Abstract
Botulinum neurotoxins (BoNTs) are deadly, toxic proteins produced by the bacterium Clostridium botulinum that can cause significant diseases in humans. The use of the toxic substances as potential bioweapons has raised concerns by the Centers for Disease Control and Prevention and the United States Military. Currently, there is no licensed vaccine to prevent botulinum intoxication. Here we present an immunogenicity study to evaluate the efficacy of novel monovalent vaccines and a trivalent cocktail DNA vaccine targeting the heavy chain C-terminal fragments of Clostridium botulinum neurotoxin serotypes A, B, and E. These synthetic DNA vaccines induced robust humoral and polyfunctional CD4+ T-cell responses which fully protected animals against lethal challenge after just 2 immunizations. In addition, naïve animals administered immunized sera mixed with the lethal neurotoxin were 100% protected against intoxication. The data demonstrate the protective efficacy induced by a combinative synthetic DNA vaccine approach. This study has importance for the development of vaccines that provide protective immunity against C. botulinum neurotoxins and other toxins.
Introduction
The neurotoxin produced by the bacterium Clostridium botulinum can cause death or paralysis in humans. In the U.S. there are approximately 145 cases of intoxication reported each year.Citation1 In addition, the threat of the use of weaponized Clostridium botulinum neurotoxin as a biowarfare agent has caused concerns.Citation2-4 However, there is currently no licensed vaccine to prevent botulinum poisoning.
There are 8 antigenically distinct serotypes (A–H) of C. botulinum.Citation2,5 The toxin types are identified serologically by neutralization with their specific antitoxin.Citation6 Five of the 8 serotypes, botulinum neurotoxin type A (BoNT/A), BoNT/B, BoNT/E, BoNT/F, and BoNT/H are known to cause human disease.Citation5,7 However, the highest incidences of human botulism are associated with BoNT/A, BoNT/B, and BoNT/E.Citation8 The botulinum toxins are produced as single-chain polypeptides which undergo cleavage by bacterial proteases to yield di-chain structures linked by a disulfide bond.Citation9 The molecule consists of a 50 kDa N-terminal light chain (LCN) metalloprotease, which is responsible for toxic activity and a 100 kDa non-toxic heavy chain (HC). The heavy chain comprises the receptor binding domain at the C-terminus (HC) and the translocation domain at the N-terminus (HN). Upon exposure, the toxin binds to receptors on peripheral nerve endings and is endocytosed into nerve cells.Citation9 In the acidic pH of the endosome, the N-terminal heavy chain (HN) aids in the translocation of the light chain (LC) across the endosomal membranes and into the cytosol.Citation9,10 The toxin then causes the proteolytic degradation of the proteins [vesicle associated membrane protein (VAMP; synaptobrevin), syntaxin, and the synaptosomal-associated protein (SNAP-25)] responsible for the release of the neurotransmitter, acetylcholine.Citation9 The prevention of neurotransmitter release at the presynaptic nerve terminals results in muscular paralysis.Citation10
Currently, the only treatments for botulinum intoxication are post-exposure antibody-based therapies. A licensed botulinum heptavalent (A, B, C, D, E, F, G) equine antitoxin (HBAT) and serotype-specific human hyperimmune globulin are being used to treat adult and infant botulism, respectively.Citation2,11-13 However, treatment with antitoxin requires rapid identification of BoNT poisoning to be effective as well as weeks to months of supportive care post-treatment. In addition, antitoxin therapy is only effective for treating the small number of life-threatening botulinum cases currently reportedCitation13 and would not be an effective prophylactic strategy to prevent intoxication of mass populations threatened with weaponized C. botulinum toxin.
The effectiveness of BoNT antiserum demonstrates that a BoNT vaccine inducing neutralizing antibodies can prevent disease upon exposure. However, since the CDC recently discontinued its use of the experimental pentavalent toxoid (A, B, C, D, and E) vaccine due to limited effectiveness and tolerability issues, there is currently no licensed vaccine to prevent botulinum poisoning.Citation2,14 In this regard, the DNA vaccine platform is an effective vaccine modality to prevent botulinum toxin poisoning. DNA vaccines are designed to specifically target antigens of interest and induce strong humoral and cellular immune responses in vaccinated hosts, providing protection from infectious challenge.Citation15-18 In addition, DNA vaccines have an unparalleled safety profile and are likely more economical to produce conceptually making them important potential experimental vaccines. The fact that DNA vaccines have been well tolerated further support the development for use anywhere a bioterrorist attack against military or civilian targets may occur.
DNA vaccines targeting the receptor-binding domain (HC) might represent an effective prophylactic vaccine to prevent botulinum poisoning. Due to its immunogenicity, the BoNT HC domain has been targeted as a recombinant antigen to generate neutralizing antibodies in DNA vaccination.Citation19-22 In addition, DNA vaccines targeting the BoNT HC fragment of serotypes A, B, and E were providing positive neutralization results.Citation23 Although protective immunity against botulinum neurotoxin poisoning is primarily antibody mediated, an evaluation of the DNA vaccine-induced CD4+ T cell response may further elucidate the mechanisms of B cell activation required to produce antigen- specific antibodies and generate memory B cell responses. However, a comprehensive study evaluating the humoral and cellular immune responses induced by a trivalent DNA vaccine targeting the BoNT HC fragments of the C. botulinum serotypes (A, B, and E) most responsible for human disease has not been reported.
Here we present an immunogenicity study to evaluate the efficacy of novel monovalent vaccines and a trivalent cocktail DNA vaccine targeting the heavy chain C-terminal fragment of C. botulinum neurotoxin serotypes A, B, and E. We show that these synthetic DNAs induced robust humoral and polyfunctional CD4+ T-cell responses and provided 100% protection against lethal challenge with the respective neurotoxin in mice. In addition, serum antibodies induced by our trivalent vaccine formulation provided 100% protection to naïve animals upon lethal toxin challenge. To our knowledge, this is the first report describing the humoral and cellular immune response generated by a BoNT trivalent DNA vaccine delivered with electroporation. In addition, this is the first report to show the ability of immunized sera from HC vaccinated animals to fully protect naïve animals from challenge with 100 LD50 BoNT/A, BoNT/B, and BoNT/E following 2 immunizations with DNA. This study has importance for the development of synthetic DNA vaccines that provide protective immunity against C. botulinum neurotoxins and other diseases caused by toxins.
Results
In vitro expression of BoNT/Hc/A, BoNT/Hc/B, and BoNT/Hc/E DNA vaccines
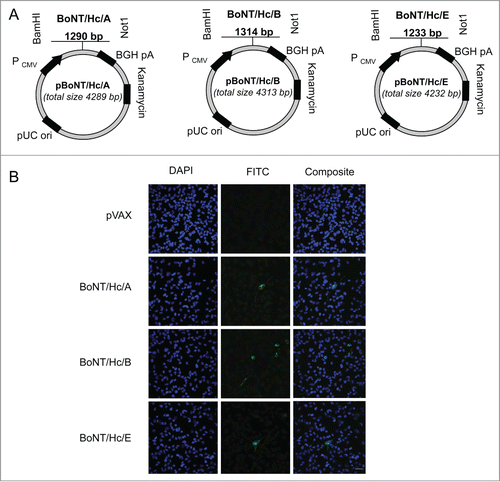
We designed 3 plasmids to target the heavy chain C-terminal fragment of BoNT serotypes A, B, and E. All heavy chain sequences were synthetically codon and RNA-optimized. We also modified potential glycosylation sites. The three components targeting the heavy chains of BoNT/A, BoNT/B, and BoNT/E were named pBoNT/Hc/A, pBoNT/Hc/B, and pBoNT/Hc/E, respectively. These BoNT/Hc/A, BoNT/Hc/B, and BoNT/Hc/E refer to the monovalent vaccine preparations (). We evaluated cellular expression of each plasmid by transfecting Rhabdomyosarcoma (RD) muscle cells with hemagglutinin (HA)-tagged BoNT/Hc/A, BoNT/Hc/B, and BoNT/Hc/E plasmids. As a negative control, we transfected RD cells with an empty vector backbone, pVAX. After 48 hr post-transfection, we evaluated expression using immunofluorescence analysis and a HA-tag antibody. Plasmid expression was confirmed with a FITC-labeled secondary antibody (green staining) (). All BoNT Hc vaccine constructs were expressed in vitro ().
Figure 1. Construction and representative in vitro expression of BoNT/Hc/A, BoNT/Hc/B, and BoNT/Hc/EDNA vaccine constructs. (A) Schematic of BoNT/Hc/A, B, or E genes cloned into the pVAX1 mammalian expression vector. The CMV promoter, BoNT Hc gene(s), BGH poly A signal, kanamycin resistance gene, and pUC origin are shown. (B) Representative in vitro expression of the hemagglutinin-tagged BoNT Hc plasmids. Expression was confirmed using transfected RD cells and a HA-tagged antibody. An empty vector (pVAX) was used as a negative control. Results were analyzed with confocal imaging. Scale bar = 100 μm.
Monovalent DNA vaccination induces strong antibody responses
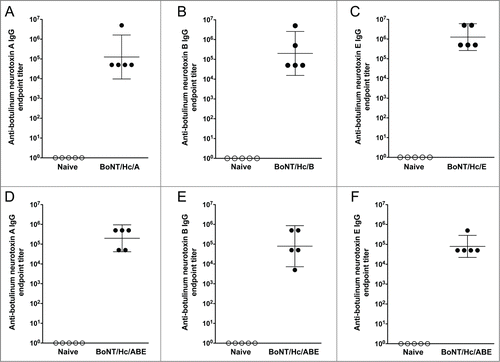
To determine the ability of the monovalent vaccines (BoNT/Hc/A, BoNT/Hc/B, and BoNT/Hc/E) to induce humoral immunity, BALB/c mice (n = 15/group) received 2 intramuscular DNA vaccinations followed by in vivo electroporation as outlined in . A preliminary study determined that a 10 μg dose of DNA for each vaccine resulted in consistent humoral responses. Therefore for these studies, 3 separate groups of mice were immunized with 10 μg of either pBoNT/Hc/A, pBoNT/Hc/B, or pBoNT/Hc/E. Three weeks after the final vaccination, sera were collected and 5 sera samples per group were used to determine binding antibody titers by ELISA. Vaccination with all monovalent antigens induced high titer antibodies (). The group geometric mean anti-neurotoxin-specific endpoint titers (with 95% confidence intervals) induced by BoNT/Hc/A, BoNT/Hc/B, and BoNT/Hc/E were 1.3 × 105 (9.7 × 103, 1.6 × 106), 2.0 × 105 (1.5 × 104, 2.6 × 106), 1.3 × 106 (2.6 × 105, 6.0 × 106), respectively. These results demonstrate the ability of the monovalent vaccine formulations to elicit high-titer antibodies after a total administration dosage of 20 μg of DNA.
Figure 2. Monovalent and trivalent study outlines. To evaluate vaccine-induced immune responses following immunization, BALB/c mice received the treatments listed at the weeks indicated for the monovalent and trivalent studies. Separate groups of animals were used in the analysis of humoral immunogenicity/challenge and cellular immunogenicity.
Figure 3. Monovalent and trivalent vaccination with BoNT/Hc/A, BoNT/Hc/B, and BoNT/Hc/E induces strong humoral immunity in mice. For the monovalent study, BALB/c mice received two 10 μg DNA immunizations spaced 3 weeks apart and sera were collected 3 weeks post-final immunization. Anti-botulinum neurotoxin titers against serotypes A (A), B (B), and E (C) for 5 animals per group were measured by ELISA. For the trivalent study, BALB/c mice (n = 5/group) received two 30 μg DNA immunizations (10 μg of each BoNT Hc construct) spaced 3 weeks apart and sera were collected 3 weeks post-final immunization. Anti-botulinum neurotoxin titers against serotypes A (D), B (E), and E (F) were measured by ELISA. Geometric mean and 95% confidence intervals are shown.
Monovalent DNA vaccination provides complete protection against lethal challenge with 102 LD50 of pure C. botulinum neurotoxin
After determining that monovalent vaccination induced high, toxin-specific antibody titers, we evaluated whether vaccinated animals directly administered 102 LD50 of pure homologous neurotoxin would be protected from intoxication. To accomplish this evaluation, animals (n =15/group) that received monovalent DNA and their respective naïve group, were i.p. injected with 200 μl of 102 LD50 of their assigned homologous neurotoxin. Animals were monitored for survival for 7 d. All vaccinated animals survived lethal neurotoxin challenge and all naïve animals succumbed to intoxication within 6 hours post-challenge ().
Table 1. Protective immune responses to monovalent vaccination with BoNT Hc vaccines
Monovalent antisera completely protects naïve mice from lethal BoNT challenge
After determining that vaccinated animals were completely protected from lethal toxin challenge, we wanted to assess whether the monovalent antisera contained antibodies potent enough to prevent intoxication of naïve mice. For this study, BALB/c mice sera from naïve and monovalent vaccinated animal groups were pooled separately and mixed 1:1 with 102 LD50 of homologous toxin representing the vaccinated antigen. Naïve BALB/c mice (n = 5/group; 6 groups total) received i.p. injections of 200 μl of the assigned sera/toxin mixture. As an experimental control, an additional 3 groups of mice (n = 5/group), received only the toxin diluent (Gel-NaH2PO4) as a negative control (data not shown). All animals were monitored for survival for 7 d post-challenge. Animals that received immune sera and homologous toxin mixtures were 100% protected against botulinum poisoning. However, animals that received naïve sera and toxins succumbed to botulinum poisoning within 6 hours (). Toxin diluent was not harmful to animals. The data demonstrate that the antibodies induced by monovalent vaccination are capable of neutralizing 100X the lethal dose of botulinum neurotoxin thereby rendering the lethal toxin no more harmful to animals than the background levels induced by a simple non-toxic diluent (Gel-NaH2PO4).
Table 2. In vivo evaluation of protection against C. botulinum neurotoxin serotypes A, B, and E with antisera from mice vaccinated with the Hc monovalent vaccines
Trivalent DNA vaccination induces strong antibody responses
Since an ideal vaccine candidate for botulinum neurotoxin would likely need to provide protection against multiple antigen serotypes, we wanted to evaluate the immunogenicity induced by trivalent vaccination. For this study, BALB/c mice (n = 5/group) received two 30 μg intramuscular DNA vaccinations (10 μg of each BoNT Hc construct) followed by in vivo electroporation. There were 3 groups of mice that received this trivalent BoNT/Hc/ABE formulation. Sera were collected 3 weeks after the final vaccination and ELISA was used to determine the binding antibody titers for each antigen. Vaccination with the trivalent vaccine formulation induced high anti-toxin-specific antibody titers (). As shown in , group geometric mean anti-toxin-specific endpoint titers (with 95% CI) were 2.0 × 105 (4.2 × 104, 9.5 × 105), 8.0 × 104 (7.2 x103, 8.7 × 105), and 8.0 × 104 (2.2 × 104, 2.8 × 105) against botulinum neurotoxin types A, B, and E, respectively. These data show that our trivalent DNA vaccine cocktail is highly immunogenic.
Trivalent DNA vaccination provides complete protection against lethal challenge with pure C. botulinum homologous neurotoxin
After determining that trivalent DNA vaccination induced high-titer toxin-specific antibodies, we next wanted to determine whether the humoral response induced was capable of protecting animals from intoxication. To evaluate protection, animals from the trivalent study described in the previous section, received i.p. injections of 200 μl of 102 LD50 of either C. botulinum neurotoxin type A, B, or E along with their respective naïve group. Animals were monitored for survival for 7 d. Similar to the monovalent study, all vaccinated animals survived lethal neurotoxin challenge whereas all naïve animals succumbed to intoxication within 6 hours post-challenge ().
Table 3. Protective immune responses to trivalent vaccination with BoNT Hc vaccines
Monovalent and trivalent vaccination induces robust and specific humoral immunity against botulinum A, B, and E antigens
As a final evaluation of humoral immunogenicity, we wanted to compare the immune responses generated against each toxin antigen when animals were administered the monovalent vaccine versus the trivalent cocktail. This comparison of anti-botulinum neurotoxin endpoint titers is shown in . Here we report that although the average anti-toxin endpoint titers decreased when animals were administered the trivalent cocktail, these differences were not significant for neurotoxin serotype A (P = 0.49), serotype B (P = 0.38), or serotype E (P = 0.09) when compared to titers from animals administered the monovalent formulation.
Figure 4. Comparison of anti-botulinum neurotoxin antibody endpoint titers induced by monovalent and trivalent vaccination. Endpoint titers obtained after 2 DNA immunizations with the monovalent and trivalent vaccines are compared. The geometric mean and 95% confidence intervals are shown. Significance was measured at P< 0.05.
Trivalent vaccination with BoNT/Hc/ABE induces antigen-specific polyfunctional CD4+ T cell immunity
Given the importance of CD4+ T helper 1 (Th1) cells in the activation of B cells to produce antigen-specific antibodies, we wanted to evaluate the cytokine frequencies and phenotypic profiles of specific CD4+ T cells following trivalent DNA immunization. To accomplish this, we vaccinated 3 separate groups of BALB/c mice (n = 5/group) with the trivalent BoNT/Hc/ABE formulation following the previously described regimen (). Three weeks after the final vaccination, we measured the ability of vaccine-induced Ag-specific T cell populations to secrete IFN-γ, TNF-α, and IL-2 in response to ex vivo Hcs BoNT/A, BoNT/B, or BoNT/E peptide stimulation in the harvested splenocytes. Our gating strategy for intracellular cytokine flow cytometry analysis is depicted in . Trivalent vaccination induced toxin-specific CD4+ T cells producing total IFN-γ (BoNT/Hc/A: 0.37%; BoNT/Hc/B: 0.38%; BoNT/Hc/E: 0.20%), total TNF-α (BoNT/Hc/A: 0.51%; BoNT/Hc/B: 0.49%; BoNT/Hc/E: 0.28%), total IL-2 (BoNT/Hc/A: 0.37%; BoNT/Hc/B: 0.32%; BoNT/Hc/E: 0.17%), and dual IFN-γ/TNF-α (BoNT/Hc/A: 0.36%; BoNT/Hc/B: 0.36%; BoNT/Hc/E: 0.18%) (). Overall, we observed that antigen stimulation with Hcs BoNT/A, BoNT/B, or BoNT/E resulted in the production of multifunctional CD4+ T cell cytokines.
Figure 5. Cytokine frequencies and phenotypic profiles of specific CD4+ T cells following DNA immunization. Cytokine recall responses to BoNT/Hc/ABE were measured 3 weeks after the last immunization by ICS and flow cytometry. (A) The gating strategy used to analyze the frequency of CD4+ T cells positive for IFN-γ, TNF-α, and IL-2 cytokines. (B) Multiparameter flow cytometry was used to determine the percentages of multifunctional CD4+ T cell cytokine profiles of BoNT/Hc/ABE. The bar chart shows the percentage of specific CD3+CD4+ T cells displayed as IFN-γ, TNF-α, and IL-2 triple, double, or single positive CD4+ T cells. Pie charts show the relative proportion of each cytokine subpopulation to BoNT/Hc/A, B, and E stimulation. Background staining from cells stimulated with medium alone was subtracted. Data represent the mean ± SEM of 5 mice per group with +P< 0.01 using Student's t-test compared to the naïve group.
Discussion
In 2011, the CDC discontinued its use of the investigational pentavalent (ABCDE) botulinum toxoid (PBT) vaccine. As a result, there is no licensed vaccine to prevent intoxication from the botulinum serotypes most responsible for human disease, serotypes A, B, and E.Citation24 However, the threat of the use of weaponized C. botulinum neurotoxin remains a concern. Although post-exposure delivery of antitoxins is effective for the treatment of rare cases of life-threatening botulism, such therapies would be inadequate to prevent intoxication of large populations threatened by the dissemination of botulinum toxins during a bioterrorism event. Therefore, there is a need to proactively develop new therapies and vaccination strategies that can prevent intoxication. Although multiple botulinum toxin vaccines are currently in development,Citation25-28 the safety, immunogenicity, and stability of DNA make this vaccine platform an additional option to specifically target botulinum antigens and allow for stable delivery anywhere a bioterrorist attack may occur. In addition, the use of DNA vaccines targeting the HC of botulinum neurotoxin serotypes A, B, and E has shown positive neutralization results.Citation23 The present study was initiated to expand upon this initial studyCitation23 of a BoNT DNA vaccine and further evaluate the DNA vaccine-induced humoral and cellular responses generated by monovalent and trivalent vaccination with BoNT HC fragments of C. botulinum serotypes A, B, and E. By evaluating the CD4+ T cell response, we wanted to clarify the mechanisms of B cell activation required to produce antigen-specific neutralizing antibodies and generate memory B cell responses. In addition, by targeting the BoNT HC of the serotypes most responsible for human disease, we wanted to determine if the DNA vaccine modality would be effective as a prophylactic botulinum toxin vaccine.
We generated 3 DNA vaccine constructs targeting the botulinum neurotoxin heavy chain C-terminal fragment of serotypes A, B, and E and named the constructs pBoNT/Hc/A, pBoNT/Hc/B, and pBoNT/Hc/E, respectively. Vaccine groups were named based upon the single construct administered. Using a previously determined optimal dosage (data not shown), we vaccinated groups of animals with each construct. All immunized animals produced high-titer antigen-specific antibodies and were completely protected against a lethal dose of toxin. The data show that DNA antisera neutralized the lethal toxin dose.
Since the current treatments for botulinum poisoning involve the delivery of antibodies to neutralize circulating toxins, we wanted to evaluate if our DNA antisera could neutralize the lethal dose of toxin and provide naïve animals protection from intoxication. By setting up an in vivo mouse protection assay in which naïve or immune sera were mixed in a 1:1 ratio with antigen specific neurotoxins and then injected into a naïve host, we were able to determine that sera collected from immunized mice were capable of completely neutralizing a lethal dose of toxin. Our results build on prior work in this area with EP delivered DNACitation23 with some differences. We used a lower vaccine dose in the present studies, and we also report for the first time the T cell responses important in the generation of immunity to these vaccines. Furthermore the challenge doses for toxin were higher in this present study than in the prior report. In the present study however, due to limited amounts of sera collected from vaccinated animals for this monovalent study, serum neutralization titers were not determined. Since we have determined that immunization with BoNT Hcs A, B, and E results in the production of antisera that can effectively neutralize 100 LD50 BoNT holotoxin, future studies will involve the measurement of serum neutralizing antibody titers and the incremental increase of toxin dose to determine the highest efficacy achieveable with this DNA vaccine approach.
From our monovalent study, we determined that the heavy chain C-terminal fragment of botulinum serotypes A, B, and E are immunogenic and vaccination with DNA constructs expressing each serotype results in high-titer, toxin-neutralizing antibodies. Since an ideal vaccine candidate for botulinum neurotoxin would likely need to provide protection against multiple antigens, we wanted to evaluate the effectiveness of a trivalent formulation of our vaccines. For the trivalent study, 6 groups of mice were vaccinated with a cocktail containing 10 μg of each construct and the resulting cocktail was named, BoNT/Hc/ABE. After two immunizations with BoNT/Hc/ABE, 3 groups of mice were evaluated for humoral immunogenicity and challenge and 3 groups of mice were evaluated for T cell responses.
Similar to the monovalent study, animals vaccinated with the trivalent vaccine produced high-titer antigen-specific antibodies and were 100% protected against the lethal challenge dose of serotype-specific holotoxin. Given the protective efficacy we observed for our monovalent and trivalent DNA vaccines, we wanted to compare the antitoxin specific endpoint titers for each antigen. Similar to other reports of the BoNT HC,Citation23,29,30 we report that antitoxin titers for each antigen decreased when antigens were delivered in the trivalent formulation although this difference was not significant. The decrease in antigen endpoint titers could indicate the presence of antigen competition in the trivalent vaccine preparation. However, although there was a decrease in antitoxin titer for each antigen in the trivalent vaccine, this decrease was not significant enough to affect the overall level of protective efficacy of the vaccine. We concluded that our monovalent and trivalent vaccine formulation could be used as an efficient vaccine modality to induce botulinum toxin-specific neutralizing antibodies and provide 100% protection from lethal challenge. In the current study, this multivalent formulation did not target botulinum serotype F. A recent report evaluating Semliki Forest virus replicon vectors targeting botulinum serotypes A, B, E, and F along with tetanus toxin has shown complete protection against 100 LD50 of toxins.Citation31 For the present studies, we did not include serotype F as the highest incidences of human botulism are associated with the more potent serotypes (A, B, and E)Citation8 and these serotypes would likely be the focus for weaponization in the event of bioterrorism.
Although it is currently accepted that the correlate of protective immunity against botulinum neurotoxin is primarily antibody mediated, we also investigated the percentages of CD4+ T cells induced by our trivalent vaccine. CD4+ T cells may contribute to the activation of B cells to produce antigen specific antibodies and more importantly is required for a long lasting memory B cell response. For this study, we evaluated the ability of Ag-specific T cell populations to secrete IFN-γ, TNF-α, and IL-2. We determined that each antigen in our trivalent cocktail induced all 3 cytokines. To our knowledge, this is the first report describing the polyfunctionality of CD4+ T cells induced by a trivalent DNA vaccine targeting the heavy chain C-terminal fragment of botulinum neurotoxin antigens A, B, and E. The high percentages of CD4+ T cells secreting IFN-γ, TNF-α, and IL-2 may explain how our DNA vaccines were able to induce high titers of antigen-specific antibodies. The high frequencies of Th1 cells secreting antitoxin cytokines provide further evidence that our DNA vaccines are capable of producing broad antigen-specific toxin immunity. By characterizing the response of cytokines associated with the activation and maintenance of humoral immunity, we can further define how the vaccines induce and mediate protection against botulinum intoxication.
The need for new, prophylactic vaccines is often not realized until there is a present threat of disease outbreak. The lack of available vaccines has recently contributed to the Ebola, Severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome coronavirus (MERS-CoV) epidemics. These disease cases highlight the need to develop new, mass vaccination strategies to prevent disease threats.
In the present study, we proactively developed DNA vaccines that are potential candidate vaccines to provide protection from intoxication with weaponized C. botulinum in the event of a bioterrorist threat. Our study demonstrates that the synthetic DNA vaccine platform has an advantage as a vaccine strategy for generating robust and protective immunity in the context of botulinum poisoning. We show that vaccination with DNA targeting the heavy chain C-terminal fragment of botulinum serotypes A, B, and E results in vaccinee antisera that can effectively neutralize toxin. In addition to inducing humoral immunity and supportive cellular responses, the vaccine elicited protection after just 2 immunizations, indicating that the DNA formulation induces optimal protective responses with minimal boosting. Further study of these DNA vaccines is needed to determine the kinetics of the protective immune response post-vaccination.
Materials and Methods
Construction and expression of BoNT/Hc/A, BoNT/Hc/B, and BoNT/Hc/E monovalent DNA vaccines
Clostridium botulinum neurotoxin heavy chain C-terminal (Hc) fragment sequences for serotypes A (YP_001386738.1), B (YP_001693307), and E (YP_001920504) were obtained from GenBank and synthetically designed and codon and RNA optimized for expression in mammals. The Hc sequences were synthesized into separate pUC57 vectors with an IgE leader sequence to increase secretion and a poly A tail to end translation. Each insert was cloned into a pVAX expression promotor between the BamHI and Not1 sites (). The DNA constructs were cloned by GenScript (Piscataway, NJ) and amplified by Aldevron (Fargo, ND). The resulting purified DNA plasmids were formulated with sterile water and used in animal vaccinations.
To confirm expression of each of the monovalent BoNT DNA vaccines, Rhabdomyosarcoma (RD) cells (2 × 105 cells) (ATCC, CCL-136) were seeded in 2-chamber tissue culture treated glass slides (BD Falcon) and transfected with hemagglutinin (YPYDVPDYA)-tagged BoNT/Hc/A, BoNT/Hc/B, or BoNT/Hc/E using Turbofectin (Origene, TF81001). RD cells were used as an experimental control and were transfected with pVAX. Transfected cells were maintained in culture in Dulbecco's Modified Eagle's Medium (DMEM; Gibco-Invitrogen, 11965–084) supplemented with 10% fetal calf serum (FBS) (Atlas Biologicals, Inc.., F0500-A) and 1% penicillin-streptomycin solution (10,000 U/ml) (Invitrogen, Inc.., 15140–122). Forty-eight hours post-transfection, cells were fixed with 2% paraformaldehyde, washed in 1X phosphate buffered saline (PBS) (Gibco-Invitrogen, 14190–136) and incubated with goat anti-mouse HA tag antibody (Abcam, ab18181) 1:100 dilution in primary standard solution (PSS) (0.1% BSA, 0.2% saponin, and 0.02% sodium azide in PBS) (37°C, 1.5 h). Cells were washed in PBS and incubated with goat polyclonal secondary antibody to mouse IgG conjugated to fluorescein isothiocyanate (FITC) (Abcam, ab6785) 1:100 dilution in PSS for 1 h at room temperature. After washing, cell nuclei were counterstained with Hoechst reagent (Sigma-Aldrich, H6024) and slides were mounted with fluoromount G (Electron Microscopy Sciences, 17984–25). Expression of the BoNT constructs was confirmed by confocal imaging. Confocal images were acquired using the Zeiss LSM 510 NLO/META Confocal Microscope at the Cell and Developmental Biology Microscopy Core, University of Pennsylvania, PA, USA.
Neurotoxins for challenge
Purified Clostridium botulinum holotoxin for serotypes A (Lot #061013–01), B (Lot #061013–01), and E (Lot #061013–01) were purchased from Metabiologics, Inc.. (Madison, WI). Standard toxin preparations for BoNT/A, BoNT/B, and BoNT/E were produced from C. botulinum "Hall," "Okra," and "Alaska" strains, respectively. The toxicity of each toxin was tested by mouse bioassays and the identity of each toxin was confirmed by antitoxin neutralization (Metabiologics, Inc.). Toxin purity was confirmed by SDS-PAGE (Metabiologics, Inc.). Holotoxin type E was activated by treatment with 10% (wt/vol) trypsin (37°C, 1 h) prior to use. Specific toxin activities for type A, B, and E holotoxins were 2.5 × 108 MLD50/mg, 8 × 107 MLD50/mg, and 6.0 × 107 MLD50/mg (QC sample activated with trypsin), respectively, as reported by Metabiologics. Toxin samples were diluted in phosphate-gelatin buffer [30 mM sodium phosphate, (pH 6.2), 2% gelatin] and immediately used in animal challenge studies.
Animals and vaccinations
Female BALB/c mice (6 to 8 weeks of age) were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed in a temperature-controlled, light-cycled facility and received food and water ad libitum. All experiments were performed in accordance with the guidelines of the National Institutes of Health (NIH) (Bethesda, MD) and the University of Pennsylvania (Philadelphia, PA) Institutional Animal Care and Use Committee (IACUC #804951).
For the monovalent study, mice were divided into 6 groups (n = 15 / group). Each vaccinated group was assigned an age-matched naïve group. The vaccinated groups received 2 immunizations spaced 3 weeks apart with 10 μg of either BoNT/Hc/A, BoNT/Hc/B, or BoNT/Hc/E via intramuscular injection into the tibialis anterior muscle. Intramuscular injection was immediately followed by electroporation (EP). Briefly, mice received 2 constant-current pulses of 0.2 A delivered through a triangular 3-electrode array consisting of 26-gauge solid stainless steel electrodes. Pulses were 52 ms in length separated by a one second delay. All in vivo electroporation procedures were performed using the CELLECTRA® 3P electroporation device (Inovio Pharmaceuticals, Inc.., Plymouth Meeting, PA). Sera samples were collected 3 weeks after the last immunization and 5 samples from each group were used to evaluate humoral immune responses.
For the trivalent study, mice were divided into 6 groups (n = 5 / group). Each vaccinated group was assigned an age-matched naïve group. The vaccinated groups received a cocktail vaccine (BoNT/Hc/ABE) containing 10 μg of BoNT/Hc/A, 10 μg of BoNT/Hc/B, and 10 μg BoNT/Hc/E. Mice received 2 immunizations spaced 3 weeks apart via intramuscular injection into the tibialis anterior muscle. Intramuscular injection was immediately followed by EP using the CELLECTRA® 3P device. Sera samples were collected 3 weeks after the last immunization and used to evaluate humoral immune responses.
Antitoxin serum antibody endpoint titer measurements
To determine antitoxin-specific sera antibody titers, C. botulinum type A, B and E neurotoxin coated plates were obtained from Metabiologics, Inc.. (Madison, WI). The coated plates were absorbed with the respective purified botulinum neurotoxin and the non-specific binding sites were pre-blocked according to established methods.Citation32 Plates were incubated with serial dilutions of immunized and naïve sera (37°C, 1.5 h). Plates were washed with PBS-T (0.05% Tween 20 in PBS) and incubated with goat anti-mouse IgG-HRP (Santa Cruz Biotechnology, Inc.., sc-2055) at a 1:5000 dilution in 1% FBS in PBS-T (0.05% Tween 20 in PBS). After washing, the enzyme substrate SigmaFAST O-phenylenediamine dihydrochloride (OPD) (Sigma-Aldrich, P1987) was added. The development was stopped with the addition of 100 μl 1N H2SO4 and optical density was determined at 450 nm. Endpoint titers were determined as previously described.Citation33 Endpoint titers are reported as the reciprocal of the last dilution that had an absorbance above the upper prediction limit.
Determination of vaccine efficacy
Four weeks after the last vaccination, mice were challenged with 102 LD50 (100 LD50) of the respective activated homologous neurotoxin. Mice received the challenge dose of toxin in a final volume of 200 μl via intraperitoneal (i.p.) injection. All mice were challenged along with their assigned naïve group and survival was monitored for 7 d. Surviving animals were humanely euthanized after one week.
Neurotoxin neutralization assay
To determine if antibodies induced by vaccination with the monovalent vaccines, BoNT/Hc/A, B, or E, were capable of neutralizing a lethal dosage of toxin, pooled-immunized antisera from 2X vaccinated animals and naïve animals were mixed 1:1 with 100 LD50 of the respective homologous neurotoxin. Sera/toxin mixtures were incubated at 37°C, 1 hr. Naïve animals were then administered 200 μl of the sera/toxin mixtures via (i.p.) injection. Survival was monitored for 7 d and surviving animals were humanely euthanized after one week.
Splenocyte harvest
To evaluate the cellular immune response to each antigen, separate groups of mice (3 groups; n = 5/group) administered the trivalent vaccine, BoNT/Hc/ABE, were humanely euthanized 3 weeks after the final immunization and spleens were harvested from each animal. Spleens were placed in RPMI 1640 medium (Mediatech, MT10–040-CM) supplemented with 10% fetal calf serum (FCS), 1% penicillin-streptomycin (10,000 U/ml) (Invitrogen), and 1X β-mercaptoethanol (Invitrogen, 21985–023). Spleens were disrupted using a Stomacher machine (Steward Laboratory Systems, Bohemia, NY) and the cellular product was strained using a 40 μg cell strainer (BD Biosciences). Red blood cells were lysed with ACK lysis buffer (Lonza, 10–548E). The remaining cells were washed with 1X PBS, resuspended in supplemented RPMI medium, restrained using a cell strainer, and used in intracellular cytokine staining (ICS) assays.
Intracellular cytokine staining
Lymphocytes were isolated and processed from the spleen and intracellular staining was performed as previously described.Citation34 The following antibodies were used for surface staining: LIVE/DEAD Fixable Violet Dead Cell stain kit (Invitrogen), CD19 (V450; clone 1D3; BD Biosciences), CD4 (FITC; clone RM4–5; BD Biosciences), and CD8 (APC-Cy7; clone 53–6.7; Abcam). For intracellular staining the following antibodies were used: IFN-γ (APC; clone XMG1.2; Biolegend), TNF-α (PE; clone MP6-XT22; ebioscience), IL-2 (PeCy7; clone JES6-SH4; ebioscience), and CD3 (PerCP/Cy5.5; clone 145–2C11; Biolegend). All data were collected using a LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star, Ashland, OR) and SPICE v5.2 (free available from http://exon.niaid.nih.gov/spice/). Boolean gating was performed using FlowJo software to examine the polyfunctionality of the T cells from vaccinated animals. For flow cytometry, cells were gated on singlets using SSC-H by SSC-A followed by gating on LIVE-DEAD (dump channel), CD3+ CD4+ CD8− T and CD3+ CD8+ CD4− T cells to examine the CD4+ and CD8+ T-cell populations secreting IFN-γ, TNF-α, and IL-2 cytokines. Cells 2 × 106/wells cells were stimulated with their respective botulism pooled peptide for 5 hours and 500,000 events were collected by the LSRII.
Statistical analysis
Data were analyzed using a Student's t-test. All statistics were performed with GraphPad Prism® v. 5.0b program (GraphPad Software, Inc..). Significance was measured at P ≤ 0.05.
Disclosure of Potential Conflicts of Interest
D.B.W. has grant funding, participates in industry collaborations, receives speaking honoraria, and collects fees for consulting. He serves on scientific review committees and advisory boards. Remuneration includes direct payments, stock, or stock options. In the interest of disclosure, he therefore notes potential conflicts associated with this work with Pfizer, Bristol Myers Squibb, Inovio, Touchlight, oncosec, Merck, VGXI, and possibly others. Licensing of technology from his laboratory has created over 100 jobs in the private sector in the biotech/pharma industry. The other authors declare no competing financial interests. No writing assistance was utilized in the production of this manuscript.
Supplemental_Figure_1.doc
Download MS Word (25 KB)Acknowledgments
The authors thank Metabiologics, Inc. for technical support and the University of Pennsylvania Cell and Developmental Biology (CDB) Microcopy Core staff for their generous technical assistance with microcopy imaging.
Funding
This work was supported by a Sponsored Research Agreement with Inovio Pharmaceuticals entitled, "Protein-Cellular Interactions" (University of Pennsylvania award #10007526) and by a UNCF/Merck Postdoctoral Fellowship Award to V.L. Scott.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- (CDC) CfDCaP. Botulism. National Center for Emerging and Zoonotic Infectious Diseases Atlanta, GA: CDC.gov, 2014
- Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, et al. Botulinum toxin as a biological weapon: medical and public health management. JAMA 2001; 285:1059-70; PMID:11209178; http://dx.doi.org/10.1001/jama.285.8.1059
- Hanson D. Botulinum toxin: a bioterrorism weapon. Emerg Med Serv. 2004 Apr; 33(4):55-9
- Smith LA. Botulism and vaccines for its prevention. Vaccine 2009; 27 Suppl 4:D33-9; PMID:19837283; http://dx.doi.org/10.1016/j.vaccine.2009.08.059
- Barash JR, Arnon SS. A novel strain of clostridium botulinum that produces type B and type H botulinum toxins. J Infect Dis 2013; 209:183-91; PMID:24106296; http://dx.doi.org/10.1093/infdis/jit449
- Sugiyama H. Clostridium botulinum neurotoxin. Microbiol Rev 1980; 44:419-48; PMID:6252433
- Prevention CfDCa. Handbook for epidemiologists, clinicians, and laboratory workers Atlanta, GA: US. Department of Health and Human Services, Public Health Service, 1998; Available at: http://www.cdc.gov/ncidod/dbmd/diseaseinfo/files/botulism.pdf
- Rainey GJ, Young JA. Antitoxins: novel strategies to target agents of bioterrorism. Nat Rev Microbiol 2004; 2:721-6; PMID:15372082; http://dx.doi.org/10.1038/nrmicro977
- Brunger AT, Breidenbach MA, Jin R, Fischer A, Santos JS, Montal M. Botulinum neurotoxin heavy chain belt as an intramolecular chaperone for the light chain. PLoS Pathog 2007; 3:1191-4; PMID:17907800; http://dx.doi.org/10.1371/journal.ppat.0030113
- Koriazova LK, Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat Struct Biol 2003; 10:13-8; PMID:12459720; http://dx.doi.org/10.1038/nsb879
- Nowakowski A, Wang C, Powers DB, Amersdorfer P, Smith TJ, Montgomery VA, Sheridan R, Blake R, Smith LA, Marks JD. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc Natl Acad Sci U S A 2002; 99:11346-50; PMID:12177434; http://dx.doi.org/10.1073/pnas.172229899
- Shukla HD, Sharma SK. Clostridium botulinum: a bug with beauty and weapon. Crit Rev Microbiol 2005; 31:11-8; PMID:15839401; http://dx.doi.org/10.1080/10408410590912952
- FDA. FDA approves first botulism antitoxin for use in neutralizing all seven known botulinum nerve serotypes silver spring, MD: US. Food and Drug Administration, 2013
- Siegel LS. Human immune response to botulinum pentavalent (ABCDE) toxoid determined by a neutralization test and by an enzyme-linked immunosorbent assay. J Clin Microbiol 1988; 26:2351-6; PMID:3235662
- Dhama K, Mahendran M, Gupta PK, Rai A. DNA vaccines and their applications in veterinary practice: current perspectives. Vet Res Commun 2008; 32:341-56; PMID:18425596; http://dx.doi.org/10.1007/s11259-008-9040-3
- Mahon BP, Moore A, Johnson PA, Mills KH. Approaches to new vaccines. Crit Rev Biotechnol 1998; 18:257-82; PMID:9887505; http://dx.doi.org/10.1080/0738-859891224167
- Morrow MP, Weiner DB. DNA drugs come of age. Sci Am 303:48-53; PMID:20583666; http://dx.doi.org/10.1038/scientificamerican0710-48
- Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, Giffear M, Pankhong P, Khan AS, Broderick KE, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med, 2012; 4:155ra138.
- Amersdorfer P, Wong C, Chen S, Smith T, Deshpande S, Sheridan R, Finnern R, Marks JD. Molecular characterization of murine humoral immune response to botulinum neurotoxin type A binding domain as assessed by using phage antibody libraries. Infect Immun 1997; 65:3743-52; PMID:9284147
- Clayton MA, Clayton JM, Brown DR, Middlebrook JL. Protective vaccination with a recombinant fragment of Clostridium botulinum neurotoxin serotype A expressed from a synthetic gene in Escherichia coli. Infect Immun 1995; 63:2738-42; PMID:7790092
- Pless DD, Torres ER, Reinke EK, Bavari S. High-affinity, protective antibodies to the binding domain of botulinum neurotoxin type A. Infect Immun 2001; 69:570-4; PMID:11119555; http://dx.doi.org/10.1128/IAI.69.1.570-574.2001
- Tavallaie M, Chenal A, Gillet D, Pereira Y, Manich M, Gibert M, Raffestin S, Popoff MR, Marvaud JC. Interaction between the two subdomains of the C-terminal part of the botulinum neurotoxin A is essential for the generation of protective antibodies. FEBS Lett 2004; 572:299-306; PMID:15304366; http://dx.doi.org/10.1016/j.febslet.2004.06.094
- Trollet C, Pereira Y, Burgain A, Litzler E, Mezrahi M, Seguin J, Manich M, Popoff MR, Scherman D, Bigey P. Generation of high-titer neutralizing antibodies against botulinum toxins A, B, and E by DNA electrotransfer. Infect Immun 2009; 77:2221-9; PMID:19237523; http://dx.doi.org/10.1128/IAI.01269-08
- CDC. Notice of CDC's discontinuation of investigational pentavalent (ABCDE) botulinum toxoid vaccine for workers at risk for occupational exposure to botulinum toxins. MMWR Morb Mortal Wkly Rep 2011; 60:1454-5.
- Miethe S, Rasetti-Escargueil C, Liu Y, Chahboun S, Pelat T, Avril A, Frenzel A, Schirrmann T, Thullier P, Sesardic D, et al. Development of neutralizing scFv-Fc against botulinum neurotoxin A light chain from a macaque immune library. MAbs 2014; 6:446-59; PMID:24492304; http://dx.doi.org/10.4161/mabs.27773
- Shearer JD, Manetz TS, House RV. Preclinical safety assessment of recombinant botulinum vaccine A/B (rBV A/B). Vaccine 2012; 30:1917-26; PMID:22269871; http://dx.doi.org/10.1016/j.vaccine.2012.01.035
- Pier CL, Tepp WH, Bradshaw M, Johnson EA, Barbieri JT, Baldwin MR. Recombinant holotoxoid vaccine against botulism. Infect Immun 2008; 76:437-42; PMID:17967862; http://dx.doi.org/10.1128/IAI.00843-07
- Kobayashi R, Kohda T, Kataoka K, Ihara H, Kozaki S, Pascual DW, Staats HF, Kiyono H, McGhee JR, Fujihashi K. A novel neurotoxoid vaccine prevents mucosal botulism. J Immunol 2005; 174:2190-5; PMID:15699151; http://dx.doi.org/10.4049/jimmunol.174.4.2190
- Ravichandran E, Al-Saleem FH, Ancharski DM, Elias MD, Singh AK, Shamim M, Gong Y, Simpson LL. Trivalent vaccine against botulinum toxin serotypes A, B, and E that can be administered by the mucosal route. Infect Immun 2007; 75:3043-54; PMID:17371853; http://dx.doi.org/10.1128/IAI.01893-06
- Zichel R, Mimran A, Keren A, Barnea A, Steinberger-Levy I, Marcus D, Turgeman A, Reuveny S. Efficacy of a potential trivalent vaccine based on Hc fragments of botulinum toxins A, B, and E produced in a cell-free expression system. Clin Vaccine Immunol 2010; 17:784-92; PMID:20357058; http://dx.doi.org/10.1128/CVI.00496-09
- Yu YZ, Liu S, Ma Y, Gong ZW, Wang S, Sun ZW. Pentavalent replicon vaccines against botulinum neurotoxins and tetanus toxin using DNA-based Semliki Forest virus replicon vectors. Hum Vaccin Immunother 2014; 10:1874-9; PMID:25424795; http://dx.doi.org/10.4161/hv.28937
- Ferreira JL, Maslanka S, Johnson E, Goodnough M. Detection of botulinal neurotoxins A, B, E, and F by amplified enzyme-linked immunosorbent assay: collaborative study. J AOAC Int 2003; 86:314-31; PMID:12723917
- Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods 1998; 221:35-41; PMID:9894896; http://dx.doi.org/10.1016/S0022-1759(98)00170-7
- Villarreal D, Wise MC, Walters JN, Reuschel E, Choi MJ, Obeng-Adjei N, Yan J, Morrow MP, Weiner DB. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res 2014; 74(6):1789-800; PMID:24448242
