Abstract
Vaccinations against hepatitis A virus (HAV) and hepatitis B virus (HBV) are recommended for patients with chronic liver disease (CLD), yet implementation of these recommendations is lacking. This study reviewed HAV and HBV antibody testing and vaccination status of patients with CLD. In 2008, we began using pre-printed liver order sets, which included vaccination options. We compared Scott & White liver clinic CLD patient records from 2005 (238) with patient records from 2008 (792). Screening rates for immunity and vaccination rates of those lacking immunity were calculated. In 2005, 66% of CLD patients were screened for HAV immunity. In 2008, 56% of CLD patients were screened. The HAV vaccination completion rate was 37% in 2005, while in 2008, the rate was 46%. In 2005, 66% of CLD patients were screened for HBV immunity; in 2008, 56 % CLD patients were screened. The HBV vaccination completion rate was 26% in 2005 compared with 36% in 2008. Although there was a lower percentage of screening in 2008, the overall number of patients tripled between 2005 and 2008. There was a significant increase in the total number of patients screened and vaccinated in 2008. Some physicians may have vaccinated their patients without checking for immunity. In January 2008, we implemented pre-printed order sets with checkboxes to help remind providers to order labs to screen for immunity against HAV and HBV and to order vaccinations for those who lacked immunity. The use of these sets may have aided in the increase of vaccination completion rates.
Abbreviations
| HAV | = | Hepatitis A virus |
| HBV | = | hepatitis B virus |
| CLD | = | chronic liver disease |
| NASH | = | nonalcoholic steatohepatitis |
| HCV | = | hepatitis C virus |
Introduction
Chronic liver disease (CLD)1 is a continuously growing problem in the United States. Annually, over 30,000 deaths and 500,000 hospitalizations are attributed to complications of CLD and cirrhosis.Citation2 Patients with CLD have higher morbidity and mortality from superimposed hepatitis than individuals without pre-existing cirrhosis.Citation3,4 CLD patients with additional liver insults, including alcohol and drugs, are at an even higher risk for decompensation from superimposed hepatitis. The Advisory Committee on Immunization Practices of Centers for Disease Control and Prevention has published recommendations concerning HAV and HBV vaccinations for disease prevention in cirrhotic patients.Citation5,6
Vaccinations should be administered in the early stages of CLD because the vaccine has been shown to have less immunogenicity as liver disease progresses. Healthy individuals, who receive the HAV vaccination series, benefit from a seroconversion rate of nearly 100%.Citation6 In comparison, patients with compensated CLD require a second vaccination to reach a similiar seroconversion rate.Citation7 Patients with decompensated CLD who receive only one HAV vaccination attain a 25–50% seroconversion rate.Citation8-10 Because of this low seroconversion rate, it is necessary to target patients with compensated CLD for HAV and HBV vaccinations to prevent superimposed hepatitis before the disease progresses to the advanced stages. It is also necessary to enact a proper screening method for such patients and to ensure compliance regarding their vaccination series. Although little is known about the rates and barriers of compliance with these recommendations, previous studies reveal that rates of compliance are highest for patients seen in specialist offices.Citation11,12
Patient education, physician pre-printed order sets for CLD patients, and streamlining follow-up vaccination visits may increase screening and compliance, which may significantly reduce the healthcare costs of treating superimposed hepatitis infections.
The objective of this study was to review HAV and HBV antibody testing and vaccination status of patients with CLD in the Scott and White liver clinic. In January 2008, we attempted to improve vaccination rates by using pre-printed order sets with reminder checkboxes to order serum antibody testing and HAV and HBV vaccinations for our CLD patients who had negative results. For each clinic visit, physician providers used this new order set to order labs and imaging for that day and for the next follow-up appointment. We compared cohorts from 2005, before the intervention, and 2008, after the intervention, to determine if we had made significant improvement in screening for HAV and HBV immunity and/or vaccinating if indicated. We hoped that the visual reminders on the pre-printed orders would significantly improve the results.
Results
Screening for Immunity
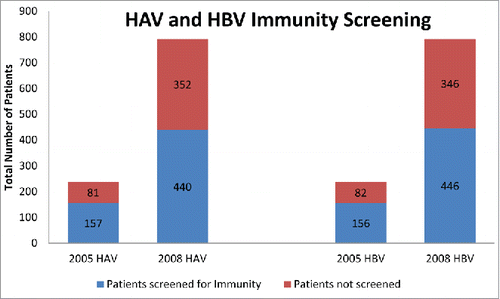
We compared Scott & White liver clinic CLD patient records from 2005 (238) with patient records from 2008 (792). In the 2005 cohort, 157 (66%) CLD patients were screened for HAV immunity; in 2008, 440 (56%) CLD patients were screened. In the 2005 cohort, 156 (66%) CLD patients were screened for HBV immunity. And, in and in 2008, 447 (56%) CLD patients were screened. Although the screening percentage was higher in 2005 compared with 2008, almost triple the numbers of patients were seen in the clinic in 2008 when compared with 2005. In addition, the patients seen in 2008 included many of the patients who were already captured from the 2005 data (). According to the 2008 data, having a diagnosis of hepatitis C virus (HCV) infection as well as patients with other liver diseases, including post-transplant status, increased the likelihood of being screened for immunity to both HAV and HBV (p < 0.0001) ()
Table 1. Hepatitis A virus antibody testing according to presence/absence of each Chronic Liver Disease – 2008 (A) Hepatitis A (N=792Footnote)
Table 2. Hepatitis B virus antibody testing according to presence/absence of each Chronic Liver Disease -2008 (A) Hepatitis B (N=792Footnote)
Completion of Hepatitis A Vaccine series
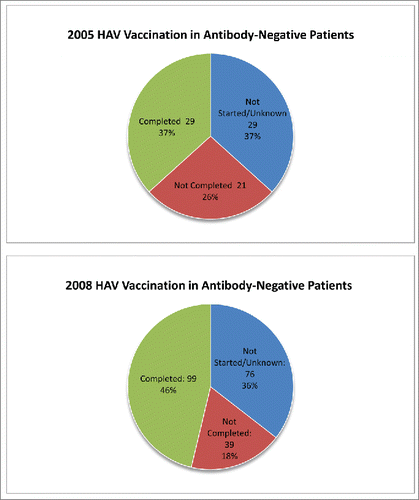
Of the patients screened for HAV immunity in 2005, 79 patients (50.3%) were deemed susceptible to HAV and 29 (37%) of these subjects completed their HAV vaccinations. Patients who were noted to have a primary care visit within the last 6 months of the hepatology visit were also more likely to complete the HAV vaccination series. In 2008, 214 (49%) of CLD patients screened for HAV antibody were negative for HBV immunity and of these patients, 99 (46%) completed their vaccination series ()
.Completion of Hepatitis B Vaccine series
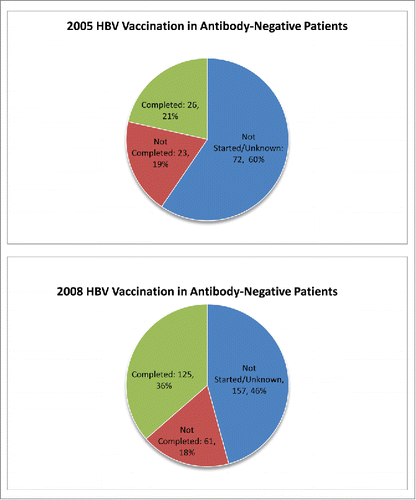
In 2005, 121 CLD patients (77.5%) were negative for HBV immunity and only 26 (21%) of these patients completed the HBV vaccination series. Another 19% received at least one injection of the HBV vaccination series. None of the variables analyzed reached statistical significance for completing the vaccination series; however, a statistical association was noted. Of the patients who completed the series, 84% had visited their primary care physician within 6 months. In 2008, 446 (56%) CLD patients received HBV immunity screening and of those, 343 (77%) were negative for the HBV antibody, indicating a need for the HBV vaccination series. Of the patients who had negative screening results, 125 (36%) completed the vaccination series ()
.Overall Observations
The percentage of patients screened for immunity to HAV and HBV unfortunately decreased after the intervention from 66% (2005) to 56% (2008). However, the raw number of patients screened increased from 157 to 440 for HAV and 156 to 447 for HBV and the raw number and percentage of those who completed their vaccinations increased. Among all CLD patients, those with HCV infection were the most likely to be screened for immunity to HAV and HBV. Of the 244 patients who had HCV, 64% were also tested for HAV and HBV (). There was no statistical difference in screening or vaccination completion rates in regards to age, gender, ethnicity, insurance status, marriage status, history of drug use, or whether the patient was cared for by a fellow or senior staff.
Discussion
Although the percentage of screening patients for immunity to HAV and HBV was higher in 2005, the volume of screened and vaccinated patients was much higher in 2008. For example, the number of patients who completed their vaccination against HBV in 2008 was higher than the number even found susceptible to HBV in 2005. One major change implemented in 2008 was the availability of pre-printed order sets for CLD patients. These order sets served as reminders to check for immunity and vaccinate if indicated. The decrease in percentage screening rate in 2008 may have occurred partly because not all the physicians were consistently using the order sets at that time. Also, some physicians may have vaccinated without screening and we did not account for this possibility. In contrast, the completion rates for vaccinations were higher in 2008. Perhaps the pre-printed order sets served as a reminder for physicians to vaccinate the patients once their antibodies were reported as negative.
Rates of vaccination of susceptible subjects with CLD improved from 2005 to 2008, but are less than ideal. Multiple barriers remained in place which could prevent vaccination completion. First, the completion of HAV and HBV vaccination series required multiple visits. The percentage of completed vaccinations was less in HBV immunization compared with HAV. This outcome is expected, due to the number of vaccinations needed (3 for HBV, and 2 for HAV), thus making compliance somewhat easier with the HAV vaccine. The incorporation of the combination vaccinations could help with this factor as well. Mail, email, phone call or automatic text messaging reminders sent to patients to receive their vaccinations could also help. Furthermore, at the time the study was conducted, patients had to report to 2 different clinics: one for vaccination ordering and another for vaccination receiving. Some clinic locations were still using paper charts to record vaccinations. Also, many of these patients traveled a great distance to see a hepatology specialist; if these patients opted to have their vaccinations performed by their local provider, this information would not have been captured. The lack of definitive electronic record keeping could have also lowered the percentages of patients screened and vaccinated, as some may have been performed and not recorded properly. Being able to order and administer vaccinations in a single hepatology clinic may improve vaccination administration and compliance.
Current guidelines call for HAV and HBV vaccinations in susceptible patients with CLD, but do not specify the etiology of CLD. Rates of anti-HAV seroprevalence is observed to be approximately 55% for patients with CLD,Citation4,13 and a pre-screening strategy for vaccination is known to be cost effective.14 This may account for the observation that patients with a diagnosis of HCV were more likely to be screened for immunity to HAV. It may reflect bias in interpretation of the current guidelines and a clarification of the diagnoses categorized to CLD may improve compliance among health care providers.
Our study had several limitations and we cannot definitively conclude that the pre-printed order sets alone led to improved vaccination completion rates. Surely, there were differences between the 2 cohorts that could not be accounted for in this study. One significant limitation involves the fact that some of the patients in the 2008 cohort were likely the same patients from the 2005 cohort. We did not identify these patients individually, so there was potential for some overlap between the 2 groups. Some patients who were found to be non-immune and vaccinated in 2005 cohort would have also been recorded as screened in the 2008 cohort. So while the number of patients tripled between the 2 cohorts, we acknowledge that some were inevitably included in both cohorts, and not all of the 2008 patients were unique to that cohort. One could argue that patients who were studied in both cohorts had more chances to be screened and vaccinated compared with those studied in only one of the cohorts, and this clearly could affect the results. Improved health care education in the 3 y time span between to 2 cohorts, better patient follow-up, improved communication between clerical/nursing/scheduling staff and patients, etc, could all potentially contribute to improved vaccination completion rates, and were not accounted for in this study.
This study was also limited because patients were classified by diagnosis; and although the presence of cirrhosis was noted, there was no account for severity of disease. Since patients with decompensated disease are less likely to develop immunity to HAV and HBV after vaccination,Citation9,10 a pre-selection bias for screening and/or vaccinating may exist and was not detected by the studied selection criteria. In addition, predicted life expectancy may have influenced a provider's decision regarding whether or not to screen for immunity and/or vaccinate certain patients. We also did not test for serologic evidence of immunity after vaccinations were completed. Between 10–15% of patients who are properly vaccinated against HBV are still susceptible to getting the virus. This can be due to lack of immune response to vaccination or from acquisition of partial immunity (decreased chance of infection but not absolute protection).
Another imperfection in our data tables involves the statistically significant p value for “other liver diseases.” It is not surprising that having chronic HCV would significantly increase the likelihood that a patient would be screened for HAV and HBV immunity. However, it was unexpected that the category of “other liver diseases” would include patients that were significantly more likely to be tested than those in other categories. It was difficult to find any diagnosis trends in this non-specific category, as it included the remaining patients that didn't have an easily identifiable diagnosis. One possible explanation is that this section contained many patients that were status post liver transplant, therefore expected to be screened for HAV and HBV at a very high percentage during the transplant evaluation process. However, we did not have a separate category specifically for post-transplant patients.
Missed opportunities for vaccination call for further education of health care providers and affected patient populations regarding the current vaccination guidelines. In the last 10 years, we have moved from free-style written orders to check box order sets to a new full electronic medical record with computer order entry. We intend to continue using these technological advancements to provide the best possible health care for CLD patients. Perhaps we could add automated alerts or click down menus as a quality control measure to remind providers to screen for immunity to HAV and HBV in CLD patients and to provide vaccinations if indicated. We hope this continues to be a topic of quality improvement at our institution.
Materials and Methods
In 2013, we received Institutional Review Board approval to review the records of patients with CLD from 2005 and 2008, who met inclusion criteria, defined as established continuity subjects, having >1 visit with a hepatologist or hepatology fellow, and a CLD diagnosis from Scott & White Hepatology Clinic. CLD diagnoses included chronic HBV infection, chronic HCV infection, nonalcoholic steatohepatitis (NASH), alcoholic liver disease, hemochromatosis, autoimmune liver disease, primary biliary cirrhosis, autoimmune hepatitis, primary sclerosing cholangitis, cryptogenic cirrhosis, or other liver disease, including post-transplant status. Chronic HCV infection was defined as having a positive HCV RNA viral load on serum blood test. Patients seen solely in consultation were excluded. Patients were considered susceptible to HAV if they had a negative HAV total antibody and were considered susceptible to HBV if they had a negative HBV surface antibody. Chronic HBV carriers were excluded from this portion. We did not differentiate between unvaccinated patients and previously vaccinated patients who had lost immunity or never mounted a proper immune response. For those who were deemed susceptible to HAV or HBV, vaccination status was recorded to determine if they properly completed the necessary vaccine series. HAV immunity was defined as the qualitative presence of anti-HAV antibody on serum blood test. HBV immunity was defined as greater than 10m [IU]/ml anti-HBV surface antibody on serum blood test. Vaccination status was noted as complete, did not complete, did not start, or unknown. Vaccine completion was defined as receiving 2 HAV vaccinations, 3 HBV vaccinations, or 3 twinrix vaccinations within a 12-month period.
2005 Cohort
A total of 238 patients from January 1 to December 31, 2005 met the inclusion criteria. Their records were reviewed for immunity to or vaccination against HAV and HBV. A dedicated hepatology clinic was initiated 1 y prior to the cohort. During this time, the practice pattern involved a robust, yet not universal, electronic medical record with written orders on a blank order sheet filled out by the provider at the end of every visit.
2008 Cohort
A total of 792 patients visited the hepatology clinic from January 1 to December 31, 2008. Inclusion criteria were the same as previously listed in the 2005 cohort. Therefore, some of the same patients from 2005 were included in this study. These patient's records were reviewed to ascertain their immunity to or vaccination against HAV and HBV. Beginning in January 2008, a pre-printed chronic liver disease order sheet with checkboxes was routinely used by practitioners. These data were then compared with data from 2005 to determine any degree of improvement in screening for immunity and vaccinating when indicated.
Statistical Analysis
The chi square test and Fisher's exact test were used to assess the association between HAV and HBV antibody testing and the presence of different chronic liver diseases. Frequencies and percentages are reported for descriptive purposes. P-values < 0.05 are considered to be statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- “Recommended Adult Immunization Schedule - United States, 2015.” Centers for Disease Control and Prevention, 05 Feb. 2015. Web. 14 May 2015; www.cdc.gov
- Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung HC. Deaths; final data for 2009. Natl Vital Stat Rep 2011; 60:1-116; PMID:24974587
- Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, Ferraro T, Concia E. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med 1998; 338:286-90; PMID:9445408; http://dx.doi.org/10.1056/NEJM199801293380503
- Keeffe EB. Is hepatitis A more severe in patients with chronic hepatitis B and other chronic liver diseases? Am J Gastroenterol 1995; 90:201-5; PMID:7847285
- Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang ST, Moyer LA, Bell BP, Alter MJ. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2005; 54(RR-16):1-31; PMID:16371945
- Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP, Prevention of hepatitis A through active or passive immunization (ACIP). MMWR Recomm Rep 2006; 55(RR-7):1-23; PMID:16708058
- Keeffe EB, Iwarson S, McMahon BJ, Lindsay KL, Koff RS, Manns M, Baumgarten R, Wiese M, Fourneau M, Safary A, et al. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology 1998; 27:881-6; PMID:9500723; http://dx.doi.org/10.1002/hep.510270336
- Smallwood GA, Coloura CT, Martinez E, Stieber AC, Heffron TG. Can patients awaiting liver transplantation elicit an immune response to the hepatitis A vaccine? Transplant Proc 2002; 34:3289-90; PMID:12493448; http://dx.doi.org/10.1016/S0041-1345(02)03572-8
- Dumot JA, Barnes DS, Younossi Z, Gordon SM, Avery RK, Domen RE, Henderson JM, Carey WD. Immunogenicity of hepatitis A vaccine in decompensated liver disease. Am J Gastroenterol 1999; 94:1601-4; PMID:10364031; http://dx.doi.org/10.1111/j.1572-0241.1999.01150.x
- Arguedas MR, Johnson A, Eloubeidi MA, Fallon MA, Fallon MB. Immunogenicity of hepatitis A vaccination in decompensated cirrhotic patients. Hepatology 2001; 34:28-31; PMID:11431730; http://dx.doi.org/10.1053/jhep.2001.25883
- Shim M, Khaykis I, Park J, Bini EJ. Susceptibility to hepatitis A in patients with chronic liver disease due to hepatitis C virus infection: missed opportunities for vaccination. Hepatology 2005; 42:688-95; PMID:16104047; http://dx.doi.org/10.1002/hep.20830
- Jacobs RJ, Meyerhoff AS, Saab Sl. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci 2005; 50:1525-1531; PMID:16110847; http://dx.doi.org/10.1007/s10620-005-2873-5
- Saab S, Lee C, Shpaner A, Ibrahim AB. Seroepidemiology of hepatitis A in patients with chronic liver disease. J Viral Hepat 2005; 12:101-5; PMID:15655056; http://dx.doi.org/10.1111/j.1365-2893.2005.00551.x
- Duncan M, Hirota WK, Tsuchida A. Prescreening versus empirical immunization for hepatitis A in patients with chronic liver disease: a prospective cost analysis. Am J Gastroenterol 2002; 97:1792-5; PMID:12135037; http://dx.doi.org/10.1111/j.1572-0241.2002.05844.x