Abstract
It is debated whether patients with celiac disease (CD) have non-protective antibody responses to HBV vaccination more frequently than non-affected subjects. To perform a literature review and meta-analysis on protective response to HBV vaccination in CD patients. RCTs and observational controlled studies were eligible. Outcome of interest was an anti-HBs (HBsAb) titer ≥10 IU/L after last vaccine dose. Comparative index was rate ratio (RR). Heterogeneity between studies was addressed and funnel plots were analyzed. Meta-regression models were applied to investigate effect size due to study-specific variables. Twelve retrospective studies on a total of 1,447 participants and 4 prospective studies on 184 subjects were selected. The RR was 0.732 (95% C.I.: 0.664-0.808) and 0.777 (95% C.I.: 0.629-0.960) in the prospective and retrospective studies, respectively. The I2, indicating heterogeneity, was 51.1% in retrospective, 39.8% in prospective studies. Non-protective antibody responses occurred more frequently in patients than controls. Due to limitations in the available studies, additional trials to evaluate post-vaccination HBsAb titer in CD patients are needed.
Introduction
In many countries HBV vaccine is routinely administered to infants and at-risk adults in order to reduce HBV-related cirrhosis and hepatocarcinoma. Long-term protection and immune memory to HBsAg are provided to vaccinees,Citation1 even though a lack of, or a low response to vaccination is reported to occur in 4% to 10% of healthy subjects.Citation2
A defective response has been correlated with age, smoking, obesity, male gender, and to the presence of specific human leukocyte antigen (HLA) molecules, including HLA-DQ2, which is expressed in almost all patients with celiac disease (CD).
Celiac disease is an immuno-mediated disorder triggered by gluten intake, which may develop at any age with a high polymorphic clinical presentation. The worldwide prevalence of CD is estimated to be 0.6% −1% in the general population and it should be taken into account that, for each person diagnosed with CD, at least 5 subjects have not yet been identified, mainly in the adult age group.Citation3-5
Whether patients with CD have non-protective antibody responses to HBV vaccination more frequently than non-affected subjects is still debated in the literature.Citation6-12
This issue has not yet been addressed by systematic reviews or meta-analyses. The results of a meta-analysis dealing with the immune response to HBV vaccination in celiac disease could provide information for clinical practice guidelines for CD patient management, since HBV infection is a relevant topic in all age groups, and a crucial issue for people belonging to high risk groups.
Objectives
The aim of this meta-analysis was to analyze the serological response to HBV vaccine in CD patients in comparison to non-affected subjects. We included participants in all age groups who regularly completed a primary HBV vaccination schedule in their lives.
Materials and Methods
Inclusion criteria
Both randomized controlled trials and observational studies evaluating the antibody response to hepatitis B vaccines in patients with CD were considered eligible for inclusion in the analysis. Studies were included if they were published up to March 2015, in the English or Italian language, and provided as full-text, or letters to the Editor and conference abstracts.
Patients who underwent primary HBV vaccination with a 3-dose or a 4-dose schedule, or booster doses, as a child or as an adult were considered, irrespective of type, dosage, route, or site of injection.
Data on rate of protective response to HBV vaccine (defined as anti-HBs titer ≥10UI/L) both in patients and in the control group had to be clearly provided. For retrospective studies, time elapsed from the last vaccine dose had to be specified, but a minimum-maximum time interval was not defined previously in the review protocol. For prospective studies, HBV antibody testing had to be performed 2-6 weeks after the last vaccine dose.
Exclusion criteria
Exclusion criteria were formulated as follows: 1) studies written other than in the English or Italian language; 2) pre-clinical studies (laboratory or animal models); 3) studies without relevant outcomes (epidemiology, etiology, management and genetics of celiac disease; HBV vaccination in patients with diseases other than celiac disease; vaccines administered in patients with celiac disease other than those for HBV); 4) reviews, commentaries, papers without original data, duplicate publications; 4) studies without a controlled design; 5) studies in which patients with celiac disease were identified without applying ongoing standardized diagnostic criteria; 6) studies with participants who did not complete a primary HBV vaccination, i.e. who received <3 doses of vaccine; 7) studies in which the rate of protective response to HBV vaccine for one or more groups of participants was lacking; 8) prospective studies in which HBV titer was evaluated earlier than 2 weeks, or more than 6 weeks, after dose administration.
Literature search
The search was carried out up to March 2015 on Pubmed, Embase, MEDLINE Ovid, Web of Science, and Scopus database. Reference lists of all included studies and qualitative topic reviews were also scanned for additional references.
MeSH terms used for Pubmed search were “Adaptive Immunity” AND “Celiac Disease,” “Immunity, Humoral” AND “Celiac Disease,” “Vaccination” AND “Celiac Disease,” “Hepatitis B Vaccines” AND “Celiac Disease,” “Diet, Gluten-Free” AND “Vaccination,” “HLA-DQ Antigens” AND “Vaccination,” “HLA-DQ2 antigen” [Supplementary Concept] AND “Vaccination.” Different combinations of the keywords “immunization,” “coeliac,” “celiac,” “vacc*,” “HB*,” “hepatitis B vacc*,” “sprue,” “gluten” were used for searching other databases.
Study selection and data collection
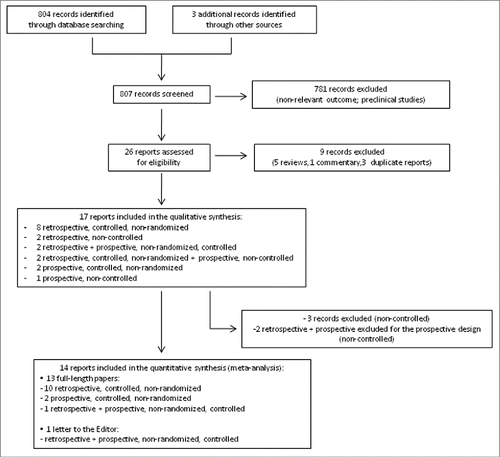
The process for study selection is shown in the flow diagram (). Only studies with a controlled design (vaccinees with CD versus non-affected vaccinees) were included in the meta-analysis.
Two authors independently extracted data using standardized, pre-piloted data collection forms. Disagreements were resolved by discussion and by consultation with a third author, where necessary.
Collection forms included name of the first author, publication year, name of journal, participants (total number, demographics, health status (patient with CD or non-affected control), intervention (type of vaccine, route, number of doses, dosage per single administration, brand), results (number of subjects who developed a protective antibody titer, time interval from the last vaccine administration), statistical analysis, funding sources. Where clarification on published data was required, study authors were contacted for the relevant information.
The methodology of trials was assessed by NOS and STROBE scales by 2 independent evaluators; areas of disagreement were arbitrated by a third. Studies reporting both a retrospective and a prospective cohort of patients and controls were managed separately.
The Cochrane Collaboration's tool for randomized clinical trials was applied for the assessment of bias risk in individual studies.
Summary measures and synthesis of results
We tabulated the extracted information using Microsoft Excel spreadsheets and then submitted to meta-analytical evaluation using Stata 13.1. The event of interest was protective antibody response to HBV vaccination, as assessed by testing serum anti-HBs titer. The outcome of interest was an anti-HBs titer ≥10 IU/L after the last dose of the primary vaccination or after a booster dose. The comparative index under evaluation was the rate ratio (RR), expressed as immunization rate in the exposed population divided by the same rate in the control population.
The heterogeneity between studies was addressed according to the DerSimonian-Laird method for random effects, calculating the tauCitation2 (the between-studies element of the variance) and applying this to the final evaluation of the total variance.Citation13 Moreover, the I2 inconsistency index was employed to express heterogeneity.Citation14
Meta-analytical pooling was also applied to seroconversion rates and converted to logits.
Bias risk assessment across studies and other analyses
Graphical funnel plots were generated to inspect visually for publication bias. The statistical methods for detecting funnel plot asymmetry were the Begg and Mazumdar rank correlation tests and the Egger et al. regression asymmetry test.Citation15,16
The significance of a possible influence on the effect size due to the study design, i.e., a possible difference between retrospective and prospective studies, was investigated by the meta-regression method. Age of vaccination of patients with CD and time interval in antibody titer determination after completion of the primary vaccination or booster in patients were also used in meta-regression models to detect possible changes in the effect size.
Results
Study selection and study characteristics
As shown in the flow diagram (), 26 potentially relevant clinical trials were identified and retrieved for more detailed evaluation. Twenty-five studies were published in English, one in Italian. Of these, 9 studies were excluded because they were reviews, commentaries or duplicate reports. Therefore, we included 17 randomized clinical trials in the qualitative synthesis.Citation6-12,17-26
Three studies were further excluded because they lacked a control arm; Citation17-19 2 other studies, reporting both a retrospective and a prospective cohort of patients, were considered only for the retrospective design, since the prospective cohort lacked a control arm.Citation20,21 As a result, 14 studies were considered for the meta-analysis. Since 2 studies reporting both a retrospective and a prospective cohort of patients and controls were managed as separated reports,Citation11,22 16 studies are listed in the forest plot (12 with retrospective and 4 with prospective design).
Data were published as full-length papers or, in one case, as a letter to the Editor.
summarizes the main characteristics of included studies. HBV vaccine was the recombinant type in all studies, but different brands of vaccine, dosage, or number of doses previously received by participants (primary vaccination or booster) were reported. Dosages ranged from 5 µg to 20 µg, in single or multiple administration, given intramuscularly in all cases.
Table 1. Characteristics of the 16 studies included in the meta-analysis
All studies were controlled and non-randomized in design. Six studies (38%) described the setting of recruitment in details. Eligibility criteria for selection and recruitment of both patients and controls were addressed clearly in 9/16 studies (56%). Reasons for dropouts were described in 4 studies, and overall adequate handling of incomplete outcome data was 7/16 (44%).
Risk of bias was high in all studies since randomization was not performed. Moreover, information about blinding of outcome assessors was lacking.
Results of individual studies and meta-analysis
Data were evaluated from a total of 1,447 participants in the retrospective study group (832 patients, 615 controls), and from 184 participants in the prospective study group (101 patients, 83 controls).
In the retrospective studies, the mean rate of protective antibody titer was 53.6% (95% C.I.: 53.8%–65.2%) in patients, and 82.1% (95% C.I.: 77.1%-86.2%) in controls. In the prospective studies, the mean rate was 65.8% (95% C.I.: 43.1%-83.0%) in patients with CD, and 89.7% (95% C.I.: 80.3%-95.0%) in controls. The forest plot for the RR is reported in .
Figure 2. Forest plot. The horizontal axis reports the rate ratio (RR) of the anti-HBV vaccination in CD vs. control subjects. The studies are grouped according to the design, retrospective (retro) or prospective (pro). A left-shift of the means implies a negative influence of CD on the immunization process.
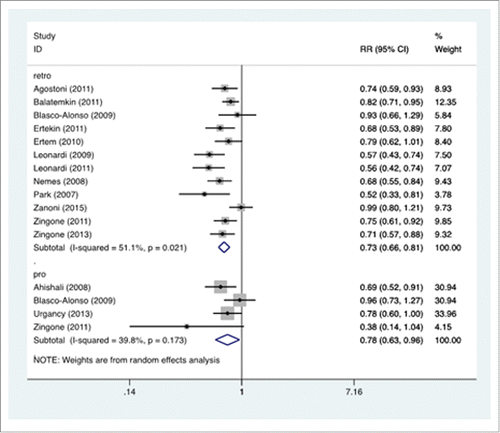
The RR was 0.732 (95% C.I.: 0.664-0.808) in the retrospective studies, 0.777 (95% C.I.: 0.629-0.960) in the prospective studies. The significance test (null hypothesis, RR = 1) yielded p = 0.000 for retrospective studies, p = 0.019 for prospective studies.
The I2 was 51.1% in the retrospective, 39.8% in the prospective studies.
Publication bias assessment and additional analyses
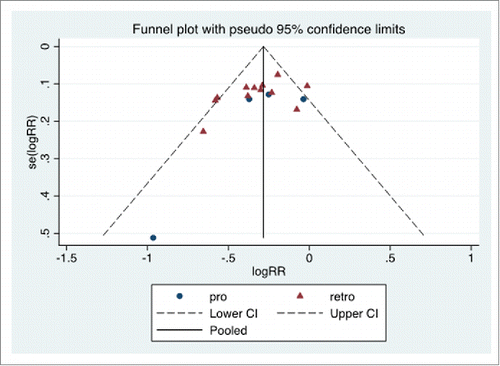
The funnel plot is reported in . No inferential evidence of publication bias was detected.
Figure 3. Funnel plot. Prospective (pro) and retrospective (retro) studies. The pooled logRR (natural logarithm of RR) is indicated (solid line), as the 95% C.I. limits (dashed lines). The y-axis reports the standard error of logRR, se(logRR).
No significant effect by the study design was detected by the meta-regression. Moreover, the age at vaccination and the time interval between vaccination and antibody titer assay did not appear to influence effect size.
Discussion
The aim of this meta-analysis was to analyze the serological response to HBV vaccine in patients with CD in comparison to non-affected subjects. The results of this meta-analysis showed that patients with CD have a statistically significant lower rate of protective antibody titer in comparison to non-affected controls, both in retrospective and in prospective studies. As a consequence, a large number of patients with CD may be considered as non-responders to HBV vaccination, even though the cumulative rate ratio for a protective response to HBV vaccination for CD patients is higher in the “prospective study” stratum, as compared to the “retrospective study” stratum.
Some limitations of this meta-analysis, however, need to be acknowledged. First of all, we could include only observational cohort studies in this review, since randomized controlled trials were lacking. Observational studies may lack the experimental element of a random allocation to an intervention, nevertheless they can be regarded as a useful tool in order to assess the effectiveness of an intervention in a community, as opposed to the special setting of controlled trials.Citation27,28 Moreover, recruitment of vaccinees in the first year of age tends to exclude any serious bias. Another limitation is the low number of studies and participants in the “prospective study” stratum, so information from a limited number of studies and participants should be extrapolated carefully.
Regarding heterogeneity among studies, mainly for samples size, vaccination schedule, and time interval between vaccination and blood sample collection for antibody titer determination, we applied meta-regression models to investigate a role for those variables. The analysis did not reveal any influence of those variables on the effect size. Taking into account that a meta-regression analysis might be not adequately powerful to reveal the effect of study-specific covariates, and to reverse the concept that “one should never use a non-significant finding to conclude that the true means in subgroups are the same, or that a covariate is not related to the effect size,” Citation29 some considerations may be added.
Firstly, the cumulative rate ratio of protective HBV antibody titer between CD patients and non-affected subjects is higher in prospective studies than in retrospective studies. This may be due to a faster decline in time of HBV antibody titer in CD patients, as reported by some authors,Citation30 rather than a defective primary antibody response to vaccination of patients with CD. Furthermore, we should consider that some non-affected subjects do not respond to HBV vaccination either. As a consequence, a genetic feature different from HLA-DQ2 haplotype and occurring more frequently in CD patients than in healthy people might suggest a defective response to HBV vaccination and cannot be excluded.
Conclusion
This meta-analysis showed that patients with CD have a statistically significant lower rate of protective antibody titer, as compared to non-affected controls, but the analysis has some limitations, due to the lack of randomized, controlled, studies, a reduced number of studies with prospective design and a moderate degree of heterogeneity among studies. Therefore, more studies with a prospective design and, possibly, randomized, controlled, clinical trials are needed for patients with celiac disease in order to clarify this topic. However, since HBV vaccination is routinely administered to children with less than 12 months of age, and CD is diagnosed after the first year of age in the great majority of cases, an approach might be to offer extensively a HBV antibody titer determination to children newly diagnosed with CD previously vaccinated as soon as possible. Moreover, an early evaluation of HBV antibody titer in young patients with CD, ideally until 1 y after the completion of vaccination schedule, could provide useful information.
In fact, if evidence for defective protection by HBV vaccination were to be confirmed in patients with CD, personalized vaccine schedules for HBV vaccination and a follow-up of HBV serological protection might be established for the management of these patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dr. Mario Cruciani for collaboration and Prof. Mark J. Newman for manuscript revision.
Funding
This study was financially supported by the Regional Public Health Authority.
References
- Spada E, Roman∫ L, Tosti ME, Zuccaro O, Paladini S, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, et al. Hepatitis B immunity in teenagers vaccinated as infants: an Italian 17-year follow-up study. Clin Microbiol Infect 2014; 20(10):O680-6; PMID:24528380; http://dx.doi.org/10.1111/1469-0691.12591
- World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec 2009; 84:405-419; PMID:19817017
- Lionetti E, Catassi C. New clues in celiac disease epidemiology, pathogenesis, clinical manifestations, and treatment. Int Rev Immunol 2011; 30(4): 219-31; PMID:21787227; http://dx.doi.org/10.3109/08830185.2011.602443
- Tovoli F, Masi C, Guidetti E, Negrini G, Paterini P, Bolondi L. Clinical and diagnostic aspects of gluten related disorders. World J Clin Cases 2015; 3(3):275-84; PMID:25789300; http://dx.doi.org/10.12998/wjcc.v3.i3.275
- Troncone R, Jabri B. Coeliac disease and gluten sensitivity. J Intern Med 2011; 269(6): 582-90; PMID:21481018; http://dx.doi.org/10.1111/j.1365-2796.2011.02385.x
- Park SD, Markowitz J, Pettei M, Weinstein T, Sison CP, Swiss SR, Levine J. Failure to respond to hepatitis B vaccine in children with celiac disease. J Pediatr Gastroenterol Nutr 2007; 44(4):431-5; PMID:17414139; http://dx.doi.org/10.1097/MPG.0b013e3180320654
- Agostoni CV, Boccazzi A, Pontari S, Bedogni G, Prampolini L, Garotta M, Torresani E, Lunghi G. Inadequate seroconversion rates in celiac disease after 3 doses of hepatitis B vaccine, administered at 3, 5 and 11 months of life. J Pediatr Infect Dis 2011; 173-176
- Balamtekın N, Uslu N, Baysoy G, Saltik-Temızel I, Demır H, Yüce A. Responsiveness of children with celiac disease to different hepatitis B vaccination protocols. Turk J Gastroenterol 2011; 22(1):27-31; PMID:21480107
- Ertekin V, Tosun MS, Selimoglu MA. Is there need for a new hepatitıs B vaccine schedule for children with celiac disease?. Hepat Mon 2011; 11(8):634-7; PMID:22140387; http://dx.doi.org/10.5812/kowsar.1735143X.1129
- Ahishali E, Boztas G, Akyuz F, Ibrisim D, Poturoglu S, Pinarbasi B, Ozdil S, Mungan Z. Response to hepatitis B vaccination in patients with celiac disease. Dig Dis Sci 2008; 53(8):2156-9; PMID:18157638; http://dx.doi.org/10.1007/s10620-007-0128-3
- Blasco-Alonso J, Espinosa MG, Navas VM, Sierra C, Serrano J, Barco A. Hepatitis b vaccine nonresponse in healthy and celiac children. Efficacy of a single booster dose. [E-letter], Pediatrics (August 3, 2009), http://pediatrics.aappublications.org/content/121/6/e1570.abstract/reply#pediatrics_el_44877
- Zanoni G, Contreas G, Valletta E, Gabrielli O, Mengoli C, Veneri D. Normal or defective immune response to Hepatitis B vaccine in patients with diabetes and celiac disease. Hum Vaccin Immunother 2015; 11(1):58-62; PMID:25483516; http://dx.doi.org/10.4161/hv.34309
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7(3):177-88; PMID:3802833; http://dx.doi.org/10.1016/0197-2456(86)90046-2
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414):557-60; PMID:12958120; http://dx.doi.org/10.1136/bmj.327.7414.557
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50(4):1088-101; PMID:7786990; http://dx.doi.org/10.2307/2533446
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315(7109):629-34; PMID:9310563; http://dx.doi.org/10.1136/bmj.315.7109.629
- Noh KW, Poland GA, Murray JA. Hepatitis B vaccine nonresponse and celiac disease. Am J Gastroenterol 2003; 98(10):2289-92; PMID:14572581; http://dx.doi.org/10.1111/j.1572-0241.2003.07701.x
- Leonardi S, Pratic∫ AD, Lionetti E, Spina M, Vitaliti G, La Rosa M. Intramuscular vs intradermal route for hepatitis B booster vaccine in celiac children. World J Gastroenterol 2012 Oct 28; 18(40):5729-33; PMID:23155313; http://dx.doi.org/10.3748/wjg.v18.i40.5729
- Walkiewicz-Jedrzejczak D, Egberg M, Nelson C, Eickoff J. Evaluation of the response to vaccination with epatiti B vaccine in pediatric patients diagnosed with celiac disease. SAGE Open Med 2014. http://dx.doi.org/10.1177/2050312114563346
- Ertem D, Gonen I, Tanidir C, Ugras M, Yildiz A, Pehlivanoğlu E, Eksioglu-Demiralp E. The response to hepatitis B vaccine: does it differ in celiac disease? Eur J Gastroenterol Hepatol 2010; 22(7):787-93; PMID:19584738; http://dx.doi.org/10.1097/MEG.0b013e32832e9d41
- Nemes E, Lefler E, Szegedi L, Kapitány A, Kovács JB, Balogh M, Szabados K, Tumpek J, Sipka S, Korponay-Szabó IR. Gluten intake interferes with the humoral immune response to recombinant hepatitis B vaccine in patients with celiac disease. Pediatrics 2008; 121(6):e1570-6; PMID:18519462; http://dx.doi.org/10.1542/peds.2007-2446
- Zingone F, Morisco F, Zanetti A, Roman∫ L, Portella G, Capone P, Andreozzi P, Tortora R, Ciacci C. Long-term antibody persistence and immune memory to hepatitis B virus in adult celiac patients vaccinated as adolescents. Vaccine 2011; 29(5):1005-8; PMID:21129395; http://dx.doi.org/10.1016/j.vaccine.2010.11.060
- Leonardi S, Spina M, Spicuzza L, Rotolo N, La Rosa M. Hepatitis B vaccination failure in celiac disease: is there a need to reassess current immunization strategies?. Vaccine 2009; 27(43):6030-3; PMID:19682619; http://dx.doi.org/10.1016/j.vaccine.2009.07.099
- Leonardi S, Longo R, Cotugno M, Tardino L, Spina M, Lionetti E, La Rosa M. [Vaccination and celiac disease: results of a retrospective study]. Minerva Pediatr 2011; 63(5):363-7; PMID:21946447
- Zingone F, Capone P, Tortora R, Rispo A, Morisco F, Caporaso N, Imperatore N, De Stefano G, Iovino P, Ciacci C. Role of gluten intake at the time of hepatitis B virus vaccination in the immune response of celiac patients. Clin Vaccine Immunol 2013; 20(5):660-2; PMID:23446217; http://dx.doi.org/10.1128/CVI.00729-12
- Urganci N, Kalyoncu D. Response to hepatitis A and B vaccination in pediatric patients with celiac disease. J Pediatr Gastroenterol Nutr 2013; 56(4):408-11; PMID:23132166; http://dx.doi.org/10.1097/MPG.0b013e31827af200
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008-12; PMID:10789670; http://dx.doi.org/10.1001/jama.283.15.2008
- Lau J, Ioannidis JP, Schmid CH. Summing up evidence: one answer is not always enough. Lancet 1998; 35: 123-7; PMID:9439507; http://dx.doi.org/10.1016/S0140-6736(97)08468-7
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. (eds.) John Wiley & Sons, Incorporated 2009; p. 211.
- Wainwright RB, Bulkow LR, Parkinson AJ, Zanis C, McMahon BJ. Protection provided by hepatitis B vaccine in a Yupik Eskimo population-results of a 10-year study. J Infect Dis 1997; 175(3):674-7; PMID:9041341; http://dx.doi.org/10.1093/infdis/175.3.674