Abstract
Montanide ISA™51 (ISA 51) is a vaccine adjuvant which has been tested in therapeutic and prophylactic vaccine trials. The aim of this review is to present a comprehensive examination of the safety and tolerability of ISA 51 containing vaccines. A systematic literature search was conducted in PubMed, EMBASE and clinicaltrials.gov. Eligible studies were categorized into: (A) uncontrolled studies with non-healthy subjects, (B) controlled studies with non-healthy subjects, and (C) controlled studies with healthy subjects. Reported adverse events (AEs) were assessed. 91 studies were included in our review. Generally observed AEs included injection site reaction; injection site pain; myalgia; headache; gastro-intestinal disorders; fatigue and fever - regardless of the administration route and subject characteristic. Specific AEs, e.g. injection site reactions and rash, were more frequently reported from subjects receiving ISA 51-adjuvanted vaccines than from subjects receiving antigen or ISA 51 only. The reported AEs were mainly mild to moderate in intensity. Serious AEs (SAEs) were reported in 27% of the uncontrolled trials and 2 trials conducted with healthy subjects. Notably, 2 other trials conducted with healthy subjects were stopped due to unacceptable AEs. Some studies indicate that the mixing procedure of antigen and adjuvant might influence the occurrence of AEs. Reports on SAEs and premature termination of 2 trials advise caution when using ISA 51. Yet, AEs might be preventable by proper mixing of vaccine and adjuvant to a stable emulsion. Trials including an active control group are needed for a fair evaluation of adjuvant safety.
Abbreviations
| AE | = | adverse event |
| AIDS | = | acquired immune deficiency syndrome |
| APC | = | antigen-presenting cell |
| CTL | = | cytotoxic T-lymphocyte |
| HIV | = | human immunodeficiency virus |
| GI | = | gastro-intestinal |
| i.d. | = | intradermal |
| i.m. | = | intramuscularly |
| ISA 51 | = | Montanide ISA™51 |
| MedRA | = | Medical Dictionary for Regulatory Activities |
| SAE | = | serious adverse event |
| s.c. | = | subcutaneously |
| W/O | = | water-in-oil. |
Introduction
Adjuvants are substances which are not antigenic themselves but help to induce strong and efficacious immune responses toward specific antigens.Citation1,2 Adjuvants enhance immune responses by at least one of the following mechanisms: (1) accelerate the onset of an immune response by activating innate immune responses or targeting antigens to professional antigen-presenting cells (APCs); (2) enhance the overall magnitude of an immune response, including the frequency of memory B and T cells; (3) elicit an immune response for a longer duration; (4) skew an immune response toward the desired directions (Th1, Th2, Th17, or balanced Th1/Th2) and (5) modulate the specificity, affinity and isotype of the elicited antibodies. Based on their modes of action, adjuvants can be classified into immune modulators, delivery vehicles or carriers with immune stimulatory effect.Citation1-4
Montanide ISA™ 51 (Seppic, France), a water-in-oil (W/O) emulsion composed of a mineral oil and a surfactant from the mannide monooleate family, is an adjuvant carrier with immune stimulatory effect.Citation2-6 When mixed with antigens in a ratio of 50/50 v/v (1:1), ISA 51 enhances antigen-specific antibody titers and cytotoxic T-lymphocyte (CTL) responses.Citation7 The immune enhancing effect of ISA 51 is suggested to be associated with depot formation (and thus slow the release of antigens at the immunization site), inflammation (which stimulates the recruitment of APCs) and lymphocyte-trapping (which stimulates the accumulation of lymphocytes in draining lymph nodes).Citation8,9 It has been given to thousands of subjects subcutaneously (s.c.) or intramuscularly (i.m.) in vaccine trials requiring cellular immune responses, such as vaccine trials for cancer, human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) and malaria.Citation5,6 A therapeutic lung cancer vaccine containing ISA 51 as emulsion adjuvant has been recently licensed in Cuba.Citation10 Recently, ISA 51 was also tested in influenza vaccine trials.Citation11,12
The inclusion of a vaccine adjuvant should be justified by a favorable risk/benefit ratio; the risk including local and systemic Adverse Events (AEs).Citation3 To our knowledge, this is the first systematic review on the assessment of the safety and tolerability of ISA 51 and ISA 51-adjuvanted vaccines.
Results
Search results and study selection
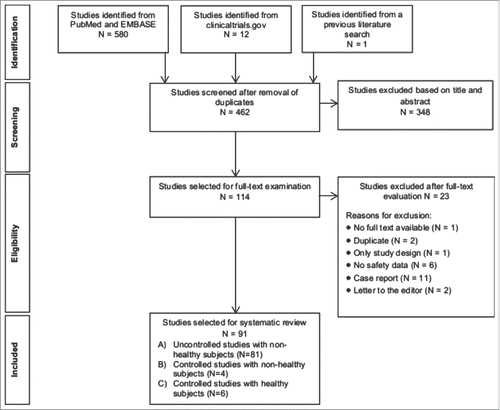
A systematic literature search performed on 9th February 2014 identified 580 studies from PubMed and EMBASE (). Additional, 12 trials on ISA 51-adjuvanted vaccine were identified from clinicaltrials.gov and one study obtained from a previous literature search on universal influenza vaccines. After removing duplicate entries, 462 studies were evaluated for inclusion based on the title and/or abstract, after which 114 potentially relevant studies were included for full-text examination; the majority of excluded studies were reviews or animal studies and 6 studies were not reported in English. From the 114 studies, full-text was unavailable for one studyCitation13, 2 studies were duplicates published under different titlesCitation14,15, one study only described the study designCitation16 and 6 studies did not report safety, tolerability and/or toxicity resultsCitation7,17-21. These together with 11 case reportsCitation22-32 and 2 letters to the editor were excludedCitation33,34. Ultimately, a total of 91 studies were included in the systemic safety review. The full list of the 91 eligible studies including citations is provided in Supplemental Table 1.
Characteristics of included studies
Uncontrolled studies with non-healthy subjects
Of the 91 studies, 81 studies were conducted in non-healthy subjects without a control group. Except for the study by Pinto et al. which was conducted in HIV-infected subjects Citation35, all studies were conducted in cancer patients. Among these studies, 14% were pilot studies (11/81), 38% were phase I trials (31/81), 12% were phase I/II trials (10/81), 30% were phase II trials (24/81) and 2% were phase III trials (2/81) - 3 studies (4%) did not specify the trial phase. Among the trials, different administration routes were used, including s.c. (56/81, 69%), i.m. (5/81, 6%), intradermal (i.d.) (10/81, 12%), intracutaneous (2/81, 2%) and a combination of i.d. and s.c. (8/81, 10%).
Controlled studies with non-healthy subjects
Four studies were conducted in non-healthy adults (aged ≥18 years) and included control group(s). In the phase Ib trial of Boffito et al., HIV-positive males were randomized to receive a single s.c. administration of HIV vaccines at different doses, alone or adjuvanted with ISA 51.Citation36 The study also included placebo (saline only) and adjuvant (ISA 51) controls. In the phase I trial of Sabbatini et al. and phase II trials of Goldinger et al. and NCT00273910, cancer patients were administered with multiple dosages of cancer vaccines with or without ISA 51.Citation37-39 Sabbatini et al. included ovarian cancer patients, while both Goldinger et al. and NCT00273910 included melanoma patients. All subjects in Sabbatini et al. received s.c. administrations. The same route was used for the administration of ISA 51-adjuvatend cancer vaccines in the other 2 trials. In Goldinger et al. and NCT00273910, the active control group (containing the same vaccine antigen but without ISA 51 or with a licensed adjuvant) received plain vaccine antigens given by the intranodal or the i.d. route respectively. The main characteristics of the 4 studies are summarized in . In total the studies included 212 non-healthy subjects of whom 50 were treated with vaccine antigens only (arm 1) and 77 were treated with ISA 51-adjuvanted cancer vaccines (arm 2 and also arm 3 for NCT00273910). In addition, 11 subjects received saline buffer alone or emulsified with ISA 51 in the trial of Boffito et al., and 74 subjects received the non- or ISA 51-adjuvanted antigen with imiquimod or poly ICLC in the other studies. The methodological quality of 3 of the 4 trials was judged to be poor with Jadad scores of 1, 1 and 2 for the trial performed by Goldinger et al., Sabbatini et al. and NCT00273910, respectively (; Table S2). Only the study performed by Boffito et al. was of good quality and reached a Jadad score of 4.
Table 1. Main characteristics of the controlled studies with non-healthy subjects
Controlled studies with healthy subjects
Six trials were conducted in healthy adults (aged ≥18 years) and included control group(s).Citation11,12,40-43 I.m. administration was used in 5 studies, Pleguezuelos et al. used s.c. administration. The phase I trials from Graham et al., Herrera et al. and Wu et al. included an adjuvant (ISA51) control group. Subjects in the Graham et al. study were randomized to receive a cocktail of HIV peptides (1 mg or 4 mg) adjuvanted with ISA 51 or ISA 51 emulsion alone. Herrera et al. and Wu et al. both tested a malaria antigen. Subjects were randomized to receive ISA 51 emulsion only or a mixture of synthetic peptides (50 µg or 100 µg per peptide) or one of 2 recombinant surface proteins (Pvs25 or Pfs25) adjuvanted with ISA 51, respectively.Citation41,43 Subjects in the Herrera et al. study were also randomized to receive the tested antigen adjuvanted with Montanide ISA 720Citation41. The phase I/II trial by Atsmon et al. and the phase I trial from Pleguezuelos et al. randomized subjects to receive a placebo, ISA51 emulsion only, or different doses of influenza antigens (250 µg or 500 µg of recombinant protein or polypeptides harboring diverse influenza B and T cell epitopes) with or without ISA 51.Citation11,12 In the study of Atsmon et al. 2 immunizations were given and in the study of Pleguezuelos et al. one immunization was given. Subjects in the phase I study of Pialoux et al. all received 2 dosages of vCP125 antigens before they were randomized to receive 2 dosages of rgp160 antigens adjuvanted with alum or ISA 51.Citation42 The main study characteristics of the 6 studies are summarized in . In total there were 231 healthy adults of which 53 were treated with vaccine antigens alone or with a licensed vaccine adjuvant (arm 1 for Atsmon et al., Pialoux et al. and Pleguezelos et al.), 31 with only ISA 51 emulsion (arm 2 or 3) and 117 were treated with ISA 51-adjuvanted vaccines. In addition, 14 subjects received saline buffer only and 16 subjects received ISA 720-adjuvanted vaccines. Except for the trial performed by Pialoux et al. which has a Jadad score of 2, all the other trials had a score ≥ 3 (; Table S3).
Table 2. Main characteristics of the controlled studies with healthy subjects
Adverse events
Uncontrolled studies with non-healthy subjects
The AE reporting methods vary among the identified trials. For those where specific AEs were reported, commonly reported AEs include injection site reaction, fatigue, fever, gastro-intestinal (GI) disorders including diarrhea, nausea and vomiting, and injection site or local erythema. Most of the observed AEs were not immunization route specific (). Nevertheless, chills were more commonly reported in trials using the i.m. route (80%), and headache was more commonly reported in trials using other administration route(s) (40%). The severity of reported AEs were mainly mild (grade I) to moderate (grade II). Severe (grade III) AEs, such as fever, headache, fatigue, nausea, hyperglycemia, neutropenia, were reported in 32% of the trials (26/81); 27% of the trials (22/81) reported serious (grade IV) AEs (SAEs), such as neutropenia, hyperglycemia, hepatic toxicity and renal failure. A possible cause due to co-intervention (e.g., GM-CSF, IL-2 or gemcitabine) and/or underlying disease could not be excluded.
Table 3. Frequency of commonly reported adverse events from uncontrolled studies with non-healthy subjects and the frequency of serious adverse events
Controlled studies with non-healthy subjects
In the study of Boffito et al. a total of 476 AEs were reported from different treatment group with equal frequency. Most of the reported AEs were mild. Moderate and severe AEs were more often reported from the study groups receiving a high antigen dose. Boffito et al. identified five SAEs, namely gastroenteritis, Kaposi's sarcoma, perinanal abscess, Shigellosis and lower abdominal pain, which were all considered not to be caused by the HIV vaccine. The safety reporting system used by Boffito et al. did not allow us to extract information on the number of subjects experiencing a specific AE and the total number of AEs experienced in the study. As stated by Boffito et al. the most frequent AEs experienced in this study were injection site pain (12,8%), headache (9,2%) and fatigue (9%).Citation36
The cancer vaccine trials performed by Goldinger et al., Sabbatini et al. and NCT00273910 included an active control group and reported the number of subjects experiencing a specific AE after the study treatment. The study from Goldinger et al. only reported the occurrence of AEs with an incidence >3 while studies from Sabbatini et al. and NCT00273910 both reported the occurrence of all AEs. summarizes the AEs reported in at least 2 of these trials. In general, treatment related AEs were more often observed in the ISA 51-adjuvanted vaccine group than in the active control group. Specific AEs including injection site reaction, pruritus, myalgia and skin and subcutaneous tissue disorders were more commonly reported from the ISA 51-adjuvanted vaccine group. Other AEs reported only by a single study include injection site rash, pain and erythema, skin neoplasm excision, skin nodule, oral herpes, sweating, decreased serum phosphate, hypersensitivity, vomiting, nausea and diarrhea. Notably, most of the AEs reported by subjects in the NCT00273910 trial were reported from subjects receiving the ISA 51-adjuvanted study vaccine prepared by vortex. In this trial, 19 and 14 cancer patients received ISA 51-ajduvanted study antigens prepared by vortex and 2-syringe mixing respectively. All patients receiving the adjuvanted study antigens developed injection site reaction regardless of the preparation process. However, AEs such as leukocyte count decrease, GI disorders, chills, fatigue, sweating and myalgia were only reported from the group receiving the vaccine prepared by vortex mixing. In addition, pruritus was reported in 5 and one patient(s) in the group of vortex and 2-syringe mixing, respectively. Rash was reported in 2 and one patient(s) in the 2 treatment groups, respectively.
Table 4. Frequency of adverse events reported in at least 2 controlled studies with non-healthy subjects
While the severity of the AEs reported from NCT00273910 was not available, most of the reported AEs reported from Goldinger et al. and Sabbatini et al. were mild or moderate. SAEs were only reported by Goldinger et al., which were 6 hospitalizations due to tumor progression. None of them was considered related to the study treatment.
Controlled studies with healthy subjects
In the trial of Graham et al., the frequency of the subjects experiencing a specific AE was not given. AEs were classified as local (including pain, tenderness, erythema and induration) or systemic (including malaise, myalgia, headache, fever and nausea) and were graded by severity.Citation40 Most of the reported AEs were mild. Moderate AEs were more commonly reported from the high antigen dose group (where the amount of ISA 51 was the same as in the low antigen dose group). Severe (systemic) AEs were only reported from the high dose antigen group after the second immunization. Subject dropouts were reported from those receiving the ISA 51-adjuvanted HIV vaccines, at both low and high antigen doses. The dropouts were caused by the painful swelling which occurred after the first immunization. The trial was prematurely terminated (after the second immunization) due to the development of sterile abscesses at the injection site in 4 out of the 21 subjects (19,0%) who received the ISA 51-adjuvanted vaccine. None of subjects in the adjuvant control (ISA 51 in saline buffer) developed sterile abscess and AEs above an intensity of mild.
Sterile abscess was also observed in the trial performed by Wu et al. of which the number of volunteers in each vaccination group experiencing a solicited AE was reported. One out of the first 30 (3,3%) enrollees from the ISA 51-adjuvanted vaccine group reported sterile abscess. Aside from frequent occurrence of injection site pain, subjects in both the ISA 51-adjuvanted vaccine groups and adjuvant control group experienced local pain, tenderness and erythema, headache, malaise, nausea and leukopenia. AEs only reported by subjects in the ISA 51-adjuvanted vaccine group included local induration and swelling, arthralgia and myalgia. Although most of the reported AEs were mild, Wu et al. observed severe (grade III) systemic AEs in the group of high Pvs25 antigen dose adjuvanted with ISA 51. In addition, erythema nodosum (an inflammatory panniculitis) were reported from 2 out of the 10 subjects (20,0%) receiving the high dose Pvs25 malaria antigen adjuvanted with ISA 51. The erythema nodosum reactions were judged to be probably related to vaccination and were the cause for the premature termination of the study. Wu et al. observed no significant differences in the incidence of AEs observed after the first and the second immunization. None of the participants in the adjuvant control groups developed AEs above moderate.Citation43
The prophylactic vaccine trials performed by Atsmon et al., Herrera et al. and Pialoux et al. reported the number of AEs after each immunization Citation11,41,42. For the trial of Pleguezuelos et al., only the number of systemic AEs that occurred after a single injection could be extracted. summarizes the reported AEs from the ISA 51-adjuvanted vaccine group and the different control groups which were reported in at least 2 of the 4 trials. The reported treatment related AEs were in general evenly distributed between the ISA 51-adjuvanted vaccine groups, the active control groups and the adjuvant control groups. Only injection site pain or tenderness were more commonly reported in the ISA 51-adjuvanted vaccine group compared to the control groups. Other AEs reported only by a single study include local swelling and erythema, redness, warmth, fatigue, rhinorrhea/nasal congestion, diarrhea, dizziness, abdominal pain, adenopathy, asthenia and malaise. The AEs reported by the 4 studies were classified as mild and moderate. Only Pleguezuelos et al. and Herrera et al. reported AEs with an intensity above moderate; 2 severe AEs (appendicitis and pre-syncope) and 2 cases of SAEs (chronic cholecysitis and acute urolithiasis), respectively. In both studies these events were considered not directly caused by the vaccine.Citation12,41
Table 5. Frequency of adverse events reported in at least 2 controlled studies with healthy subjects
Discussion
We have assessed the safety and tolerability of ISA 51 as a vaccine adjuvant. A total of 91 clinical trials on ISA 51-adjuvanted vaccines which provided information on safety, tolerability and/or toxicity were reviewed. Of these 91 trials, 81 were uncontrolled studies with non-healthy subjects, 4 were controlled studies with non-healthy subjects and 6 were controlled studies with healthy subjects.
Commonly reported AEs from uncontrolled trials included fever, fatigue, GI disorders, injection site erythema and injection site reaction. These AEs were also reported from controlled trials. For controlled trials with non-healthy subjects the frequency of AEs was generally higher in the ISA 51-adjuvanted vaccine group compared with the active control group. However, for controlled studies with healthy subjects the reported AEs were in general evenly distributed between the treatment groups except for injection site pain or tenderness. These AEs were more commonly reported in the ISA 51-adjuvanted vaccine group compared to the active and adjuvant control groups. Since the immune response and the sensitivity to pain and discomfort can be expected to be different between non-healthy and healthy subjects, we classified the reviewed studies into 2 subject backgrounds. However, the reported AEs were found to be neither subject specific nor immunization route specific.
In general, the observed AEs from controlled trials with non-healthy as well as healthy individuals were mild to moderate in intensity. However, it should be noted that 2 controlled studies on ISA 51-adjuvanted vaccines in healthy subjects were terminated prematurely due to unacceptable AEs.Citation40,43 The study of Wu et al. was stopped after the 2 reported cases of erythema nodosum, which was not observed from other ISA 51-adjuvanted vaccine studies. The trial by Graham et al. was stopped after 4 cases of sterile abscess, which was also reported by one subject who had erythema nodosum in the trial of Wu et al. and by subjects in 2 other uncontrolled therapeutic vaccine studies in breast cancer patients. In the study of Carr et al., one of the 21 patients (4,8%) was reported to develop a small abscess after receiving the ISA 51-adjuvanted cancer vaccine.Citation44 The same vaccine was later used in the trial by Mulens et al. where two of the 38 patients (5,3%) who received the ISA 51-adjuvanted vaccine developed severe abscess after the vaccination.Citation45 Since none of the subjects in the adjuvant control group of Graham et al. and Wu et al. was reported to have erythema nodosum, sterile abscess, and/or AEs with an intensity above mild, it is unlikely that the unwanted side effects were caused by ISA 51 alone. Wu et al. noted that the same production lot of malaria Pvs25 antigen was used to produce alum-adjuvanted Pvs25 vaccine, and was shown to be well tolerated in humans.Citation46 This ruled out the possibility that the unacceptable AEs were caused by the antigen alone. It is therefore most likely that the AEs were caused by the combination of ISA 51 and vaccine antigens. Thus, the mixing procedure for ISA 51 emulsified adjuvant and antigens and other factors involved in the final presentation of the combined product (e.g., antigen dose, dose volume, etc.) should be investigated as possible cause(s) for the observed serious AEs.
The effect of the method used for mixing ISA 51 and vaccine antigen was particularly addressed in the trial NCT00273910. In this trial, subjects receiving the ISA 51-adjuvanted study vaccine prepared by vortex experienced more AEs, e.g., pruritus, rash, GI disorders, chills, fatigue and myalgia, compared to subjects receiving the study vaccine by 2-syringe mixing. The increased frequency of AEs observed from the vortex mixing group is likely resulted from the poor quality of the mixed ISA 51/antigen emulsions. Vortex mixing probably did not generate enough shear strength to produce stable emulsions.Citation5 With unstable emulsions, coalescence and change in the droplet size will occur. The antigen is thus no longer entrapped but readily released into the injected environment. Moreover, the escaped mineral oils might cause unwanted local or systemic side effects. Therefore, mixing by syringe is recommended for small volume preparations to obtain homogenous and stable emulsions.Citation5
To properly address the effect of ISA 51 on vaccine safety and tolerability, the AEs reported from ISA 51-adjuvanted vaccine need to be compared to those from an active control group. The inclusion of an active control group, however, was found to be rare among the reviewed studies. This might be due to ethical consideration (e.g. it may be considered unethical to give patients suboptimal treatments) or due to lack of a scientific interest. Only seven out of the 91 reviewed studies has an active control group. This limited the power to detect statistically significant differences between the ISA 51-adjuvanted vaccine group and the active control group. Therefore, the incidence of AEs could only be compared in a descriptive way.
This review has several limitations. AEs reported by Goldinger et al. were pre-selected and only those with an AE incidence of more than 3 in the study population were presented. Therefore, the AEs with an incidence of zero in for the study of Goldinger et al. may not reflect the real situation. Besides, 5 of the 11 controlled studies (45,5%) included for the review had a low methodological quality based on the Jadad scores. Except the trials performed by Boffito et al., Graham et al., Herrera et al. and Pleguezelos et al. the other included controlled studies might have detection and performance biases since there were no blinding procedures for the study participants, personnel and the investigators.Citation47 Such potential biases might result in giving more attention to those study participants that receive the ISA 51-adjuvanted vaccines. This may have resulted in an overestimation of (S)AEs in the participants receiving an ISA 51-adjuvanted vaccine. Furthermore, 3 of the 6 controlled trials with non-healthy subjects may be subject to selection bias since no randomization procedure was mentioned. However, it is not clear to us what the likely direction the potential selection bias may cause.Citation47 Last but not least, different AE classifications and reporting systems used by the reviewed studies limited the possibility to present a complete overview of the occurrence and the frequency of AEs experienced by subjects who received ISA 51-adjuvanted vaccines. This could have led to an underestimation of the AEs, especially for the uncontrolled trials.
Conclusion
AEs are frequently reported among trials testing ISA 51-adjuvanted antigens including non-healthy subjects as well as healthy subjects. In addition, SAEs are frequently reported among studies with non-healthy subjects and 2 trials testing ISA 51-adjuvanted prophylactic vaccines with healthy subjects are stopped due to unacceptable AEs which were most likely caused by the combination of ISA 51 adjuvant and the vaccine antigens. Studies indicate that the mixing procedure of the study antigen with ISA 51 might influence the occurrence of AEs. Therefore, for future trials caution should be taken when mixing ISA 51 and the vaccine antigen, especially with regards to small volumes, in order to produce stable emulsions and to prevent unwanted side effects. In addition, trials including an active control group are urgently needed for a fair evaluation of adjuvant safety and tolerability and adjuvant effect on immune boosting, preferably using a standardized reporting system for vaccine related AEs such as the MedRA reporting system.
Materials and Methods
Search strategy
Vaccine trials using ISA 51 adjuvant were systematically searched. Published medical studies were obtained from searches of PubMed and EMBASE with the free-text term “ISA 51”OR“ISA51” and ‘ISA 51’OR‘ISA51’, respectively. References from the databases were extracted and imported to RefWorks where duplicate entries were removed. The clinical trial register (clinicaltrials.gov) was hand searched for unpublished trials for which study results were available. References given by the trials identified from clinicaltrials.gov were examined for relevancy. No protocol is registered for the systematic review.
Study selection
Title and abstract of the extracted references were screened for relevant studies. Studies were included if: (1) they were clinical trials; (2) they used ISA 51 as vaccine adjuvant; and (3) they were reported in English. Comment letters, reviews and conference abstracts and/or presentations were excluded. Full-text articles from the resulting pre-screened studies were obtained for the second stage evaluation. Studies were excluded if (1) they did not report safety, tolerability and/or toxicity data, (2) they were case reports or (3) letters to an editor, and (4) full-text was not available from the University Medical Center Groningen library. The screening and full-text evaluation procedures were performed independently by 2 members of the study team. Consensus on study selection was achieved. In case of disagreement, a third member of the study team made the final decision.
Data extraction
Data on study design, study population, study treatments (including trial vaccine and administration route), study endpoints and safety, tolerability and/or toxicity outcomes were extracted from the selected studies by one study team member. The extracted information was independently validated by a second team member. Trials included in the systematic review were categorized by the following subject characteristics and study design: (A) uncontrolled studies with non-healthy subjects, (B) controlled studies with non-healthy subjects and (C) controlled studies with healthy subjects. A control group could be an active control (immunization with the tested antigens without adjuvants or with adjuvants used in licensed vaccines, e.g., alum, MF-59, AS03 and AS04); a placebo-control (immunization with saline buffer); or an adjuvant control (immunization with ISA 51 in saline buffer).
Quality assessment
The methodological quality of the controlled studies selected for the review was evaluated by the Jadad score. The scoring system judged the procedure and description of randomization (0, 1 or 2 point), double-blinding (0, 1 or 2 point) and the description of withdrawals and drop outs (0 or 1 point). Using this scoring system a maximum score of 5 could be obtained; a score of ≤2 was considered as indicating poor methodological quality.Citation48
Disclosure of Potential Conflicts of Interest
This review has been written in the context of trial preparations with an ISA™51-adjuvanted universal influenza vaccine (FLU-v, SEEK, London).
Supplemental_Material.zip
Download Zip (30.5 KB)Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
This study is funded by the European Commission DG-Research and the European Member States funded Universal Influenza Vaccines Secured (UNISEC) project (FP7-Health No. 602012).
References
- WHO. Annex 1 - guidelines for the production and quality control of synthetic peptide vaccines. WHO Technical Report Studies 1999; 889:24-43
- Wilson-Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B. Vaccine adjuvants: Current challenges and future approaches. J Pharm Sci 2009; 98:1278-316; PMID:18704954; http://dx.doi.org/10.1002/jps.21523
- European Medicines Agency. Committee for medicinal products for human use guideline on adjuvants in vaccines for human use. 2004; Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/11/WC500015469.pdf
- Cox JV, Coulter AR. Adjuvants - a classification and review of their modes of action. Vaccine 1997; 15:248-56; PMID:9139482; http://dx.doi.org/10.1016/S0264-410X(96)00183-1
- Aucouturier J, Dupuis L, Deville S, Ascarateil S, Ganne V. Montanide ISA 720 and 51: A new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines 2002; 1:111-8; PMID:12908518; http://dx.doi.org/10.1586/14760584.1.1.111
- Seppic. Montanide ISA 51 VG [Internet]. Paris (France): Seppic [cited 12 February 2014]. Available from http://www.seppic.com/human-health/vaccine390adjuvant/montanide-isa-@/1018/view-1042-seproduit.html.
- Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Teates D, Neese P, Grosh WW, Petroni G, et al. Evaluation of peptide vaccine immunogenicity in draining lymph nodes and peripheral blood of melanoma patients. Int J Cancer 2001; 92:703-11; PMID:11340576; http://dx.doi.org/10.1002/1097-0215(20010601)92:5%3c703::AID-IJC1250%3e3.0.CO;2-5
- Kaeberle M. Function of carriers and adjuvants in induction of immune responses. In: Nervig R, Gough P, Kaeberle M, Whetstone C, eds. Carriers and Adjuvants for Veterinary Biologics. USA: Iowa State University Press, 1986:11-23.
- Aucouturier J, Dupuis L, Ganne V. Adjuvants designed for veterinary and human vaccines. Vaccine 2001; 19:2666-72; PMID:11257407; http://dx.doi.org/10.1016/S0264-410X(00)00498-9
- Fox CB, Haensler J. An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants. Expert Rev Vaccines 2013; 12:747-58; PMID:23885820; http://dx.doi.org/10.1586/14760584.2013.811188
- Atsmon J, Kate-Ilovitz E, Shaikevich D, Singer Y, Volokhov I, Haim KY, Ben-Yedidia T. Safety and immunogenicity of multimeric-001 - A novel universal influenza vaccine. J Clin Immunol 2012; 32:595-603; PMID:22318394; http://dx.doi.org/10.1007/s10875-011-9632-5
- Pleguezuelos O, Robinson S, Stoloff GA, Caparros-Wanderly W. Synthetic influenza vaccine (FLU-v) stimulates cell mediated immunity in a double-blind, randomized, placebo-controlled phase I trial. Vaccine 2012; 30:4655-60; PMID:22575166; http://dx.doi.org/10.1016/j.vaccine.2012.04.089
- Gringeri A, Santagostino E, Muca-Perja M, Mannucci PM, Zagury JF, Bizzini B, Lachgar A, Carcagno M, Rappaport J, Criscuolo M, et al. Safety and immunogenicity of HIV-1 tat toxoid in immunocompromised HIV-1-infected patients. J Hum Virol 1998; 1:293-8; PMID:10195254
- Slingluff Jr. CL, Lee S, Zhao F, Chianese-Bullock KA, Olson WC, Butterfield LH, Whiteside TL, Leming PD, Kirkwood JM. A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602). Clin Cancer Res 2013; 19:4228-38; PMID:23653149; http://dx.doi.org/10.1158/1078-0432.CCR-13-0002
- Melanoma Study Group of the Mayo Clinic Cancer Center, Celis E. Overlapping human leukocyte antigen class I/II binding peptide vaccine for the treatment of patients with stage IV melanoma: Evidence of systemic immune dysfunction. Cancer 2007; 110:203-14; PMID:17541944; http://dx.doi.org/10.1002/cncr.22744
- Georgoulias V, Douillard J-, Khayat D, Manegold C, Rosell R, Rossi A, Menez-Jamet J, Iche M, Kosmatopoulos K, Gridelli C. A multicenter randomized phase IIb efficacy study of vx-001, a peptide-based cancer vaccine as maintenance treatment in advanced non-small-cell lung cancer: Treatment rationale and protocol dynamics. Clin Lung Cancer 2013; 14:461-5; PMID:23647738; http://dx.doi.org/10.1016/j.cllc.2013.02.001
- Chianese-Bullock KA, Pressley J, Garbee C, Hibbitts S, Murphy C, Yamshchikov G, Petroni GR, Bissonette EA, Neese PY, Grosh WW, et al. MAGE-A1-, MAGE-A10-, and gp100-derived peptides are immunogenic when combined with granulocyte-macrophage colony-stimulating factor and montanide ISA-51 adjuvant and administered as part of a multipeptide vaccine for melanoma. J Immunol 2005; 174:3080-6; PMID:15728523; http://dx.doi.org/10.4049/jimmunol.174.5.3080
- Harris RC, Chianese-Bullock KA, Petroni GR, Schaefer JT, Brill II LB, Molhoek KR, Deacon DH, Patterson JW, Slingluff Jr. CL. The vaccine-site microenvironment induced by injection of incomplete freund's adjuvant, with or without melanoma peptides. J Immunother 2012; 35:78-88; PMID:22130163; http://dx.doi.org/10.1097/CJI.0b013e31823731a4
- Miller JS, Curtsinger J, Berthold M, Malvey K, Bliss RL, Le CT, Fautsch SK, Dudek AZ, Blazar BR, Panoskaltsis-Mortari A. Diminished neo-antigen response to keyhole limpet hemocyanin (KLH) vaccines in patients after treatment with chemotherapy or hematopoietic cell transplantation. Clin Immunol 2005; 117:144-51; PMID:16112616; http://dx.doi.org/10.1016/j.clim.2005.07.005
- Revicki DA, van den Eertwegh AJM, Lorigan P, Lebbe C, Linette G, Ottensmeier CH, Safikhani S, Messina M, Hoos A, Wagner S, et al. Health related quality of life outcomes for unresectable stage III or IV melanoma patients receiving ipilimumab treatment. Health Qual Life Outcomes 2012; 10:66; http://dx.doi.org/10.1186/1477-7525-10-66
- Salerno EP, Shea SM, Olson WC, Petroni GR, Smolkin ME, McSkimming C, Chianese-Bullock KA, Slingluff Jr. CL. Activation, dysfunction and retention of T cells in vaccine sites after injection of incomplete freund's adjuvant, with or without peptide. Cancer Immunol Immunother 2013; 62:1149-59; PMID:23657629; http://dx.doi.org/10.1007/s00262-013-1435-5
- Eikawa S, Kakimi K, Isobe M, Kuzushima K, Luescher I, Ohue Y, Ikeuchi K, Uenaka A, Nishikawa H, Udono H, et al. Induction of CD8 T-cell responses restricted to multiple HLA class i alleles in a cancer patient by immunization with a 20-mer NY-ESO-1f (NY-ESO-1 91-110) peptide. Int J Cancer 2013; 132:345-54; PMID:22729530; http://dx.doi.org/10.1002/ijc.27682
- Oka Y, Tsuboi A, Murakami M, Hirai M, Tominaga N, Nakajima H, Elisseeva OA, Masuda T, Nakano A, Kawakami M, et al. Wilms tumor gene peptide-based immunotherapy for patients with overt leukemia from myelodysplastic syndrome (MDS) or MDS with myelofibrosis. Int J Hematol 2003; 78:56-61; PMID:12894852; http://dx.doi.org/10.1007/BF02983241
- Saitoh A, Narita M, Watanabe N, Tochiki N, Yamahira A, Nakamura T, Kaji M, Masuko M, Furukawa T, Toba K, et al. WT1 peptide vaccination in a CML patient: Induction of effective cytotoxic T lymphocytes and significance of peptide administration interval. Med Oncol 2011; 28:219-30; PMID:20107936; http://dx.doi.org/10.1007/s12032-010-9425-3
- Shirakata T, Oka Y, Nishida S, Hosen N, Tsuboi A, Oji Y, Murao A, Tanaka H, Nakatsuka S, Inohara H, et al. WT1 peptide therapy for a patient with chemotherapy-resistant salivary gland cancer. Anticancer Res 2012; 32:1081-5; PMID:22399636
- Takahashi N, Ohkuri T, Homma S, Ohtake J, Wakita D, Togashi Y, Kitamura H, Todo S, Nishimura T. First clinical trial of cancer vaccine therapy with artificially synthesized helper/killer-hybrid epitope long peptide of MAGE-A4 cancer antigen. Cancer Sci 2012; 103:150-3; PMID:22221328; http://dx.doi.org/10.1111/j.1349-7006.2011.02106.x
- Tsuboi A, Oka Y, Nakajima H, Fukuda Y, Elisseeva OA, Yoshihara S, Hosen N, Ogata A, Kito K, Fujiki F, et al. Wilms tumor gene WT1 peptide-based immunotherapy induced a minimal response in a patient with advanced therapy-resistant multiple myeloma. Int J Hematol 2007; 86:414-7; PMID:18192109; http://dx.doi.org/10.1007/BF02983998
- Tsuboi A, Oka Y, Osaki T, Kumagai T, Tachibana I, Hayashi S, Murakami M, Nakajima H, Elisseeva OA, Fei W, et al. WT1 peptide-based immunotherapy for patients with lung cancer: Report of two cases. Microbiol Immunol 2004; 48:175-84; PMID:15031530; http://dx.doi.org/10.1111/j.1348-0421.2004.tb03503.x
- Valmori D, Dutoit V, Ayyoub M, Rimoldi D, Guillaume P, Lienard D, Lejeune F, Cerottini J-, Romero P, Speiser DE. Simultaneous CD8+ T cell responses to multiple tumor antigen epitopes in a multipeptide melanoma vaccine. Cancer Immun 2003; 3:1-14
- Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother 2006; 55:1294-8; PMID:16315030; http://dx.doi.org/10.1007/s00262-005-0102-x
- Alvarez MC, Macias AA, Saurez MG, Fernandez ML, Lage DA. Bronchial carcinoid tumor treated with interferon and a new vaccine against NeuGcGM3 antigen expressed in malignant carcinoid cells. Cancer Biol Ther 2007; 6:853-5; PMID:17611391; http://dx.doi.org/10.4161/cbt.6.6.4186
- Oji Y, Oka Y, Nishida S, Tsuboi A, Kawakami M, Shirakata T, Takahashi K, Murao A, Nakajima H, Narita M, et al. WT1 peptide vaccine induces reduction in minimal residual disease in an imatinib-treated CML patient. Eur J Haematol 2010; 85:358-60; PMID:20633041; http://dx.doi.org/10.1111/j.1600-0609.2010.01497.x
- Maeda T, Hosen N, Fukushima K, Tsuboi A, Morimoto S, Matsui T, Sata H, Fujita J, Hasegawa K, Nishida S, et al. Maintenance of complete remission after allogeneic stem cell transplantation in leukemia patients treated with wilms tumor 1 peptide vaccine. Blood Cancer J 2013; 3:e130; PMID:23912610; http://dx.doi.org/10.1038/bcj.2013.29
- Maslak PG, Dao T, Gomez M, Chanel S, Packin J, Korontsvit T, Zakhaleva V, Pinilla-Ibarz J, Berman E, Scheinberg DA. A pilot vaccination trial of synthetic analog peptides derived from the BCR-ABL breakpoints in CML patients with minimal disease. Leukemia 2008; 22:1613-6; PMID:18256684; http://dx.doi.org/10.1038/leu.2008.7
- Pinto LA, Berzofsky JA, Fowke KR, Little RF, Merced-Galindez F, Humphrey R, Ahlers J, Dunlop N, Cohen RB, Steinberg SM, et al. HIV-specific immunity following immunization with HIV synthetic envelope peptides in asymptomatic HIV-infected patients. AIDS 1999; 13:2003-12; PMID:10546852; http://dx.doi.org/10.1097/00002030-199910220-00002
- Boffito M, Fox J, Bowman C, Fisher M, Orkin C, Wilkins E, Jackson A, Pleguezuelos O, Robinson S, Stoloff GA, et al. Safety, immunogenicity and efficacy assessment of HIV immunotherapy in a multi-centre, double-blind, randomised, placebo-controlled phase ib human trial. Vaccine 2013; 31:5680-6; PMID:24120550; http://dx.doi.org/10.1016/j.vaccine.2013.09.057
- Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, Jungbluth AA, Ritter G, Aghajanian C, Bell-McGuinn K, et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res 2012; 18:6497-508; PMID:23032745; http://dx.doi.org/10.1158/1078-0432.CCR-12-2189
- Goldinger SM, Dummer R, Baumgaertner P, Mihic-Probst D, Schwarz K, Hammann-Haenni A, Willers J, Geldhof C, Prior JO, Kundig TM, et al. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8+ T-cell responses in melanoma patients. Eur J Immunol 2012; 42:3049-61; PMID:22806397; http://dx.doi.org/10.1002/eji.201142361
- National Cancer Institute. Evaluation of the impact of adjuvants accompanying peptide immunization in high-risk melanoma (NCT00273910). United Staes of America: U.S. National Institutes of Health [cited 15 February 2014]. Available from https://clinicaltrials.gov/ct2/show/NCT00273910?term=NCT00273910&rank=1.
- Graham BS, Mcelrath MJ, Keefer MC, Rybczyk K, Berger D, Weinhold KJ, Ottinger J, Ferarri G, Montefiori DC, Stablein D, et al. Immunization with cocktail of HIV-derived peptides in montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity. PLoS ONE 2010; 5:e11995; http://dx.doi.org/10.1371/journal.pone.0011995
- Herrera S, Fernandez OL, Vera O, Cardenas W, Ramirez O, Palacios R, Chen-Mok M, Corradin G, Arevalo-Herrera M. Phase I safety and immunogenicity trial of plasmodium vivax CS derived long synthetic peptides adjuvanted with montanide ISA 720 or montanide ISA 51. Am J Trop Med Hyg 2011; 84:12-20; PMID:21292873; http://dx.doi.org/10.4269/ajtmh.2011.09-0516
- Pialoux G, Excler J-, Riviere Y, Gonzalez-Canali G, Feuillie V, Coulaud P, Gluckman J-, Matthews TJ, Meignier B, Kieny M-, et al. A prime-boost approach to HIV preventive vaccine using a recombinant canarypox virus expressing glycoprotein 160 (MN) followed by a recombinant glycoprotein 160 (MN/LAI). AIDS Res Hum Retroviruses 1995; 11:373-81; PMID:7598771; http://dx.doi.org/10.1089/aid.1995.11.373
- Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and pvs 25 formulated with montanide ISA 51. PLoS ONE 2008; 3:e2636; http://dx.doi.org/10.1371/annotation/c1aa88dd-4360-4902-8599-4d7edca79817
- Carr A, Rodriguez E, Arango MDC, Camacho R, Osorio M, Gabri M, Carrillo G, Valdes Z, Bebelagua Y, Perez R, et al. Immunotherapy of advanced breast cancer with a heterophilic ganglioside (NeuGcGM3) cancer vaccine. J Clin Oncol 2003; 21:1015-21; PMID:12637465; http://dx.doi.org/10.1200/JCO.2003.02.124
- Mulens V, De La Torre A, Marinello P, Rodriguez R, Cardoso J, Diaz R, O'Farrill M, Macias A, Viada C, Saurez G, et al. Immunogenicity and safety of a NeuGcGM3 based cancer vaccine: Results from a controlled study in metastatic breast cancer patients. Hum Vaccines 2010; 6:736-44; http://dx.doi.org/10.4161/hv.6.9.12571
- Malkin EM, Durbin AP, Diemert DJ, Sattabongkot J, Wu Y, Miura K, Long CA, Lambert L, Miles AP, Wang J, et al. Phase 1 vaccine trial of Pvs25H: A transmission blocking vaccine for plasmodium vivax malaria. Vaccine 2005; 23:3131-8; PMID:15837212; http://dx.doi.org/10.1016/j.vaccine.2004.12.019
- Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Cochrane Handbook for Systemic Reviews of Interventions [Internet]. Higgins JPT, Green S, eds. [2011, cited 10 December 2014]. Available from http://handbook.cochrane.org/.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996; 17:1-12; PMID:8721797; http://dx.doi.org/10.1016/0197-2456(95)00134-4