigen presentation, in vivo Langerin−CD11b+ DCs were observed to be uniquely capable of presenting soluble antigens, such as house dust mite antigen,Citation113 ovalbumin,Citation114-116 or part of the class II MHC Eα chain,Citation117 to CD4+ T cells. In the context of intramuscular vaccination with alum, CD11b+ migDC were capable of cross-presentation of soluble OVA to CD8+ T cells, though in these studies, CD64+ monocyte derived DCs, were equally capable of presenting antigen.Citation116
CD11b+ migDC also have a role in CD4+ T cell priming during infection. Langerin−CD11b+ DCs present antigen derived from Herpes Simplex Virus to CD4+ T cellsCitation71 while also maintaining a role as the major cytokine producing population of skin DCs. In response to influenza vaccination, Langerin−CD11b+ DCs produced higher levels of the chemokines MIP1α, MIP1β, and RANTES than CD103+ DCs, although the CD103+ DCs (to be discussed next) were identified as key presenters of influenza derived antigens.Citation118 In accordance with their role as both cytokine producers and antigen presenters, CD11b+ migDC can also skew CD4+ T cells toward a TH2 phenotype, either in the context of a TH2 inducing adjuvant (papain) or a TH2 driving infection (Nippostrongylus brasiliensis).Citation62 Additionally, CD11b+ migDC are required for the generation of a protective TH17 response to Aspergillus fumigatus challenge,Citation119 demonstrating their highly versatile capacity to shape the resulting T cell response.
Langerin+CD103+ DCs
This dermal migDC population is associated with a cross-presentation function and identified by co-expression of Langerin and the integrin CD103. These cells depend on the transcription factor BATF3,Citation120 as well as the transcriptional regulator IRF8.Citation121 Although CD103+ DC, like other DC, depend on Flt3L and are significantly reduced in numbers in the Flt3L knockout mouse, upon Flt3L treatment they do not expand as significantly as CD11b+ and CD11b− dermal DC, suggesting that once they have differentiated from pre-DC in the tissue, they either lose Flt3L dependence or become dependent on or susceptible to pathways opposing Flt signaling.Citation39 CD103+ DCs have been ascribed a unique capacity for cross-presentation, as facilitated by high expression of the cell death receptor, DNGR-1 (CLEC9A).Citation75 Indeed, CD103+ DCs play a key role in cross-presentation of antigens derived from self,Citation106,122 as well as those derived from Herpes Simplex Virus-1122, influenza,Citation123,124 vaccinia virus,Citation74 and C. Albicans.Citation70 Concordant with this specialized function, CD103+ DCs have decreased endosomal acidification when compared to Langerin−CD11b+ DCs, allowing CD103+ DCs to preserve the integrity of acquired antigens for a longer period of time.Citation81,123 In the case of influenza, 2 studies have independently demonstrated that CD103+ DCs are the primary APC responsible for the generation of the adaptive immune responses. However, in one study, CD103+ DCs were not productively infected by the virus, and thus were utilizing cross-presentation,Citation123 while in the other study, CD103+ DC were found to be infected with the virus, and thereby directly presenting antigens.Citation125 In both studies, type I IFN signaling was capable of preventing further viral infection, though further studies will be necessary to determine which presentation pathway (direct or cross) is the primary route of antigen presentation in this infection. Additionally, a recent study has also identified the potential of CD103+ DC to skew the T cell response to Leishmania, despite being dispensable for antigen presentation.Citation126 In humans, a similar population of DCs has been identified, based on expression of BDCA-3 or CD141.Citation29 In agreement with the murine studies, human BDCA-3+ DCs are uniquely proficient in cross-presentation of antigens.Citation29
In the setting of cancer, CD103+ DCs were just reported to infiltrate tumors in 2, separate murine models; ectopically implanted B16 melanoma, as well as spontaneously arising mammary tumors driven by expression of the mouse mammary tumor virus promoter.Citation81 In agreement with these findings, CD103+ DC also infiltrated human metastatic melanoma. In this important study, the authors generated a signature associated with either CD103+ tumor-infiltrating DCs or CD11b+ tumor-infiltrating DCs, and found that a high CD103 to CD11b signature ratio correlated with a better prognosis in a clinical retrospective analysis of breast cancer, head and neck squamous cell carcinoma, or lung adenocarcinoma.Citation81 However, it was not clear whether this stratification was driven more by the CD103+ DC or by the CD11b+ side of this ratio, as in human tumor analysis, PDL1+ tolerizing interstitial myeloid cells (represented here within the denominator) correlate with response to immunotherapy.Citation127,128 Also, while a mechanism for DC recruitment was not demonstrated in this study, tumors secrete high levels of CCL19 and CCL21 (the ligands for CCR7), which could potentially attract migratory DCs as well as other CCR7 dependent populations such as regulatory T cells (as discussed below).Citation129 Also it is unclear as to whether intra-tumoral DCs were originally skin-derived and infiltrated the tumor, or were recruited from the bone marrow derived pre-DC circulating through blood, which then entered the tumor and there differentiated into a tissue-resident DC. Many questions remain concerning the interactions between these DCs and tumors, but it is clear that CD103+ DCs are critical for the generation of cytolytic CD8+ T cell responses to a variety of pathogens, and their unique cross-presentation capability offers distinct potential in the presentation of tumor-derived antigens.
Together, skin migDC form a network of sentinel cells, with each population playing a unique role in shaping the response to a variety of pathogens. Understanding the unique functional properties associated with individual DC subsets will allow for further fine tuning of the immune response being generated, either by specifically targeting one population using antibody therapies as discussed previously or by understanding key pathways utilized by these individual DC populations during their licensing. However, in order to fully utilize the potential of the skin DC network, future studies are necessary to understand the coordination of these subsets, since, in vivo, each population plays a distinct role in shaping pathogen specific responses, as seen by the coordination of CD103+ migDC and LC during infection with C. Albicans, or the compensatory roles of CD103+ migDC and CD11b+ migDC in response to influenza. While individual DC populations are capable of generating an adaptive immune response, the interactions between the members of the skin DC network tailors the response specifically toward an individual pathogen, and thereby shapes the resultant immunological memory. The power to fine tune the immune response by harnessing these DC interactions will be indispensable for future vaccines.
Skin Resident Dendritic Cells in Immune Regulation
As early as the late 1990s, it had been hypothesized that DCs played an active role in the generation and maintenance of self tolerance, and that migratory DC in particular may be especially well suited for this function.Citation130 Indeed, DCs are capable of engulfing both apoptotic and necrotic cells, and generating MHC:peptide complexes from them.Citation131 However, only exposure to necrotic cells results in increased expression of classical DC maturation markers, such as MHC II, CD40, and CD86, as well as triggers DCs potential to activate naïve T cells.Citation132,133 MigDC are regularly exposed to normal cellular turnover (apoptotic), as mentioned above, and constitutively migrate to the cutaneous lymph node, making them an ideal candidate for DC induced tolerance to self antigens. Indeed, recent data as demonstrated presentation of murine skin-derived antigens in the non-inflamed state is restricted to migDCs, including both LCs and dermal DCs, and this presentation enforces deletional tolerance of self reactive T cells.Citation134 In the case of Leishmania infection, deletion of LCs results in a greater production of IFNγ by CD4+ T cells, as well as decreased parasite loads at the site of infection,Citation77 though it is difficult to discern if this is due to LCs playing a tolerizing role, or due to the fact that Leishmania infects directly propogates in LCs.Citation77 Additionally, murine LCs,Citation95 CD103+ DCs,Citation95 and Langerin−CD11b+ DCsCitation117,135 are all capable of generating CD4+ FoxP3+ Tregs, and a similar phenotype has been identified in human LCs.Citation84 Furthermore, in some settings, murine CD4+ T cells activated by LCs do not fully differentiate into cytokine producing effector cells or persist in vivo, despite initially dividing upon antigen encounter.Citation136 In accordance with a tolerizing role in immunity, human BDCA-3+ DCs, the counterpart to murine CD103+ DCs, have been found to produce large amounts of IL-10, present self-antigens, and induce regulatory T cells.Citation30
Recently, we found that murine migratory DC collectively and individually play a tolerogenic role during the setting of DEC205 protein vaccination.Citation39 Surprisingly, this effect occurred despite the inclusion of an adjuvant targeting TLR4 in our vaccine design, (GLA).Citation137 In these studies, LN resident DCs were responsible for the generation of adaptive immunity in response to vaccination, either by subcutaneous or intradermal administration. Despite enhanced antigen capture by migDC that had reached the LN (as compared to LN resident DC), immune responses were enhanced in CCR7 knockout animals, in which migDC could not traffic to the LN, demonstrating that migDCs were not required for productive immunity.
By using a DTR model where LC and CD103+ DCs (or just CD103+ alone in bone marrow chimera) could be transiently ablated (), we observed deletion of individual migratory DC subsets also enhanced immunity. Loss of either LC and CD103+ migDC or CD103+ migDCs alone resulted in increased IFNγ production by effector CD4+ T cells, revealing that CD103+ DCs can inhibit the immune response to a DEC205 targeted vaccination, despite the inclusion of adjuvant. In our model, an increased CD4+ T cell response also translated into increased vaccine specific IgG titers, further extending the immune regulatory function of CD103+ DCs to the humoral arm of the adaptive immune system. Furthermore, upon examination of the transcriptional signature of migDCs as compared to LN resident DCs, we found that migDCs expressed high levels of a variety of transcripts associated with immune tolerance. Parallel findings were found when comparing murine lung migDC to LN resident DCs.Citation28 Of translational importance, this tolerance signature was also present when we compared human peripheral blood DCs to skin resident DCs, suggesting that this program was conserved between mice and humans. We identified NF-κB signaling by ingenuity pathway analysis as central to this program. Another recent study has also identified a number of these tolerance associated genes when comparing migratory DC in the skin to those in the lymph node, suggesting that at least a large part of the program we identified may be amplified following migration to the cutaneous lymph node.Citation41 Collectively, overlapping features of the tolerance signature and migration signature are programmed through NF-κB signaling in DCs. DC specific loss of NF-κB signaling in mice resulted in loss of tolerance to self antigens in the steady-state, resulting in a lupus-like autoimmune phenotype marked by significantly increased lymph node size, as well as increased germinal centers and anti-nuclear antibody deposits in the kidney.Citation41 In addition to these studies, one study using gene gun based vaccination also noted higher numbers of CD4+ and CD8+ IFNγ producing cells upon ablation of dermal langerin+ DC.Citation83 However, despite having a regulatory role in the T cell response, CD103+ dermal DC and LC had a coordinated stimulatory role in the development of the antibody response in this study. The antigen amount and delivery methods may underscore the differences between these 2 studies. By subcutaneous injection we observe antigen rapidly accumulating and captured in LN within 3 hours by both resident and already migrated DC, while a gene gun based approach results in only very small doses of antigen over a longer period of time, which presumably must be actively transported by migratory DC for priming to occur.
Some recent studies have also reported that migDC are not needed to generate adaptive immunity to viral vaccines or particulate antigens. In one study, using UV-inactivated influenza as a model vaccine, UV inactivated influenza was found to drain directly to the LN following footpad or intradermal ear injection.Citation138 Additionally, removing the vaccinated ear 30 minutes after vaccination (thereby preventing new skin DC migration) had no affect on the generation of protective immunity against viral rechallenge. Another study used particulate antigen and found that a LN resident DC population (enriched in LN resident CD11b+ DC) was positioned to filter particulate antigens that drain directly from the vaccine site. Again, in this model, migDC were unnecessary for generating a T cell response.Citation139 In both these studies, as well as in our study, vaccine antigens were capable of draining freely to the local LN, without active transport from migDC. Therefore, in order to effectively target migDC, it may be important to prevent this type of antigen draining. These observations are important to note for future vaccine design as they suggest that targeting vaccinations to migDCs may not be ideal in all contexts, and that a unique set of licensing requirements exist for migDCs.
Recent work has also begun to address the role of migDC in the generation of anti-tumor immunity, as discussed earlier. In a murine melanoma model, B16, it has been demonstrated that expression of CCL21 increases tumor growth, while a tumor line where CCL21 was genetically ablated resulted in decreased tumor growth in a T cell dependent manner.Citation129 However, while the authors attributed this to increased infiltration of the tumor by regulatory T cells, skin migDC also express high levels of the receptor for CCL21, namely CCR7, which could be driving the increase in Treg formation. Indeed, when CCR7 signaling was decreased either through the use of a knockdown tumor cell line, or CCR7 knockout host mice, significantly less CD11c+ cells were found within the tumor, despite increased tumor specific CD8+ T cell infiltration. Furthermore, both CD103+ and CD11b+ migrate into skin tumors in mice, and are capable of transporting antigen to the draining LN,Citation81 though their status (stimulatory vs. tolerizing) following interaction with a neoplasm is just beginning to be addressed.Citation81 How skin cancer affects migDC programming and how in turn these populations contribute to cancer surveillance and therapy is an important area of future research, and could be a contributor to immune priming vs. tolerance to tumors by the immune system.
Conclusions
The skin is a unique microenvironment that interacts with pathogens and self-antigens regularly. In order to maintain the appropriate balance between activation to pathogens and tolerance to self, a network of migDCs surveys the skin and mediates the interactions between the immune system and foreign invaders. The individual characteristics of these populations make them attractive for targeted therapy, and have the potential to further amplify or tune a vaccine to the response desired depending upon the utilized DC populations. For example, targeting a protein vaccine to CD103+ DCs could be more effective in generating a cytolytic T cells response when compared to broader DC targeting, while targeting Langerin−CD11b+ could lead to enhanced production of neutralizing antibodies. In addition, understanding how individual migDC populations coordinate responses to pathogens will allow for greater fine tuning of the response to vaccinations. However, as seen in the setting of DEC205 vaccinations, it will be important to fully understand the licensing cues that drive each of these subsets to activate adaptive immunity, so as to stimulate an optimal immune response.
Despite the need to perform more human skin based immunology, additional pre-clinical models will be invaluable to add mechanistic insight toward understanding the specific roles of individual migDC populations, as well as the function of migDCs as a whole. As a vaccination route, the skin is unique, depending predominantly on cross-presentation of antigens from infected and dying cells, instead of on direct presentation though infection of DCs. When utilized correctly, skin based vaccinations are capable of generating sterilizing and protective immunity, as seen by the overwhelming success of the smallpox vaccine. However, work in our lab and others has recently revealed that the DCs involved in generating this protection are also responsible for tightly regulating the immune system, likely to prevent horror-autotoxicus,Citation140 and may be hampering the immune response in certain settings.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 1973; 137:1142-62; PMID:4573839; http://dx.doi.org/10.1084/jem.137.5.1142
- Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C, Jorgas T, Kurz SM, Rose-John S, Yokota Y, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol 2003; 4:380-6; PMID:12598895; http://dx.doi.org/10.1038/ni903
- Anderson KL, Perkin H, Surh CD, Venturini S, Maki RA, Torbett BE. Transcription factor PU.1 is necessary for development of thymic and myeloid progenitor-derived dendritic cells. J Immunol 2000; 164:1855-61; PMID:10657634; http://dx.doi.org/10.4049/jimmunol.164.4.1855
- Guerriero A, Langmuir PB, Spain LM, Scott EW. PU.1 is required for myeloid-derived but not lymphoid-derived dendritic cells. Blood 2000; 95:879-85; PMID:10648399
- McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 2000; 95:3489-97; PMID:10828034
- Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med 1996; 184:1953-62; PMID:8920882; http://dx.doi.org/10.1084/jem.184.5.1953
- Laouar Y, Welte T, Fu X-Y, Flavell RA. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity 2003; 19:903-12; PMID:14670306; http://dx.doi.org/10.1016/S1074-7613(03)00332-7
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 2002; 17:211-20; PMID:12196292; http://dx.doi.org/10.1016/S1074-7613(02)00365-5
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 2003; 3:984-93; PMID:14647480; http://dx.doi.org/10.1038/nri1246
- Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol 2011; 29:163-83; PMID:21219184; http://dx.doi.org/10.1146/annurev-immunol-031210-101345
- Colonna M, Trinchieri G, Liu Y-J. Plasmacytoid dendritic cells in immunity. Nat Immunol 2004; 5:1219-26; PMID:15549123; http://dx.doi.org/10.1038/ni1141
- Domínguez PM, Ardavín C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev 2010; 234:90-104; PMID:20193014; http://dx.doi.org/10.1111/j.0105-2896.2009.00876.x
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003; 19:59-70; PMID:12871639; http://dx.doi.org/10.1016/S1074-7613(03)00171-7
- Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev 2010; 234:55-75; PMID:20193012; http://dx.doi.org/10.1111/j.0105-2896.2009.00885.x
- Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev 2010; 234:18-31; PMID:20193009; http://dx.doi.org/10.1111/j.0105-2896.2009.00870.x
- Levin C, Perrin H, Combadiere B. Tailored immunity by skin antigen-presenting cells. Hum Vaccin Immunother 2015; 11:27-36; PMID:25483512; http://dx.doi.org/10.4161/hv.34299
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Förster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 2004; 21:279-88; PMID:15308107; http://dx.doi.org/10.1016/j.immuni.2004.06.014
- Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol 2014; 14:289-301; PMID:24722477
- Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, Schoenfeld D, Nomicos E, Park M; NIH Intramural Sequencing Center Comparative Sequencing Program, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013; 498:367-70; PMID:23698366; http://dx.doi.org/10.1038/nature12171
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC; NISC Comparative Sequencing Program, Bouffard GG, Blakesley RW, Murray PR, Green ED, et al. Topographical and temporal diversity of the human skin microbiome. Science 2009; 324:1190-2; PMID:19478181; http://dx.doi.org/10.1126/science.1171700
- Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med 2004; 351:2295-301; PMID:15525714; http://dx.doi.org/10.1056/NEJMoa043540
- Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med 2010; 16:224-7; PMID:20081864; http://dx.doi.org/10.1038/nm.2078
- Meyer KC, Klatte JE, Dinh HV, Harries MJ, Reithmayer K, Meyer W, Sinclair R, Paus R. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol 2008; 159:1077-85; PMID:18795933
- Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, Ueha S, Horiuchi K, Tanizaki H, Kabashima K, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol 2012; 13:744-52; PMID:22729248; http://dx.doi.org/10.1038/ni.2353
- Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity 2011; 35:572-82; PMID:22000287; http://dx.doi.org/10.1016/j.immuni.2011.08.014
- Kovalenko A, Kim JC, Kang TB, Rajput A, Bogdanov K, Dittrich-Breiholz O, Kracht M, Brenner O, Wallach D. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med 2009; 206:2161-77; PMID:19720838; http://dx.doi.org/10.1084/jem.20090616
- Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol 2014; 14:417-28; PMID:24854591; http://dx.doi.org/10.1038/nri3683
- Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol 2012; 13:888-99; PMID:22797772; http://dx.doi.org/10.1038/ni.2370
- Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 2012; 37:60-73; PMID:22795876; http://dx.doi.org/10.1016/j.immuni.2012.04.012
- Chu C-C, Ali N, Karagiannis P, Di Meglio P, Skowera A, Napolitano L, Barinaga G, Grys K, Sharif-Paghaleh E, Karagiannis SN, et al. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J Exp Med 2012; 209:935-945; PMID:22547651; http://dx.doi.org/10.1084/jem.20112583
- Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c +BDCA-1+ dendritic cells and CD163+FXIIIA + macrophages. J Clin Invest 2007; 117:2517-25; PMID:17786242; http://dx.doi.org/10.1172/JCI32282
- Yoshida K, Kubo A, Fujita H, Yokouchi M, Ishii K, Kawasaki H, Nomura T, Shimizu H, Kouyama K, Ebihara T, et al. Distinct behavior of human Langerhans cells and inflammatory dendritic epidermal cells at tight junctions in patients with atopic dermatitis. J Allergy Clin Immunol 2014; 134:856-64; PMID:25282566; http://dx.doi.org/10.1016/j.jaci.2014.08.001
- Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med 2009; 206:2937-46; PMID:19995951; http://dx.doi.org/10.1084/jem.20091527
- Ouchi T, Kubo A, Yokouchi M, Adachi T, Kobayashi T, Kitashima DY, Fujii H, Clausen BE, Koyasu S, Amagai M, et al. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J Exp Med 2011; 208:2607-13; PMID:22143886; http://dx.doi.org/10.1084/jem.20111718
- Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature 1993; 361:82-5; PMID:8421498; http://dx.doi.org/10.1038/361082a0
- Henri S, Guilliams M, Poulin LF, Tamoutounour S, Ardouin L, Dalod M, Malissen B. Disentangling the complexity of the skin dendritic cell network. Immunol Cell Biol 2010; 88:366-75; PMID:20231850; http://dx.doi.org/10.1038/icb.2010.34
- Haniffa M, Gunawan M, Jardine L. Human skin dendritic cells in health and disease. J Dermatol Sci 2015; 77:85-92; PMID:25301671; http://dx.doi.org/10.1016/j.jdermsci.2014.08.012
- Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol 1993; 151:6535-45; PMID:7504023
- Mollah SA, Dobrin JS, Feder RE, Tse SW, Matos IG, Cheong C, Steinman RM, Anandasabapathy N. Flt3L dependence helps define an uncharacterized subset of murine cutaneous dendritic cells. J Invest Dermatol 2014; 134:1265-75; PMID:24288007; http://dx.doi.org/10.1038/jid.2013.515
- Wilson NS, Young LJ, Kupresanin F, Naik SH, Vremec D, Heath WR, Akira S, Shortman K, Boyle J, Maraskovsky E, et al. Normal proportion and expression of maturation markers in migratory dendritic cells in the absence of germs or Toll-like receptor signaling. Immunol Cell Biol 2008; 86:200-5; PMID:18026177; http://dx.doi.org/10.1038/sj.icb.7100125
- Baratin M, Foray C, Demaria O, Habbeddine M, Pollet E, Maurizio J, Verthuy C, Davanture S, Azukizawa H, Flores-Langarica A, et al. Homeostatic NF-κB signaling in steady-state migratory dendritic cells regulates immune homeostasis and tolerance. Immunity 2015; 42:627-39; PMID:25862089; http://dx.doi.org/10.1016/j.immuni.2015.03.003
- Stoitzner P, Tripp CH, Douillard P, Saeland S, Romani N. Migratory Langerhans cells in mouse lymph nodes in steady state and inflammation. J Invest Dermatol 2005; 125:116-25; PMID:15982311; http://dx.doi.org/10.1111/j.0022-202X.2005.23757.x
- Kastenmüller K, Wille-Reece U, Lindsay RW, Trager LR, Darrah PA, Flynn BJ, Becker MR, Udey MC, Clausen BE, Igyarto BZ, et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J Clin Invest 2011; 121:1782-96; PMID:21540549; http://dx.doi.org/10.1172/JCI45416
- Guermonprez P, Helft J, Claser C, Deroubaix S, Karanje H, Gazumyan A, Darasse-Jèze G, Telerman SB, Breton G, Schreiber HA, et al. Inflammatory Flt3l is essential to mobilize dendritic cells and for T cell responses during Plasmodium infection. Nat Med 2013; 19:730-8; PMID:23685841; http://dx.doi.org/10.1038/nm.3197
- Muto J, Morioka Y, Yamasaki K, Kim M, Garcia A, Carlin AF, Varki A, Gallo RL. Hyaluronan digestion controls DC migration from the skin. J Clin Invest 2014; 124:1309-19; PMID:24487587; http://dx.doi.org/10.1172/JCI67947
- Anandasabapathy N, Feder R, Mollah S, Tse SW, Longhi MP, Mehandru S, Matos I, Cheong C, Ruane D, Brane L, et al. Classical Flt3L-dependent dendritic cells control immunity to protein vaccine. J Exp Med 2014; 211:1875-91; PMID:25135299; http://dx.doi.org/10.1084/jem.20131397
- Harman AN, Bye CR, Nasr N, Sandgren KJ, Kim M, Mercier SK, Botting RA, Lewin SR, Cunningham AL, Cameron PU. Identification of lineage relationships and novel markers of blood and skin human dendritic cells. J Immunol 2013; 190:66-79; PMID:23183897; http://dx.doi.org/10.4049/jimmunol.1200779
- Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med 2012; 209:1167-81; PMID:22565823; http://dx.doi.org/10.1084/jem.20120340
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010; 330:841-5; PMID:20966214; http://dx.doi.org/10.1126/science.1194637
- Heath WR, Villadangos JA. No driving without a license. Nat Immunol 2005; 6:125-6; PMID:15662437; http://dx.doi.org/10.1038/ni0205-125
- Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol 2011; 11:788-98; PMID:22025056; http://dx.doi.org/10.1038/nri3087
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhé C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 2005; 22:643-54; PMID:15894281; http://dx.doi.org/10.1016/j.immuni.2005.04.004
- Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol 2005; 169:569-76; PMID:15897263; http://dx.doi.org/10.1083/jcb.200501071
- Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med 2012; 209:1153-65; PMID:22615130; http://dx.doi.org/10.1084/jem.20112675
- Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med 2007; 204:3147-56; PMID:18086865; http://dx.doi.org/10.1084/jem.20071966
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 2005; 23:611-20; PMID:16356859; http://dx.doi.org/10.1016/j.immuni.2005.10.008
- Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 2008; 8:362-71; PMID:18379575; http://dx.doi.org/10.1038/nri2297
- Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med 2007; 204:735-45; PMID:17371928; http://dx.doi.org/10.1084/jem.20061405
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 2008; 322:1097-100; PMID:19008445; http://dx.doi.org/10.1126/science.1164206
- Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, Sher A, Ploegh HL, Murphy TL, Sibley LD, et al. CD8α(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity 2011; 35:249-59; PMID:21867928; http://dx.doi.org/10.1016/j.immuni.2011.08.008
- Tussiwand R, Lee WL, Murphy TL, Mashayekhi M, KC W, Albring JC, Satpathy AT, Rotondo JA, Edelson BT, Kretzer NM, et al. Compensatory dendritic cell development mediated by BATF-IRF interactions. Nature 2012; 490:502-7; PMID:22992524; http://dx.doi.org/10.1038/nature11531
- Gao Y, Nish SA, Jiang R, Hou L, Licona-Limón P, Weinstein JS, Zhao H, Medzhitov R. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity 2013; 39:722-32; PMID:24076050; http://dx.doi.org/10.1016/j.immuni.2013.08.028
- Macatonia SE. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med 1987; 166:1654-67; PMID:3119761; http://dx.doi.org/10.1084/jem.166.6.1654
- Hayashi M, Higashi K, Kato H, Kaneko H. Assessment of preferential Th1 or Th2 induction by low-molecular-weight compounds using a reverse transcription-polymerase chain reaction method: comparison of two mouse strains, C57BL/6 and BALB/c. Toxicol Appl Pharmacol 2001; 177:38-45; PMID:11708898; http://dx.doi.org/10.1006/taap.2001.9286
- Van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 2009; 182:5836-45; PMID:19380832; http://dx.doi.org/10.4049/jimmunol.0802999
- Holzmann S, Tripp CH, Schmuth M, Janke K, Koch F, Saeland S, Stoitzner P, Romani N. A Model System Using Tape Stripping for Characterization of Langerhans Cell-Precursors In Vivo. J Invest Dermatol 2004; 122:1165-74; PMID:15140219; http://dx.doi.org/10.1111/j.0022-202X.2004.22520.x
- Kashem SW, Igyártó BZ, Gerami-Nejad M, Kumamoto Y, Mohammed J, Jarrett E, Drummond RA, Zurawski SM, Zurawski G, Berman J, et al. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity 2015; 42:356-66; PMID:25680275; http://dx.doi.org/10.1016/j.immuni.2015.01.008
- Bonifazi P, Zelante T, D'Angelo C, De Luca A, Moretti S, Bozza S, Perruccio K, Iannitti RG, Giovannini G, Volpi C, et al. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol 2009; 2:362-74; PMID:19421183; http://dx.doi.org/10.1038/mi.2009.17
- Haley K, Igyártó BZ, Ortner D, Bobr A, Kashem S, Schenten D, Kaplan DH. Langerhans cells require MyD88-dependent signals for Candida albicans response but not for contact hypersensitivity or migration. J Immunol 2012; 188:4334-9; PMID:22442445; http://dx.doi.org/10.4049/jimmunol.1102759
- Igyártó BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 2011; 35:260-72; PMID:21782478; http://dx.doi.org/10.1016/j.immuni.2011.06.005
- Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med 2003; 197:153-62; PMID:12538655; http://dx.doi.org/10.1084/jem.20021109
- Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 2006; 25:153-62; PMID:16860764; http://dx.doi.org/10.1016/j.immuni.2006.04.017
- Eidsmo L, Allan R, Caminschi I, van Rooijen N, Heath WR, Carbone FR. Differential migration of epidermal and dermal dendritic cells during skin infection. J Immunol 2009; 182:3165-72; PMID:19234214; http://dx.doi.org/10.4049/jimmunol.0802950
- Seneschal J, Jiang X, Kupper TS. Langerin+ dermal DC, but not Langerhans cells, are required for effective CD8-mediated immune responses after skin scarification with vaccinia virus. J Invest Dermatol 2014; 134:686-94; PMID:24126845; http://dx.doi.org/10.1038/jid.2013.418
- Iborra S, Izquierdo HM, Martínez-López M, Blanco-Menéndez N, Reis e Sousa C, Sancho D. The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice. J Clin Invest 2012; 122:1628-43; PMID:22505455; http://dx.doi.org/10.1172/JCI60660
- Frenz T, Waibler Z, Hofmann J, Hamdorf M, Lantermann M, Reizis B, Tovey MG, Aichele P, Sutter G, Kalinke U. Concomitant type I IFN receptor-triggering of T cells and of DC is required to promote maximal modified vaccinia virus Ankara-induced T-cell expansion. Eur J Immunol 2010; 40:2769-77; PMID:20821729; http://dx.doi.org/10.1002/eji.201040453
- Kautz-Neu K, Noordegraaf M, Dinges S, Bennett CL, John D, Clausen BE, von Stebut E. Langerhans cells are negative regulators of the anti-Leishmania response. J Exp Med 2011; 208:885-91; PMID:21536741; http://dx.doi.org/10.1084/jem.20102318
- Ritter U, Meissner A, Scheidig C, Körner H. CD8 alpha- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur J Immunol 2004; 34:1542-50; PMID:15162423; http://dx.doi.org/10.1002/eji.200324586
- Iezzi G, Fröhlich A, Ernst B, Ampenberger F, Saeland S, Glaichenhaus N, Kopf M. Lymph node resident rather than skin-derived dendritic cells initiate specific T cell responses after Leishmania major infection. J Immunol 2006; 177:1250-6; PMID:16818784; http://dx.doi.org/10.4049/jimmunol.177.2.1250
- Khanna KM, Blair DA, Vella AT, McSorley SJ, Datta SK, Lefrançois L. T cell and APC dynamics in situ control the outcome of vaccination. J Immunol 2010; 185:239-52; PMID:20530268; http://dx.doi.org/10.4049/jimmunol.0901047
- Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014; 26:638-52; PMID:25446897; http://dx.doi.org/10.1016/j.ccell.2014.09.007
- Stoitzner P, Pfaller K, Stössel H, Romani N. A close-up view of migrating Langerhans cells in the skin. J Invest Dermatol 2002; 118, 117-25; PMID:11851884; http://dx.doi.org/10.1046/j.0022-202x.2001.01631.x
- Nagao K, Ginhoux F, Leitner WW, Motegi S, Bennett CL, Clausen BE, Merad M, Udey MC. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A 2009; 106:3312-7; PMID:19218433; http://dx.doi.org/10.1073/pnas.0807126106
- Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 2012; 36:873-84; PMID:22560445; http://dx.doi.org/10.1016/j.immuni.2012.03.018
- Trumpfheller C, Longhi MP, Caskey M, Idoyaga J, Bozzacco L, Keler T, Schlesinger SJ, Steinman RM. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Intern Med 2012; 271:183-92; PMID:22126373; http://dx.doi.org/10.1111/j.1365-2796.2011.02496.x
- Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med 2004; 199:815-24; PMID:15024047; http://dx.doi.org/10.1084/jem.20032220
- Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, Park CG, Steinman RM. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol 2008; 180:3647-50; PMID:18322168; http://dx.doi.org/10.4049/jimmunol.180.6.3647
- Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ, et al. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood 2005; 106:1278-85; PMID:15878980; http://dx.doi.org/10.1182/blood-2005-01-0318
- Sancho D, Mourão-Sá D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest 2008; 118:2098-110; PMID:18497879; http://dx.doi.org/10.1172/JCI34584
- Charalambous A, Oks M, Nchinda G, Yamazaki S, Steinman RM. Dendritic cell targeting of survivin protein in a xenogeneic form elicits strong CD4+ T cell immunity to mouse survivin. J Immunol 2006; 177:8410-21; PMID:17142738; http://dx.doi.org/10.4049/jimmunol.177.12.8410
- Cheong C, Choi JH, Vitale L, He LZ, Trumpfheller C, Bozzacco L, Do Y, Nchinda G, Park SH, Dandamudi DB, et al. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood 2010; 116:3828-38; PMID:20668230; http://dx.doi.org/10.1182/blood-2010-06-288068
- Mahnke K, Qian Y, Fondel S, Brueck J, Becker C, Enk AH. Targeting of antigens to activated dendritic cells in vivo cures metastatic melanoma in mice. Cancer Res 2005; 65:7007-12; PMID:16061687; http://dx.doi.org/10.1158/0008-5472.CAN-05-0938
- Ring S, Maas M, Nettelbeck DM, Enk AH, Mahnke K. Targeting of autoantigens to DEC205+ dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J Immunol 2013; 191:2938-47; PMID:23945139; http://dx.doi.org/10.4049/jimmunol.1202592
- Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity 2004; 20:695-705; PMID:15189735; http://dx.doi.org/10.1016/j.immuni.2004.05.002
- Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, Merad M, Steinman RM. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest 2013; 123:844-54; PMID:23298832
- Caskey M, Trumpfheller C, Pollak S, Sinnenberg L, Hurley A, Pring J, Shimeliovich I, Yipp B, Anandasabapathy N, Mehandru S, et al. In vivo targeting of HIV gag to dendritic cells in combination with poly ICLC is safe and immunogenic in healthy volunteers. Retrovirology 2012; 9:O51; http://dx.doi.org/10.1186/1742-4690-9-S2-O51
- Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, Chuang E, Sanborn RE, Lutzky J, Powderly J, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med 2014; 6:232ra51; PMID:24739759; http://dx.doi.org/10.1126/scitranslmed.3008068
- Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res 2013 19:4917-24; PMID:23868869; http://dx.doi.org/10.1158/1078-0432.CCR-12-1972
- Shen X, Wong SBJ, Buck CB, Zhang J, Siliciano RF. Direct priming and cross-priming contribute differentially to the induction of CD8+ CTL following exposure to vaccinia virus via different routes. J Immunol 2002; 169:4222-9; PMID:12370352; http://dx.doi.org/10.4049/jimmunol.169.8.4222
- Zahner SP, Kel JM, Martina CA, Brouwers-Haspels I, van Roon MA, Clausen BE. Conditional deletion of TGF-βR1 using Langerin-Cre mice results in Langerhans cell deficiency and reduced contact hypersensitivity. J Immunol 2011; 187:5069-76; PMID:21998450; http://dx.doi.org/10.4049/jimmunol.1101880
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kündig TM, Frei K, Ginhoux F, Merad M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 2012; 37:1050-60; PMID:23177320; http://dx.doi.org/10.1016/j.immuni.2012.11.001
- Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, Kissenpfennig A, Barbaroux JB, Groves R, Geissmann F. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med 2009; 206:3089-100; PMID:19995948; http://dx.doi.org/10.1084/jem.20091586
- Seré K, Baek JH, Ober-Blöbaum J, Müller-Newen G, Tacke F, Yokota Y, Zenke M, Hieronymus T. Two distinct types of langerhans cells populate the skin during steady state and inflammation. Immunity 2012; 37:905-16; PMID:23159228; http://dx.doi.org/10.1016/j.immuni.2012.07.019
- Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat Immunol 2006; 7:265-73; PMID:16444257; http://dx.doi.org/10.1038/ni1307
- Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol 2006; 126:787-96; PMID:16439974; http://dx.doi.org/10.1038/sj.jid.5700107
- Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, Devilard E, Viret C, Azukizawa H, Kissenpfennig A, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med 2010; 207:189-206; PMID:20038600; http://dx.doi.org/10.1084/jem.20091964
- Banchereau J, Thompson-Snipes L, Zurawski S, Blanck JP, Cao Y, Clayton S, Gorvel JP, Zurawski G, Klechevsky E. The differential production of cytokines by human Langerhans cells and dermal CD14(+) DCs controls CTL priming. Blood 2012; 119:5742-9; PMID:22535664; http://dx.doi.org/10.1182/blood-2011-08-371245
- Romano E, Cotari JW, Barreira da Silva R, Betts BC, Chung DJ, Avogadri F, Fink MJ, St Angelo ET, Mehrara B, Heller G, et al. Human Langerhans cells use an IL-15R-α/IL-15/pSTAT5-dependent mechanism to break T-cell tolerance against the self-differentiation tumor antigen WT1. Blood 2012; 119:5182-90; PMID:22510877; http://dx.doi.org/10.1182/blood-2011-09-382200
- Tussiwand R, Everts B, Grajales-Reyes GE, Kretzer NM, Iwata A, Bagaitkar J, Wu X, Wong R, Anderson DA, Murphy TL, et al. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity 2015; 42:916-28; PMID:25992862; http://dx.doi.org/10.1016/j.immuni.2015.04.017
- Klechevsky E. Human dendritic cells - stars in the skin. Eur J Immunol 2013; 43:3147-55; PMID:24222336; http://dx.doi.org/10.1002/eji.201343790
- Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology 2013; 140:22-30; PMID:23621371; http://dx.doi.org/10.1111/imm.12117
- Bajaña S, Roach K, Turner S, Paul J, Kovats S. IRF4 promotes cutaneous dendritic cell migration to lymph nodes during homeostasis and inflammation. J Immunol 2012; 189:3368-77; PMID:22933627; http://dx.doi.org/10.4049/jimmunol.1102613
- Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, et al. Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 2013; 38:322-35; PMID:23352232; http://dx.doi.org/10.1016/j.immuni.2012.10.016
- Nizza ST, Campbell JJ. CD11b+ migratory dendritic cells mediate CD8 T cell cross-priming and cutaneous imprinting after topical immunization. PLoS One 2014; 9; PMID:24618819; http://dx.doi.org/10.1371/journal.pone.0091054
- Del Rio M-L, Rodriguez-Barbosa J-I, Kremmer E, Förster R. CD103- and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol 2007; 178:6861-6; PMID:17513734; http://dx.doi.org/10.4049/jimmunol.178.11.6861
- Langlet C, Tamoutounour S, Henri S, Luche H, Ardouin L, Grégoire C, Malissen B, Guilliams M. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol 2012; 188:1751-60; PMID:22262658; http://dx.doi.org/10.4049/jimmunol.1102744
- McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity 2009; 30:277-88; PMID:19200757; http://dx.doi.org/10.1016/j.immuni.2008.11.013
- Beaty SR, Rose CE, Sung S-S. J. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J Immunol 2007; 178:1882-95; PMID:17237439; http://dx.doi.org/10.4049/jimmunol.178.3.1882
- Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 2013; 38:970-83; PMID:23706669; http://dx.doi.org/10.1016/j.immuni.2013.04.011
- Edelson BT, Bradstreet TR, KC W, Hildner K, Herzog JW, Sim J, Russell JH, Murphy TL, Unanue ER, Murphy KM. Batf3-dependent cd11b low/- peripheral dendritic cells are gm-csf-independent and are not required for th cell priming after subcutaneous immunization. PLoS One 2011; 6; PMID:22065991; http://dx.doi.org/10.1371/journal.pone.0025660
- Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med 2009; 206:3115-30; PMID:20008528; http://dx.doi.org/10.1084/jem.20091756
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol 2009; 10:488-95; PMID:19349986; http://dx.doi.org/10.1038/ni.1724
- Helft J, Manicassamy B, Guermonprez P, Hashimoto D, Silvin A, Agudo J, Brown BD, Schmolke M, Miller JC, Leboeuf M, et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J Clin Invest 2012; 122:4037-47; PMID:23041628; http://dx.doi.org/10.1172/JCI60659
- GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med 2008; 205:1621-34; PMID:18591406; http://dx.doi.org/10.1084/jem.20071365
- Moltedo B, Li W, Yount JS, Moran TM. Unique type I interferon responses determine the functional fate of migratory lung dendritic cells during influenza virus infection. PLoS Pathog 2011; 7:e1002345; PMID:22072965; http://dx.doi.org/10.1371/journal.ppat.1002345
- Martínez-López M, Iborra S, Conde-Garrosa R, Sancho D. Batf3-dependent CD103+ dendritic cells are major producers of IL-12 that drive local Th1 immunity against Leishmania major infection in mice. Eur J Immunol 2015; 45:119-29; PMID:25312824; http://dx.doi.org/10.1002/eji.201444651
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti-PD-1 Therapy. Clin Cancer Res 2014; 20:5064-74. doi:10.1158/1078-0432.CCR-13-3271; PMID:24714771; http://dx.doi.org/10.1158/1078-0432.CCR-13-3271
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563-7; PMID:25428504; http://dx.doi.org/10.1038/nature14011
- Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science (80) 2010; 328:749-52; PMID:20339029; http://dx.doi.org/10.1126/science.1185837
- Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med 2000; 191:411-6; PMID:10662786; http://dx.doi.org/10.1084/jem.191.3.411
- Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med 1998; 188:2163-73; PMID:9841929; http://dx.doi.org/10.1084/jem.188.11.2163
- Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 2000; 191:423-34; PMID:10662788; http://dx.doi.org/10.1084/jem.191.3.423
- Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med 1999; 5:1249-55; PMID:10545990; http://dx.doi.org/10.1038/15200
- Waithman J, Allan RS, Kosaka H, Azukizawa H, Shortman K, Lutz MB, Heath WR, Carbone FR, Belz GT. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J Immunol 2007; 179:4535-41; PMID:17878350; http://dx.doi.org/10.4049/jimmunol.179.7.4535
- Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, Malissen B. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood 2010; 115:1958-68; PMID:20068222; http://dx.doi.org/10.1182/blood-2009-09-245274
- Shklovskaya E, O'Sullivan BJ, Ng LG, Roediger B, Thomas R, Weninger W, Fazekas de St Groth B. Langerhans cells are precommitted to immune tolerance induction. Proc Natl Acad Sci U S A 2011; 108:18049-54; PMID:22006331; http://dx.doi.org/10.1073/pnas.1110076108
- Pantel A, Cheong C, Dandamudi D, Shrestha E, Mehandru S, Brane L, Ruane D, Teixeira A, Bozzacco L, Steinman RM, et al. A new synthetic TLR4 agonist, GLA, allows dendritic cells targeted with antigen to elicit Th1 T-cell immunity in vivo. Eur J Immunol 2012; 42:101-9; PMID:22002164; http://dx.doi.org/10.1002/eji.201141855
- Woodruff MC, Heesters BA, Herndon CN, Groom JR, Thomas PG, Luster AD, Turley SJ, Carroll MC. Trans-nodal migration of resident dendritic cells into medullary interfollicular regions initiates immunity to influenza vaccine. J Exp Med 2014; 211:1611-21; PMID:25049334; http://dx.doi.org/10.1084/jem.20132327
- Gerner MY, Torabi-Parizi P, Germain RN. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 2014; 42:172-85; PMID:25607462; http://dx.doi.org/10.1016/j.immuni.2014.12.024
- Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A 2002; 99:351-8; PMID:11773639; http://dx.doi.org/10.1073/pnas.231606698
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med 2007; 204:1653-64; PMID:17591855
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 2002; 196:1627-38; PMID:12486105; http://dx.doi.org/10.1084/jem.20021598
