Abstract
Children in early infancy do not mount effective antibody responses to many vaccines against commons infectious pathogens, which results in a window of increased susceptibility or severity infections. In addition, vaccine-preventable infections are among the leading causes of morbidity in pregnant women. Immunization during pregnancy can generate maternal immune protection as well as elicit the production and transfer of antibodies cross the placenta and via breastfeeding to provide early infant protection. Several successful vaccines are now recommended to all pregnant women worldwide. However, significant gaps exist in our understanding of the efficacy and safety of other vaccines and in women with conditions associated with increased susceptible to high-risk pregnancies. Public acceptance of maternal immunization remained to be improved. Broader success of maternal immunization will rely on the integration of advances in basic science in vaccine design and evaluation and carefully planned clinical trials that are inclusive to pregnant women.
Introduction
Infectious diseases remain a major cause of morbidity and mortality in children between the ages of 0 to 4 y More than 5 million child-related deaths occur worldwide from vaccine-preventable diseases.Citation1 Vaccination can avert the occurrence of severe infections and alleviate their devastating consequences. However, newborn infants do not efficiently develop protective immunity in response to many vaccines.Citation2 Scheduled vaccination against common infections, such Hepatitis B, pertussis and Haemophilus influenzae, usually commences at a few months to several years after birth,Citation3 leaving a critical window of vulnerability. For this reason, immunization of pregnant women has emerged as an alternative strategy to combat neonatal infection. It relies on the transfer of maternal vaccine-induced humoral immunity to the fetus during gestation and breastfeeding to confer early immune protection until routine vaccination of the child is initiated. In addition, maternal immunization also generates immune protection to the pregnant mother, who are at increased risk of a variety of infections due to the unique immune alternations that occur during pregnancy.Citation4-6 Several successful maternal vaccines, such as Tetanus-Diphtheria-Pertussis (Tdap) vaccine and inactivated influenza vaccine (IIV), are now universally recommended by the Center for Disease Control and prevention (CDC) to all pregnant women.Citation7 However, significant gaps exist in our knowledge of the efficacy and safety of many other vaccines with existing or novel formulations. This review surveys the current profile of maternal vaccine recommendations from the World Health Organization (WHO) and the CDC, vaccine use, and discusses the scientific and clinical advances and challenges in understanding the benefits and risks of maternal vaccination, with the goal of shedding light on the direction of developing safer and more efficient maternal vaccines to combat a broader range of infections.
Literature Search Strategy
We synthesized an outline of the review based on current recommendations and concerns of maternal vaccination. Following the outline a systematic literature search was performed (up to February 2015) in PUBMED using keywords and terms: “maternal vaccination” and “vaccine antibody production,” “vaccine safety” and “neonatal Fc receptor;” which were relevant to each section of our search outline. The search was performed without limitations to species and diseases. Articles were cited based on relevance and quality as interpreted by all authors. Moreover, relevant abstracts from recent meetings were also included. Based on the reviewed information and recent progress in vaccinology and reproductive immunology, we formulated a perspective on future directions for maternal vaccination.
Current Recommendation and Optimal Schedule
The World Health Organization (WHO) and the Advisory Committee on Immunization Practices (ACIP) at the CDC consider maternal immunization a high priority. shows the present guidelines in the United States for immunization of pregnant women are issued from the ACIP. Since there is no evidence of adverse pregnancy outcomes when given inactive vaccines (viral, bacterial and toxoid), both organizations recommend vaccinating during pregnancy especially when there is explicit risk to exposure.Citation8 Maternal vaccination aims protect the both the mother and neonate. As of 2013, 2 vaccines for pertussis and influenza are recommended by the ACIP and WHO to be administered to all women of reproductive age before, during or after pregnancy.
Table 1. Current recommendations of maternal immunization by the Center of Disease Control in the United States
Children under the age of 6 months, and pregnant women are at the greatest risk for hospitalization and death due to pertussis and influenza.Citation9,10 In the United States (US), the earliest recommended immunization series for pertussis is 2 months of age, which is depended on passive maternal antibody exchange. At the time of birth, pregnant women have relatively low concentration of maternal pertussis antibodies.Citation11-13 Expectant mothers can be vaccinated for pertussis preferably between 27 and 36 weeks of gestation in the US,Citation14 between 28 and 32 (up to 38) weeks in the United Kingdom (UK),Citation15 and between 28 and 38 weeks in New Zealand.Citation16 In the UK and New Zealand, the vaccination is funded by governmental initiatives. In Australia, third trimester Dtap vaccination is also recommended for women during each pregnancy,Citation17 although the vaccine is currently not funded under the Australian National Immunization Program, but is free under some state and territory initiatives. Vaccination resulted in high concentrations of pertussis antibodies for the first 2 months of life which did not alter infant response to routine recommended vaccinations.Citation10,18 Pertussis vaccination reports show no negative consequences to neonate regardless of maternal vaccination schedule and benefits the fetus fold2- through passive immunity and cocooning where the neonate is protected through contact immunization.Citation10,19 However, a major concern about maternal pertussis vaccination is blunting of the infants response to their routine vaccinations.
Unlike pertussis, there is considerable evidence that pregnant women are at increased risk for more serve illness, miscarriages, premature and stillborn births associated with influenza infection.Citation9 Due to the maternal exposure to influenza, WHO recommends that pregnant women should have the highest priority, since children under the age of 6 months are not eligible to receive influenza vaccines.Citation1 ACIP recommends influenza vaccination as early as the first trimester, when other risk factors, such as chronic disease, can complicate over the course of the flu.Citation20 Reports have shown that prenatal vaccinations reduce the incidence of hospitalization up to 48% in children for 12 months in the US and upwards of 63% have been reported in Bangladesh.Citation21,22 Moreover, several other inactivated vaccines, including Hepatitis A and B and meningococcal, are recommended for women before, during or after pregnancy when risk factors exist.Citation7 Therefore, more case-by-case studies are needed that use inactivated vaccines with novel adjuvants to assess potential risks.Citation23
Live attenuated vaccines are generally not recommended for use in pregnancy due to the potential risk of introducing to the developing fetus a live pathogen that could acquire secondary mutations causing the reversion to virulence, and the concern over attenuated pathogens causing severe complications in immunocompromised pregnant women. In general, there is a lack of widely conclusive safety studies. Certain live attenuated vaccines, such as Measles-Mumps-Rubella, Yellow Fever and Pneumococcus, are only administered if there is a high risk of exposure to disease in which the mother or child could be in danger ()
Table 2. Summary based on CDC and WHO of the potential risk of vaccine-preventable diseases on pregnancy
Global profile of vaccine usage in the obstetric population
Successful implementation of maternal immunization primarily relies upon the ability of medical professionals to actively educate and implement immunization services to the general public. Even with encouraging data from post licensure studies of the safety of maternal influenza and Tdap vaccines, there are still uncertainties among the public in the understanding of maternal immunologic protection and traditional immunization schedule. For example, in Europe, only 62% of the countries recommend a seasonal influenza vaccine to pregnant woman; moreover, among these countries, only a third recommend influenza vaccination for all pregnant women.Citation24 While in the United States, maternal influenza vaccination rate has been estimated to be around only 50%.Citation25 Currently there is little information available regarding the rate of Tdap and other vaccinations. To increase the number of pregnant women getting vaccinated, care providers play an important role in promoting vaccine acceptance. Women have reported their doctors as being their most trusted source for information. Therefore, a major barrier in vaccinating pregnant women is the lack of provider recommendation. Additional issues include that obstetric care providers are not set up to routinely administer vaccines as part of antenatal care provision in all models of care, and that not all recommended maternal vaccines are provided free in all countries, which further hampers acceptance, especially by low-income populations. Given the increased risk of vaccine-preventable diseases during pregnancy, an avant-garde means is needed to increase vaccine coverage. For example, the uses of modern technologies, such as “educational text messages,” is an innovative systematic strategy to prompt women to communicate with their providers.Citation26 Likewise, healthcare professionals should assume responsibility for keeping themselves abreast of the scientific knowledge on the protective efficacy and possible adverse effect in the short- and long-term health status of various pregnant and pediatric populations as it pertains to maternal vaccinations.Citation27
Transfer of Maternal Immunity via Placenta and Breastfeeding
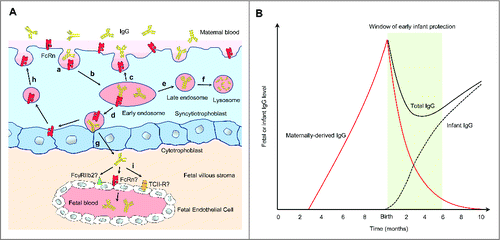
Underpinning the idea of maternal immunization is that the transport of vaccine-induced maternal immunoglobulin G (IgG) antibody across the placenta by neonatal Fc receptor (FcRn) expressed on trophoblasts across,Citation28-30 confers fetal and early infant immune protection (). The amount of IgG transferred across the placenta to the fetus correlates with IgG concentration in the maternal circulation, gestational age, maternal health and IgG subclass, with IgG1 and IgG4 preferentially transported over IgG3 and IgG2.Citation31 Therefore, vaccines that contain protein antigens, such as Tdap, that elicit predominant production of IgG1 and IgG3 are generally more efficient than polysaccharide vaccines that mainly elicit the production of IgG2.Citation32,33 IgG transfer from the mother to the fetus can occur as early as gestational week 13, with the largest amount transferred during the third trimester of pregnancy ().Citation34,35 Additional passive immunity in the form of vaccine-induced IgA, IgG and IgM secreted into colostrum and breast milk is transferred during breastfeeding.
Figure 1. Transport of maternal IgG via the placenta to the fetus and its function in early infant immune protection. (A) Villous syncytiotrophoblasts are in direct contact with the maternal blood and express FcRn. Syncytiotrophoblasts internalize maternal IgG (a) into early endosomes, where the acidic pH induces IgG to bind FcRn with high affinity (b). IgG-FcRn complexes can either be recycled back to the maternal blood, where IgG dissociates (c) or undergo transcytosis to the fetal side of the syncytiotrophoblast surface (d). Unbound IgG in early endosomes can be recycled back to the maternal blood (c) or targeted to late endosomes (e) and subsequently lysosomes for degradation (f). Transcytosed IgG is released from FcRn under neutral pH (g) and enters the fetal villous stroma. FcRn on the fetal side of syncytiotrophoblasts can be retrieved back to the maternal side for additional rounds of IgG transport (h). FcγRIIb2, FcRn, or possibly transcobalamin II receptor (TCII-R) expressed on the fetal endothelium may mediate the transport of IgG into fetal circulation, but the exact mechanism and the relative contribution of these pathways remain unknown (i). (B) The placental transfer of maternal IgG to the fetus is detectable as early as gestational week 13Citation35 With increased placental FcRn expression, IgG transport steadily increases as the pregnancy progresses, with the largest amount transferred during the third trimester. Although maternal IgG wanes after birth, it provides the infant with immune protection during a critical window after birth (green) before the infant produces significant amount of antibodies upon vaccination.
Benefits of Immunizing Pregnant Women
Maternal immunization presents unique benefits to the mother, fetus and the newborn by inducing active immunity in the mother and the transfer of passive humoral immunity across the placenta to the fetus. The fetus is susceptible to infections during pregnancy, perhaps due to the immaturity of the fetal immune system,Citation36 and its tendency to mount tolerogenic responses.Citation37-40 Newborn infants mount sub-optimal immune responses to many viral, bacterial and fungal pathogens, rendering them prone to more severe or prolonged infections than adults.Citation37,41 The heightened neonatal susceptibility was attributed to a less intact mucosal barrier, a lack of existing immunological memory, the immaturity of the neonatal immune system and its propensity to mount tolerogenic responses.Citation39,42 Despite recent efforts in breaking neonatal tolerance to induce efficient vaccine response in infants,Citation43-54 fetal and neonatal tolerance may have important physiological functions by promoting fetal-maternal tolerance, avoiding harmful fetal inflammation during development and suppressing detrimental inflammation during mucosal colonization after birth.Citation55-58 In England, Amirthalingham et. al (2014) sought to determine the effectiveness of the pertussis vaccination program for pregnant women and infants between January 2008 and September 2014. By comparing vaccine status of mothers in confirmed cases with estimated of vaccine coverage for the national population of pregnant women, they noticed greater than 90% vaccine effectiveness based on national vaccine coverage and confirmed infant cases. They concluded that the vaccines effectiveness is due to both passive antibody transport and reduction of maternal exposure. Therefore, immunizing pregnant women can circumvent the multiple disadvantages of attempting to directly immunize neonates immediately after birth.
Another important, albeit less obvious, benefit of maternal vaccination may involve the function of maternal antibodies in promoting the immune maturation and modulation in infants. Animal studies found that maternally derived antibodies have a profound and long-lasting effect in promoting the development of infant B cells and their antibody diversification and production, as well as the selection against potentially autoreactive B cell populations.Citation59-61 Maternal antibodies acquired from milk during breastfeeding can provide mucosal protection by neutralizing pathogenic virulence factors in the intestinal lumen and inhibiting the adhesion and invasion of pathogens.Citation62,63 They may further facilitate mucosal antigen sampling and the development of immune tolerance for commensal microbes and food antigens during the critical window shortly after birth. In this case, maternal antibodies may contribute to the association of breastfeeding with reduced childhood disorders, such as allergic diseases.Citation64
Risks of Immunizing Pregnant Women
Concern over pregnancy outcome and infant health
In the past, researchers have been reluctant to include pregnant women in clinical studies because of the potential harm to the fetus. Though this is amicable, their exclusion has limited the growth of knowledge about vaccine safety and efficacy for pregnant women and the fetus.Citation65 As mentioned before, major risks of immunizing pregnant women include the possibility of introducing to the developing fetus a live pathogen, which could acquire secondary mutations leading to a reversion to virulence. Live attenuated vaccines may also cause potentially severe complications in immunocompromised pregnant subjects. Therefore, they are generally contraindicated in pregnant women. As recommended by the CDC and WHO, in case of a live viral vaccine, such as MMR and varicella, being inadvertently administered to a pregnant woman, or should a woman becomes pregnant within 4 weeks after vaccination, she should be counseled about the theoretical concern for the fetus, although inadvertent administration of these vaccines should not be considered a reason to terminate pregnancy.Citation66 There is no evidence showing an increased risk of vaccinating pregnant women with inactivated vaccines or toxoids. Therefore, their benefit outweighs the potential risk if the vaccine-preventable infection poses a significant risk to the pregnant woman, the fetus and baby.
Vaccinations for pertussis and influenza represent good and successful paradigms of maintaining the high safety standard of maternal vaccines, which the safety research of other maternal vaccines can follow. Until recently, both vaccines were extensively tested in non-pregnant populations before being licensed for use in pregnant women.Citation67 Secondly, large scale post-licensure surveillance on pregnancy outcome and infant health are being carried out following their recommended use in pregnant women by ACIP and WHO. Post-licensure monitoring is necessary, as pre-licensure trials may not have detected rare events or adequately represented special populations. Major challenges (i.e. voluntary follow-ups and retrospective analysis) are speculative with virtually no information against biological agents, such as anthrax, or diseases which are extremely uncommon in the developed world, such as small pox and typhus. In cases where exposure is high, treatment is based heavily on theoretical benefit-to-risk ratio.Citation7 In 1999, concerns over the link of certain vaccine ingredients, such as thimerosal and mercury, to neurodevelopmental disorders, such as autism, were also raised. A recent comprehensive review concluded that the weight of evidence is that there is no link between the 2 and that better communication of the evidence is warranted.Citation68 Furthermore, the main brands of vaccines given in pregnancy, including Tdap and influenza, do not contain thimerosal. Many scientific (such as Institute of Medicine and American Congress of Obstetricians and Gynecologists), international (WHO), governmental (CDC) and civil society agencies have been vigilant about reassuring short- and long-term vaccine safety. However, more basic research and post-marketing surveillance systems are needed to ease misconceptions of any potential new vaccine ingredients.Citation69
Interference with infant response to vaccination
Certain maternal vaccine-induced antibodies transferred to the fetus have been found to inhibit the neonate's humoral immune response to vaccination.Citation70 Multiple mechanisms may underlie such inhibitory effects,Citation62 but they may vary between vaccines, vaccine doses and schedules.Citation71-73 Human maternal antibodies wane over a period of 6 to 12 months, which correlates with their amount present in the neonates at birth.Citation74-76 Interference on infant humoral immunity was found to mainly impact primary immunization in early infancy but not subsequent boosting.Citation77 Therefore, this should not be a deterrent to maternal immunization, especially if vaccination during pregnancy can mitigate high mortality and morbidity of infant infectious diseases. The pros and cons of maternal vaccination effects on infant immune response after scheduled infant immunization need to be carefully and systemically evaluated.
Conclusion and Future Directions
Maternal immunization has emerged as a worldwide public health strategy to protect both pregnant women and infants against infections. To date, the ACIP and WHO have specifically recommend immunization of all pregnant women against influenza and pertussis. Many other countries, such as the UK, Australia and New Zealand, have also similar vaccination recommendation for pregnant women. Despite the success, public acceptance to these recommended maternal vaccines remains to be increased world-wide. To this end, we believe that it is the key to better inform the public of disease risks, vaccine safety and benefits, continue to disseminate the newest scientific knowledge on maternal vaccination to physicians and encourage them to recommend to patients in all models of care, foster the universal implementation of vaccination by physicians and integrate public and private infrastructure and resources to provide financial support for vaccination programs.
In terms of the development of new maternal vaccines, a number of promising new vaccines, such as those against group B streptococcus and respiratory syncytial virus, are in the pipeline or in trial.Citation78 However, pregnant women have been traditionally excluded from many vaccine trials, which have precipitated a lack of long-term safety and efficacy data for pregnant women and the fetus. It is precisely those initial ethical and practical concerns that have become a bottle neck in maternal vaccine development and have had a direct effect on the quality of care to pregnant women and their ability to make informed decisions on immunization. Furthermore, significant gaps exist in our understanding of the efficacy and safety of vaccines in pregnant women with underlying conditions associated with increased susceptible to high-risk pregnancies. In order break such a vicious cycle, we advocate that consistent efforts are needed to open well-designed vaccine trials to safely include and monitor pregnant women. As the efficacy of maternal vaccines uniquely relies on the secretion of antibodies at the maternal-fetal interface and in the mammary gland, maternal vaccine development requires a thorough understanding of the mechanisms of mucosal immune regulation and the microbiota influence during pregnancy,Citation79,80 and mucosal immune assessment, in addition to systemic immune assessment, should be incorporated into vaccine trial protocols. Last, we believe that integration of some recent scientific advances in systems vaccinology, which has demonstrated success in systemically profiling and in certain cases predicting vaccine efficacy,Citation81-84 will improve greatly the speed, accuracy and safety of vaccines development for the pregnant population.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The authors acknowledge the funding support from the American Congress of Obstetricians and Gynecologists Industrial Award on Immunization, Burroughs Wellcome Fund Preterm Birth Initiative, National Institutes of Health U01AI95776 Young Investigator Award, Wayne State University Maternal Perinatal and Child Health Initiative, Wayne State University Office of the Vice President for Research (OVPR) and Barbara Ann Karmanos Cancer Institute (to K.C.). A.N.F. is supported partly by a fellowship from the Wayne State University OVPR.
References
- World Health Organization W. Global Immunization Data. World Health Organization 2014
- Lambert PH, Liu M, Siegrist CA. Can successful vaccines teach us how to induce efficient protective immune responses? Nat Med 2005; 11:S54-62; PMID:15812491; http://dx.doi.org/10.1038/nm1216
- Mackell SM. Vaccine Recommendations for Infants & Children. In: Brunette GW, ed. CDC Health Information for International Travel United States of America: Oxford University Press, 2014
- Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis 2006; 12:1638-43; PMID:17283611; http://dx.doi.org/10.3201/eid1211.060152
- Pazos M, Sperling RS, Moran TM, Kraus TA. The influence of pregnancy on systemic immunity. Immunol Res 2012; 54:254-61; PMID:22447351; http://dx.doi.org/10.1007/s12026-012-8303-9
- Sappenfield E, Jamieson DJ, Kourtis AP. Pregnancy and susceptibility to infectious diseases. Infect Dis Obstet Gynecol 2013; 2013:752852; PMID:23935259; http://dx.doi.org/10.1155/2013/752852
- Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women-Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013; 62:131-5; PMID:23425962
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1-64; PMID:21293327
- Brydak LB, Nitsch-Osuch A. Vaccination against influenza in pregnant women. Acta Biochimica Polonica 2014; 61:589-91; PMID:25195141
- Munoz FM, Bond NH, Maccato M, Pinell P, Hammill HA, Swamy GK, Walter EB, Jackson LA, Englund JA, Edwards MS, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. Jama 2014; 311:1760-9; PMID:24794369; http://dx.doi.org/10.1001/jama.2014.3633
- Gonik B, Puder KS, Gonik N, Kruger M. Seroprevalence of Bordetella pertussis antibodies in mothers and their newborn infants. Infect Dis Obstet Gynecol 2005; 13:59-61; PMID:16011994; http://dx.doi.org/10.1080/10647440500068289
- Healy CM, Munoz FM, Rench MA, Halasa NB, Edwards KM, Baker CJ. Prevalence of pertussis antibodies in maternal delivery, cord, and infant serum. J Infectious Diseases 2004; 190:335-40; PMID:15216470; http://dx.doi.org/10.1086/421033
- Van Savage J, Decker MD, Edwards KM, Sell SH, Karzon DT. Natural history of pertussis antibody in the infant and effect on vaccine response. J Infect Dis 1990; 161:487-92; PMID:2313127; http://dx.doi.org/10.1093/infdis/161.3.487
- (ACIP) ACoIP. Guidelines for Vaccinating Pregnant Women. In: Prevention CfDCa, ed. Atlanta, GA, USA, 2014
- Pertussis. In: Salisbury D, Ramsay M, eds. Immunisation against infectious disease: Department of Health and Public Health England:277-302
- New Zealand Immunisation Handbook 2014. New Zealand Ministry of Health:359
- The Australian Immunisation Handbook, Department of Health of the Australian Government, 2014
- Hardy-Fairbanks AJ, Pan SJ, Decker MD, Johnson DR, Greenberg DP, Kirkland KB, Talbot EA, Bernstein HH. Immune responses in infants whose mothers received Tdap vaccine during pregnancy. Pediatr Infect Dis J 2013; 32:1257-60; PMID:23799518; http://dx.doi.org/10.1097/INF.0b013e3182a09b6a
- Shakib JH, Korgenski K, Sheng X, Varner MW, Pavia AT, Byington CL. Tetanus, diphtheria, acellular pertussis vaccine during pregnancy: pregnancy and infant health outcomes. J Pediatr 2013; 163:1422-6 e1-4; PMID:23896191; http://dx.doi.org/10.1016/j.jpeds.2013.06.021
- Center for Disease Control and Prevention. Recommended adult immunization schedule-Advisory Committee on Immunization Practices (ACIP), 2015. MMWR Mob Mortal Wkly Rep 64:93–94.
- Poehling KA, Szilagyi PG, Staat MA, Snively BM, Payne DC, Bridges CB, Chu SY, Light LS, Prill MM, Finelli L, et al. Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol 2011; 204:S141-8; PMID:21492825; http://dx.doi.org/10.1016/j.ajog.2011.02.042
- Eick AA, Uyeki TM, Klimov A, Hall H, Reid R, Santosham M, O'Brien KL. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med 2011; 165:104-11; PMID:20921345; http://dx.doi.org/10.1001/archpediatrics.2010.192
- Herberts C, Melgert B, van der Laan JW, Faas M. New adjuvanted vaccines in pregnancy: what is known about their safety? Expert Rev Vaccines 2010; 9:1411-22; PMID:21105777; http://dx.doi.org/10.1586/erv.10.133
- World Health Organization. Evaluation of seasonal influenza vaccination policies and coverage in the WHO European Region Copenhagen, Denmark: WHO Regional Office for Europe 2014
- Centers for Disease Control and Prevention. Influenza vaccination coverage among pregnant women–United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2013; 62:787-92; PMID:24067583
- Kharbanda EO, Vargas CY, Castano PM, Lara M, Andres R, Stockwell MS. Exploring pregnant women's views on influenza vaccination and educational text messages. Prev Med 2011; 52:75-7; PMID:21047526; http://dx.doi.org/10.1016/j.ypmed.2010.10.009
- Moffatt K, McNally C. Vaccine Refusal: Perspectives from Pediatrics. In: Chatterjee A, ed. Vaccinophobia and Vaccine Controversies of the 21st Century. New York: Springer Science+Business Media, 2013:97-118
- Kristoffersen BK. Human placental Fcγ-binding proteins in the maternofetal transfer of IgG. APMIS 1996; 104:5-36; PMID:8944053; http://dx.doi.org/10.1111/j.1600-0463.1996.tb05583.x
- Raghavan M, Chen MY, Gastinel LN, Bjorkman PJ. Investigation of the interaction between the class I MHC-related Fc receptor and its immunoglobulin G ligand. Immunity 1994; 1:303-15; PMID:7889418; http://dx.doi.org/10.1016/1074-7613(94)90082-5
- Vaughn DE, Bjorkman PJ. Structural basis of pH-dependent antibody binding by the neonatal Fc receptor. Structure 1998; 6:63-73; PMID:9493268; http://dx.doi.org/10.1016/S0969-2126(98)00008-2
- Costa-Carvalho BT, Vieria HM, Dimantas RB, Arslanian C, Naspitz CK, Sole D, Carneiro-Sampaio MM. Transfer of IgG subclasses across placenta in term and preterm newborns. Braz J Med Biol Res 1996; 29:201-4; PMID:8731349
- Nagao AT, Friedlander-Del Nero D, Arslanian C, Carneiro-Sampaio MM. Elevated levels and different repertoire profile of colostral anti-LPS antibodies may have a significant role in compensating newborn immunity. Scand J Immunol 2001; 53:602-9; PMID:11422909; http://dx.doi.org/10.1046/j.1365-3083.2001.00921.x
- van den Berg JP, Westerbeek EA, Berbers GA, van Gageldonk PG, van der Klis FR, van Elburg RM. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr Infect Dis J 2010; 29:801-5; PMID:20803841; http://dx.doi.org/10.1097/INF.0b013e3181dc4f77
- Saji F, Koyama M, Matsuzaki N. Current topic: human placental Fc receptors. Placenta 1994; 15:453-66; PMID:7997446; http://dx.doi.org/10.1016/S0143-4004(05)80415-1
- Saji F, Samejima Y, Kamiura S, Koyama M. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod 1999; 4:81-9; PMID:10357095; http://dx.doi.org/10.1530/ror.0.0040081
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature 1953; 172:603-6; PMID:13099277; http://dx.doi.org/10.1038/172603a0
- Silverstein AM. Ontogeny of the Immune Response. Science 1964; 144:1423-8; PMID:14171536; http://dx.doi.org/10.1126/science.144.3625.1423
- Wang G, Miyahara Y, Guo Z, Khattar M, Stepkowski SM, Chen W. “Default” generation of neonatal regulatory T cells. J Immunol 2010; 185:71-8; PMID:20498359; http://dx.doi.org/10.4049/jimmunol.0903806
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol 2004; 4:553-64; PMID:15229474; http://dx.doi.org/10.1038/nri1394
- Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol 2006; 176:5741-8; PMID:16670279; http://dx.doi.org/10.4049/jimmunol.176.10.5741
- Darmstadt GL, Zaidi AKM, Stoll BJ. Neonatal Infections: A Global Perspective. In: Remington JS, Klein JO, Wilson CB, Nizet V, Maldonado YA, eds. Infectious Diseases of the Fetus and Newborn Infant. Philadelphia, PA, USA: Saunders/Elsevier 2011:24-51
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 2007; 7:379-90; PMID:17457344; http://dx.doi.org/10.1038/nri2075
- Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science 1996; 271:1728-30; PMID:8596934; http://dx.doi.org/10.1126/science.271.5256.1728
- Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 1996; 271:1723-6; PMID:8596932; http://dx.doi.org/10.1126/science.271.5256.1723
- Sarzotti M, Robbins DS, Hoffman PM. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science 1996; 271:1726-8; PMID:8596933; http://dx.doi.org/10.1126/science.271.5256.1726
- Martinez X, Brandt C, Saddallah F, Tougne C, Barrios C, Wild F, Dougan G, Lambert PH, Siegrist CA. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc Natl Acad Sci U S A 1997; 94:8726-31; PMID:9238045; http://dx.doi.org/10.1073/pnas.94.16.8726
- Hassett DE, Zhang J, Whitton JL. Neonatal DNA immunization with a plasmid encoding an internal viral protein is effective in the presence of maternal antibodies and protects against subsequent viral challenge. J Virol 1997; 71:7881-8; PMID:9311877
- Bot A, Bot S, Bona C. Enhanced protection against influenza virus of mice immunized as newborns with a mixture of plasmids expressing hemagglutinin and nucleoprotein. Vaccine 1998; 16:1675-82; PMID:9713946; http://dx.doi.org/10.1016/S0264-410X(98)00054-1
- Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci U S A 1998; 95:15553-8; PMID:9861007; http://dx.doi.org/10.1073/pnas.95.26.15553
- Jakobsen H, Saeland E, Gizurarson S, Schulz D, Jonsdottir I. Intranasal immunization with pneumococcal polysaccharide conjugate vaccines protects mice against invasive pneumococcal infections. Infect Immun 1999; 67:4128-33; PMID:10417183
- Kovarik J, Gaillard M, Martinez X, Bozzotti P, Lambert PH, Wild TF, Siegrist CA. Induction of adult-like antibody, Th1, and CTL responses to measles hemagglutinin by early life murine immunization with an attenuated vaccinia-derived NYVAC(K1L) viral vector. Virology 2001; 285:12-20; PMID:11414801; http://dx.doi.org/10.1006/viro.2001.0945
- Franchini M, Abril C, Schwerdel C, Ruedl C, Ackermann M, Suter M. Protective T-cell-based immunity induced in neonatal mice by a single replicative cycle of herpes simplex virus. J Virol 2001; 75:83-9; PMID:11119576; http://dx.doi.org/10.1128/JVI.75.1.83-89.2001
- Fadel SA, Ozaki DA, Sarzotti M. Enhanced type 1 immunity after secondary viral challenge in mice primed as neonates. J Immunol 2002; 169:3293-300; PMID:12218149; http://dx.doi.org/10.4049/jimmunol.169.6.3293
- Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, Ungaro R, Levy O, Moldawer LL. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 2008; 112:1750-8; PMID:18591384; http://dx.doi.org/10.1182/blood-2008-01-130500
- Ito M, Nakashima A, Hidaka T, Okabe M, Bac ND, Ina S, Yoneda S, Shiozaki A, Sumi S, Tsuneyama K, et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol 2010; 84:75-85; PMID:19969371; http://dx.doi.org/10.1016/j.jri.2009.09.005
- Vitoratos N, Papadias C, Economou E, Makrakis E, Panoulis C, Creatsas G. Elevated circulating IL-1beta and TNF-α, and unaltered IL-6 in first-trimester pregnancies complicated by threatened abortion with an adverse outcome. Mediators Inflamm 2006; 2006:30485; PMID:17047289; http://dx.doi.org/10.1155/MI/2006/30485
- Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med 2006; 203:973-84; PMID:16606665; http://dx.doi.org/10.1084/jem.20050625
- Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013; 504:158-62; PMID:24196717; http://dx.doi.org/10.1038/nature12675
- Fink K, Zellweger R, Weber J, Manjarrez-Orduno N, Holdener M, Senn BM, Hengartner H, Zinkernagel RM, Macpherson AJ. Long-term maternal imprinting of the specific B cell repertoire by maternal antibodies. Eur J Immunol 2008; 38:90-101; PMID:18081043; http://dx.doi.org/10.1002/eji.200737872
- Malanchere E, Huetz F, Coutinho A. Maternal IgG stimulates B lineage cell development in the progeny. Eur J Immunol 1997; 27:788-93; PMID:9079823; http://dx.doi.org/10.1002/eji.1830270330
- Lemke H, Coutinho A, Lange H. Lamarckian inheritance by somatically acquired maternal IgG phenotypes. Trends Immunol 2004; 25:180-6; PMID:15039044; http://dx.doi.org/10.1016/j.it.2004.02.007
- Faucette AN, Unger BL, Gonik B, Chen K. Maternal vaccination: moving the science forward. Hum Reprod Update 2015; 21:119-35; PMID:25015234; http://dx.doi.org/10.1093/humupd/dmu041
- Jackson KM, Nazar AM. Breastfeeding, the immune response, and long-term health. J Am Osteopath Assoc 2006; 106:203-7; PMID:16627775
- Kull I, Wickman M, Lilja G, Nordvall SL, Pershagen G. Breast feeding and allergic diseases in infants-a prospective birth cohort study. Arch Dis Child 2002; 87:478-81; PMID:12456543; http://dx.doi.org/10.1136/adc.87.6.478
- Lyerly AD, Little MO, Faden R. The second wave: Toward responsible inclusion of pregnant women in research. Int J Fem Approaches Bioeth 2008; 1:5-22; PMID:19774226; http://dx.doi.org/10.2979/FAB.2008.1.2.5
- Centers for Disease Control and Prevention. Guidelines for Vaccinating Pregnant Women 2014
- Centers for Disease C, Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:13-5; PMID:21228763
- Parker SK, Schwartz B, Todd J, Pickering LK. Thimerosal-containing vaccines and autistic spectrum disorder: a critical review of published original data. Pediatr 2004; 114:793-804; PMID:15630018; http://dx.doi.org/10.1542/peds.2004-0434
- Poland GA. MMR vaccine and autism: vaccine nihilism and postmodern science. Mayo Clin Proc 2011; 86:869-71; PMID:21878599; http://dx.doi.org/10.4065/mcp.2011.0467
- Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol 2014; 5:446; PMID:25278941; http://dx.doi.org/10.3389/fimmu.2014.00446
- Siegrist CA. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 2003; 21:3406-12; PMID:12850349; http://dx.doi.org/10.1016/S0264-410X(03)00342-6
- Bjorkholm B, Granstrom M, Taranger J, Wahl M, Hagberg L. Influence of high titers of maternal antibody on the serologic response of infants to diphtheria vaccination at three, five and twelve months of age. Pediatr Infect Dis J 1995; 14:846-50; PMID:8584309; http://dx.doi.org/10.1097/00006454-199510000-00005
- Slack MH, Schapira D, Thwaites RJ, Schapira C, Bamber J, Burrage M, Southern J, Andrews N, Miller E. Acellular pertussis vaccine given by accelerated schedule: response of preterm infants. Arch Dis Child Fetal Neonatal Ed 2004; 89:F57-60; PMID:14711858; http://dx.doi.org/10.1136/fn.89.1.F57
- Leuridan E, Hens N, Hutse V, Aerts M, Van Damme P. Kinetics of maternal antibodies against rubella and varicella in infants. Vaccine 2011; 29:2222-6; PMID:20558248; http://dx.doi.org/10.1016/j.vaccine.2010.06.004
- Leuridan E, Van Damme P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine 2007; 25:6296-304; PMID:17629601; http://dx.doi.org/10.1016/j.vaccine.2007.06.020
- Watanaveeradej V, Endy TP, Samakoses R, Kerdpanich A, Simasathien S, Polprasert N, Aree C, Vaughn DW, Ho C, Nisalak A. Transplacentally transferred maternal-infant antibodies to dengue virus. Am J Trop Medi Hyg 2003; 69:123-8; PMID:13677366
- Glezen WP. Effect of maternal antibodies on the infant immune response. Vaccine 2003; 21:3389-92; PMID:12850346; http://dx.doi.org/10.1016/S0264-410X(03)00339-6
- Madhi SA, Leroux-Roels G, Koen A, Jose L, Cutland C, Maes C, Ter Meulen A, Wittke F, Martell L, Slobod K. Safety and Immunogenicity of an investigational maternal trivalent vaccine to prevent perinatal group B streptococcus (GBS) infection. 31th Annual ESPID Meeting. Milan, Italy 2013
- Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr 2010; 156:S8-15; PMID:20105666; http://dx.doi.org/10.1016/j.jpeds.2009.11.014
- Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity 2010; 33:479-91; PMID:21029959; http://dx.doi.org/10.1016/j.immuni.2010.09.013
- Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol 2011; 12:786-95; PMID:21743478; http://dx.doi.org/10.1038/ni.2067
- Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity 2010; 33:516-29; PMID:21029962; http://dx.doi.org/10.1016/j.immuni.2010.10.006
- Quinello C, Quintilio W, Carneiro-Sampaio M, Palmeira P. Passive acquisition of protective antibodies reactive with Bordetella pertussis in newborns via placental transfer and breast-feeding. Scand J Immunol 2010; 72:66-73; PMID:20591078
- Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S, Davis C, Schmidt DS, Johnson SE, Milton A, Rajam G, et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol 2014; 15:195-204; PMID:24336226; http://dx.doi.org/10.1038/ni.2789
