Abstract
The central nervous system (CNS) has long been regarded as an immune-privileged site, with the blood-brain barrier (BBB) limiting the entering of systemic immune cells and components. Exposure of low-energy focused ultrasound (FUS) with the presence of microbubbles has been found to provide a temporary and targeted opening of the BBB without inflicting brain damage or inflammation, and is thus an attractive means of delivering CNS therapeutic agents and raising the potential for targeted CNS immunotherapy. Based on our recent studies on enhancing brain-tumor immune-related therapy via this mechanism,Citation1 we summarize current approaches using FUS-induced BBB opening to promote immune regulation and project potential directions for FUS-induced CNS immunotherapy.
Introduction
The blood-brain barrier (BBB) is a specialized structure related to circulation with the capillary lumen in the central nervous system (CNS). It consists of tightly lined endothelial cells forming a tight junction, covered by a thick basement membrane, and is firmly supported by the astrocyte endfeet to cause high electrical resistivity. The BBB is highly selective for molecular penetration between blood circulation and extracellular fluid inside the brain parenchyma (molecules > 400 Da have difficulty penetrating the BBB). More importantly, the BBB restricts the free passage of immune cells into the CNS along with most antigens, thus endogenous CNS antigens cannot be easily detected by systemic immune cells. Therefore, the CNS has long been recognized to be an immune-privileged organ.Citation2,3
Neuroinflammation is a major cause of the BBB disruption, and contributes to undesirable pathological consequences.Citation4 For example, neuroinflammation is an major pathological effect during traumatic brain injury, and plays a key role in secondary brain injuries such as metabolic disturbances and cerebrovascular dysfunction that further increase the likelihood of tissue ischemia and brain edema.Citation5 There is evidence that Alzheimer disease (AD) is highly associated with neuroinflammatory response and there is also evidence that astrocytes and microglia are activated to secrete pro-inflammative cytokines to further worsen AD.Citation6 Previous studies have found that the neurodegeneration found in Parkinson disease is also highly correlated with CNS inflammation,Citation7 and corresponds with excessive immunological activation. To bypass the BBB but not the CNS inflammation route, the main current approach is through direct intracranial injections of immunotherapeutic agents.Citation8,9 A noninvasive, targeted, and transient BBB opening is needed to break the CNS's immune-privileged status to allow for efficient implementation of CNS immunotherapy.
Recent studies have shown that, in the presence of microbubbles, low-energy burst-tone FUS exposure can transiently increase the BBB's permeability.Citation10,11 This BBB-opening induced by FUS exposure is reversible, and does not damage neural cells when the exposure level is well controlled. Compared to alternative approaches such as modified lipophilic chemicals or hypertonic solutions infused through the carotid arteries to enhance chemotherapeutic agent delivery into the brain,Citation10 the advantages of this approach include its entirely noninvasive nature, creating a local BBB-opening that minimizes off-target effects, and the process can be reversed within several hours (offering a suitable time window for drug release). These advantages make the FUS-induced BBB opening a very attractive alternative for increasing local concentrations of therapeutic molecules in CNS.
Previously high-intensity focused ultrasound to induce hyperthermia and thermal ablations for cancer therapies have clinically shown its usefulness in triggering immune response via heat-activated or tissue-necrotic immune triggering routes.Citation12-15 Our previous paper investigated the use of FUS-induced BBB opening to serve as another potential pass way in triggering local adaptive immune response against brain tumor progression,Citation1 the first demonstration that a therapeutically-effective cell number of tumor-infiltrating lymphocytes can be directed to a tumor without impacting the systemic immune response.Citation1 Together with this finding, we summarize our findings and those from the literature () and investigate the potential for applying this technique for immune regulation and CNS immunotherapy.
Figure 1. Schematic showing FUS-induced BBB opening with its potential effect in CNS immune modulation and immunotherapy.
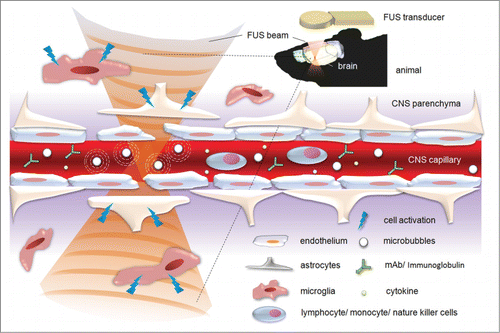
Strategies for FUS-induced BBB opening in CNS immune modulation and immunotherapy
FUS-BBB-opening triggered monocytes activation
Exposing the brain at a relatively high acoustic pressure may induce not only the BBB-opened effect but also the accompanying erythrocyte extravasations.Citation11,16,17 The leakage of pro-inflammatory molecules and chemokines into the brain milieu may in turn promote macrophage infiltration and homing. However, it is unclear whether activated macrophages originate from the circulation or in situ microglia. It is hypothesized that activated macrophages from the circulation, in concert with other immune competent cells, can infiltrate the CNS through the BBB-opened pores. Previously we have demonstrated the circulating monocytes/microphages indeed infiltrated the BBB-opened CNS tissues.Citation18 Monocytes were labeled by using superparamagnetic iron oxide (SPIO) nanoparticles, and we observed that, following excessive FUS exposure, SPIO-laden monocytes can gradually aggregate, indicated by the apparent imaging signal intensity level drop in MRI. This aggregation, however, depends on the FUS exposure level, and was only observed at heavy exposure levels which far exceeded that required for inducing BBB-opening. Yet, this suggests that the FUS-induced BBB opening (at heavy exposure levels) could recruit monocytes/macrophages into the CNS via FUS BBB-opening, and benefit the recruitment of activated macrophages such as in brain cancer treatment application.Citation19
FUS-BBB-opening triggered enhanced NK cell delivery
In addition to monocytes/ macrophages, NK cells have been found to assist in large-scale penetration into the CNS through the FUS-induced BBB opening for cancer therapy. Alkins et al. implanted HER2-expressing human breast tumor cells into nude rats; the tumor cells were labeled with SPIO nanoparticles and can therefore be tracked in-vivo via MRI.Citation20 Following targeted FUS-induced BBB opening and SPIO-labeled NK cell IV administration, the MRI showed a significant SI drop (up to 20%), indicating successful homing aggregation of the injected NK cells. With a high concentration of injected NK cells, a nearly fold5- increase of NK cells can be attracted to the tumor site than without FUS. It also suggests that FUS-induced BBB opening can successfully enhance immune cell targeting therapy for brain tumor treatment.
FUS-BBB-opening triggered enhanced antibody delivery
Monoclonal antibody therapy is a key immunotherapy approach that uses monoclonal antibodies (mAb) to specifically bind to target cells or proteins, and can eventually trigger a host immune response against disease. Monoclonal antibodies have high specificity against targeted extracellular or cell surfaces, and therefore can induce specific CNS immune modulation or immunotherapy. However, the mAb typically measures several tens of kDa, thus the BBB hampers most mAb from penetrating to the CNS. The FUS-induced BBB opening can allow mAb penetration to reach significant therapeutic levels for successful CNS disease therapy.
Kinoshita et al. previously demonstrated this approach by delivering the mAb, Herceptin (trastuzumab; Genentech).Citation21 Herceptin is a humanized mAb that targets human epidermal growth factor receptor 2 (HER2/c-erbB2) expressed in breast cancer cells, and it is desirable to delivered Herceptin directly into the brain for patients with metastatic breast cancer. When using FUS-induced BBB opening with concurrent Herceptin/ Gd-DTPA IV administration, they observed the enhancement of the MR contrast highly correlates with the Herceptin concentration. The improvement of Herceptin delivery into brain ranges from 1.5- to 3-fold higher than controls.
Similar to enhanced Herceptin delivery, Jordeo et al. showed that the same strategy that can be applied for targeting anti-amyloid β (Aβ) peptide deposited in the CNS.Citation22 It has been previously shown that direct injection of anti-Aβ mAb can be more efficient in Aβ clearance than high dose peripheral injection, and there is a strong rationale to suppose that the FUS-induced BBB opening, which allows for mAb penetration, can also be beneficial in Aβ clearance. They reported that the delivered anti-Aβ mAb (BAM-10, Mouse monoclonal against Aβ 1–40) can quickly bind to Aβ plaques. They also showed that the single BAM-10 administration can significantly reduce the pathology of compact plaques in transgenic mice either evaluated in Aβ plaque number, mean size (both ∼12% reduction) and surface area (∼23% reduction).
In their following study,Citation23 without the externally administered antibodies, the endogenous antibodies presented in the blood circulation, including Immunoglobulin G (IgG) and IgM, were able to penetrate through the opened BBB and bind to Aβ plaques, leading to Aβ solubilization, facilitating the transport of Aβ, or microglial phagocytosis.Citation24
FUS-BBB-opening triggered glial cell activation
The FUS-induced BBB opening has also been reported to facilitate neuroglial cell activation. Glial cells have long been expected to play key roles in antibody-medicated Aβ plaque clearance.Citation24 In our previous study using the FUS-induced BBB opening to enhance viral gene vector delivery to the brain, we presented evidence of increased GFAP expression in the FUS-exposed brain, indicating that FUS effectively activates astrocytesCitation25 (). Jordao et al also reported similar observation of the enriched GFAP expression in those glial cells, combined with the over-expression of ionized calcium-binding adaptor molecules 1 (Iba1), which indicates microglia activation.Citation23 In the same study, they also presented evidence of Aβ internalization of the activated microglia and astrocytes, assisting the clearing of Aβ plaque in transgenic AD mice. This phenomenon has recently been reconfirmed by Leinenga and Gotz, who found that it can restore memory functions of transgenic AD mice.Citation26 Importantly, this glial cell activation and Aβ clearance process was found to not cause neuroinflammation, as neuroinflammation has long been considered a potential pathway leading to Aβ deposition and AD progression.
Figure 2. Glial Fibrillary Acidic Protein (GFAP) immunofluorescence, neuronal nuclei (NeuN), and HE staining in the contralateral (A, B, C) and in the FUS-BBB opened brain (D, E, F). Neuron distributions (nuclei stained by NeuN; B and E) appeared similar on both sides of the brain, but concentrations of activated glial cells (stained by GFAP; A and D) were increased in the experimental lateral brain. HE-staining (C and F) showed that the tissue structure was not severely damaged by FUS treatment. Bar = 200 μm.
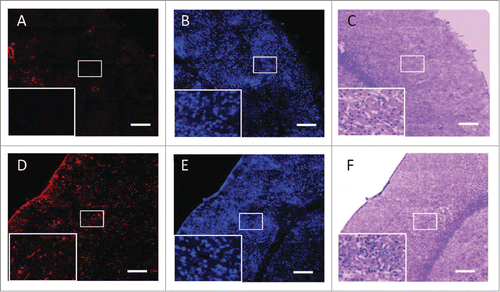
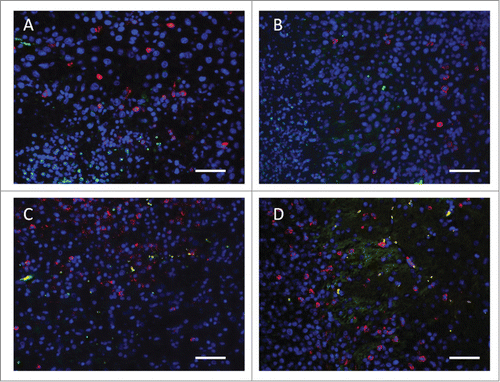
Figure 3. Representative fluorescent IHC analysis shows lymphocyte infiltration in the brain tumor and the corresponding lymphocyte population changes (particularly Treg and CTL populations). (A) Untreated brain tumor; (B) brain tumor treated with FUS-induced BBB opening; (C) brain tumor treated by IP IL-12 administration; (D) combined FUS with IL-12 administration, showing the enriched CTL infiltration and CTL/Treg ratio increase. Green: CD4+CD25+ lymphocytes (Treg); Red: CD3+CD8+ lymphocytes (CTL); Blue: DAPI-stained cell nucleus. Bar = 50 μm.
FUS-BBB-opening alone or enhanced cytokine delivery in lymphocyte activation/infiltration
We also previously evaluated the potential for FUS-induced BBB opening triggering endogenous lymphocyte infiltration.Citation1 We previously tested whether FUS-induced BBB opening in either normal or glioma-implant animals would trigger lymphocyte penetration in the target brain. Intermediate (BBB opened with brain intact) or heavy FUS exposure level (BBB opened with large-scale erythrocyte extravasations) was used, but no significant cell population changes were found either in CD3 + CD4+ (representing helper T lymphocytes; Th), CD3 + CD8+ (representing cytotoxic T lymphocytes; CTL), or CD4 + CD25+ (representing regulatory T lymphocytes; Treg) lymphocyte subgroups infiltrating into the BBB-opened brain. In addition, no noticeable changes of lymphocyte subgroups were found in lymphatic tissues. Thus we concluded that the FUS-induced BBB opening did not induce systemic immune response or it was insufficient to trigger lymphocyte infiltration alone (Fig. 3).
On the other hand, it has been verified that the local intracranial delivery of cytokines (e.g., Interleukin-12(IL-12)), could drive the immunosuppressive glioma microenvironment toward an antitumor immune response. IL-12 is physically secreted at the antigen site by immune cells such as macrophages, B-cells, and microglia. Also, in addition to being an important element in the immune system, IL-12 is a potentially powerful antitumor cytokine.Citation27,28 It has been shown that the presence of IL-12 can enhance the proliferation of T cells,Citation29,30 and also facilitate interferon (IFN)-gamma production to promote Th1-mediated antitumor cytotoxic immunityCitation31 and the associated anti-cancer immunological response. Since IL-12 has been reported to be involved in T-cell-dependent pathways and may correct the glioma-induced immunosuppression, it was hypothesized that the FUS-induced BBB opening may provide transient micro-vascular and micro-environmental changes in the tumor bed, leading to an increase in tumor cytokine/chemokine release and triggering lymphocyte infiltration.
In a parallel test in the same study,Citation1 we also evaluated the combined cytokine administration with the FUS-induced BBB opening on the lymphocyte glioma-infiltrating effect. Concurrently intraperitoneal (IP) injecting a low dose of IL-12 with the FUS-induced BBB opening can locally increase IL-12 concentrations up to 2.87-fold higher than control. This in turn induces significant CTL infiltration while still maintaining systemic immune stability, leading to glioma suppression and animal survival (a median survival improvement of over 40%).Citation1
Future clinical potential for FUS-induced neural immune modulation or immunotherapy
One important clinical application is for neurodegenerative disease treatment such as AD, it's the pathological cause of which is recognized as being highly correlated with Aβ and tau amyloid mis-folding, causing neuron death. In addition to astrocytes or microglia activation, plaque clearance has been attempted. Pro-inflammative cytokines (including TNF-α, TGF-β, and IL-6) have been found in higher concentrations in AD patients, thereby supporting the correlation of neuro-inflammation with AD.Citation32 The FUS-induced BBB opening may have potential to increase exposure of plaques to allow for antigen presentation, or for indirectly inducing innate/adoptive immune cells for plaque clearance.
The FUS-induced BBB opening may also play a key role in breaking through the immunosuppressive shield of malignant brain tumor. Several mechanisms have been proposed. First, immunosuppressive cytokines (e.g., TGF-b, IL-10, VEGF, etc.) are found in tumor microenvironments. FUS-induced BBB opening could enhance systemic delivery of either specific blocked molecules/antibodies for these cytokines or nonspecific immune-enhanced cytokines (e.g., IL-12) to overcome those immunosuppressive effects caused by tumor. Second, tumor cells typically express weak MHC with poor antigen presentation to escape immune surveillance. FUS-induced BBB opening may improve the efficiency of antigen presentation by enhancing MHC expression in tumor tissues to facilitate anti-tumor immunity, Third, macrophages or T cells tend to transform into immunosuppressive subtypes in tumor microenvironment. FUS-induced BBB opening may readjust the percentage or distribution of the macrophage toward activated type instead of immunosuppressive M2 type, while also inhibiting Treg and promoting Cytotoxic CD8+ T cell infiltration. Finally, FUS-induced BBB opening may further increase local concentrations of antibody-based tumor vaccines and immune checkpoint therapies through systemic administration, such as anti-epidermal growth factor receptor (EGFR) mAbs or anti- Cytotoxic T-lymphocyte-associated antigen 4(CTLA-4) mAbs and thus improved treatment outcomes.Citation33
In summary, the FUS-induced BBB opening offers new possibilities for CNS immune modulation through the following 3 major mechanisms: (1) by increasing local blood-brain permeability to allow penetration of circulating mAbs or cytokines that can perform immunological regulation, (2) by recruiting or adjusting desirable immune cells to infiltrate and target the lesion, and (3) by activating neuroglial cells and other innate cells to transform the microenvironment to against the disease. These three pathways indicate the possibility of future application of the FUS-induced BBB opening for neuro-immune modulation and neruo-immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Ministry of Science and Technology, TAIWAN, through grants 101-2221-E-182-002-MY3, 102-2221-E-182-020-MY3 and 103-2325-B-182A-007; National Health Research Institutes, TAIWAN, through grants. NHRI-EX103-10004NI; Chang Gung University, TAIWAN, through grant EMRPD1D0951E; and Chang Gung Memorial Hospital, TAIWAN, through grants. CORPD3E0071, CMRPD2A0033, CIRPD2E0051, CMRPD2D0111-3, CMRPG3D1341 and CMRPD2A0033.
References
- Chen PY, Hsieh HY, Huang CY, Lin CY, Wei KC, Liu HL. Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J Transl Med 2015; 13:93; PMID:25784614; http://dx.doi.org/10.1186/s12967-015-0451-y
- Wraith DC, Nicholson LB. The adaptive immune system in diseases of the central nervous system. J Clin Invest 2012; 122:1172-9; PMID:22466659; http://dx.doi.org/10.1172/JCI58648
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev 2006; 213:48-65; PMID:16972896; http://dx.doi.org/10.1111/j.1600-065X.2006.00441.x
- Wispelwey B, Lesse AJ, Hansen EJ, Scheld WM. Haemophilus influenzae lipopolysaccharide-induced blood brain barrier permeability during experimental meningitis in the rat. J Clin Invest 1988; 82:1339-46; PMID:3262627; http://dx.doi.org/10.1172/JCI113736
- Tanriverdi F, Unluhizarci K, Kelestrimur F. Persistent neuroinflammation may be involved in the pathogenesis of traumatic brain injury (TBI)-induced hypopituitarism: potential genetic and autoimmune factors. J Neurotrauma 2010; 27:301-2; PMID:19831821; http://dx.doi.org/10.1089/neu.2009.1102
- Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol 2005; 37:289-305; PMID:15474976; http://dx.doi.org/10.1016/j.biocel.2004.07.009
- Yan J, Fu Q, Cheng L, Zhai M, Wu W, Huang L, Du G. Inflammatory response in Parkinson's disease (Review). Mol Med Rep 2014; 10:2223-33; PMID:25215472
- Yoshida S, Tanaka R, Takai N, Ono K. Local administration of autologous lymphokine-activated killer cells and recombinant interleukin 2 to patients with malignant brain tumors. Cancer Res 1988; 48:5011-6; PMID:3261631
- Walls KC, Ager RR, Vasilevko V, Cheng D, Medeiros R, LaFerla FM. p-Tau immunotherapy reduces soluble and insoluble tau in aged 3xTg-AD mice. Neurosci Lett 2014; 575:96-100; PMID:24887583; http://dx.doi.org/10.1016/j.neulet.2014.05.047
- Liu HL, Hua MY, Chen PY, Chu PC, Pan CH, Yang HW, Huang CY, Wang JJ, Yen TC, Wei KC. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 2010; 255:415-25; PMID:20413754; http://dx.doi.org/10.1148/radiol.10090699
- Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 2005; 24:12-20; PMID:15588592; http://dx.doi.org/10.1016/j.neuroimage.2004.06.046
- Wu F, Wang ZB, Lu P, Xu ZL, Chen WZ, Zhu H, Huang CY, Wang JJ, Yen TC, Wei KC. Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol 2004; 30:1217-22; PMID:15550325; http://dx.doi.org/10.1016/j.ultrasmedbio.2004.08.003
- Wu F, Wang ZB, Cao YD, Zhou Q, Zhang Y, Xu ZL, Zhu XQ. Expression of tumor antigens and heat-shock protein 70 in breast cancer cells after high-intensity focused ultrasound ablation. Ann Surg Oncol 2007; 14:1237-42; PMID:17187168; http://dx.doi.org/10.1245/s10434-006-9275-6
- Lu P, Zhu XQ, Xu ZL, Zhou Q, Zhang J, Wu F. Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer. Surgery 2009; 145:286-93; PMID:19231581; http://dx.doi.org/10.1016/j.surg.2008.10.010
- Wang X, Qin J, Chen J, Wang L, Chen W, Tang L. The effect of high-intensity focused ultrasound treatment on immune function in patients with uterine fibroids. Int J Hyperthermia 2013; 29:225-33; PMID:23537008; http://dx.doi.org/10.3109/02656736.2013.775672
- Liu HL, Hsu PH, Chu PC, Wai YY, Chen JC, Shen CR, Yen TC, Wang JJ. Magnetic resonance imaging enhanced by superparamagnetic iron oxide particles: usefulness for distinguishing between focused ultrasound-induced blood-brain barrier disruption and brain hemorrhage. J Magn Reson Imaging 2009; 29:31-8; PMID:19097103; http://dx.doi.org/10.1002/jmri.21599
- Liu HL, Wai YY, Chen WS, Chen JC, Hsu PH, Wu XY, Huang WC, Yen TC, Wang JJ. Hemorrhage detection during focused-ultrasound induced blood-brain-barrier opening by using susceptibility-weighted magnetic resonance imaging. Ultrasound Med Biol 2008; 34:598-606; PMID:18313204; http://dx.doi.org/10.1016/j.ultrasmedbio.2008.01.011
- Liu HL, Wai YY, Hsu PH, Lyu LA, Wu JS, Shen CR, Chen JC, Yen TC, Wang JJ. In vivo assessment of macrophage CNS infiltration during disruption of the blood-brain barrier with focused ultrasound: a magnetic resonance imaging study. J Cereb Blood Flow Metab 2010; 30:674; PMID:20195293; http://dx.doi.org/10.1038/jcbfm.2009.251
- Chen PY, Liu HL, Hua MY, Yang HW, Huang CY, Chu PC, Lyu LA, Tseng IC, Feng LY, Tsai HC, et al. Novel magnetic/ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro Oncol 2010; 12:1050-60; PMID:20663792; http://dx.doi.org/10.1093/neuonc/noq054
- Alkins R, Burgess A, Ganguly M, Francia G, Kerbel R, Wels WS, Hynynen K. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res 2013; 73:1892-9; PMID:23302230; http://dx.doi.org/10.1158/0008-5472.CAN-12-2609
- Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A 2006; 103:11719-23; PMID:16868082; http://dx.doi.org/10.1073/pnas.0604318103
- Jordao JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, Hynynen K, Aubert I. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease. PloS One 2010; 5:e10549; PMID:20485502; http://dx.doi.org/10.1371/journal.pone.0010549
- Jordao JF, Thevenot E, Markham-Coultes K, Scarcelli T, Weng YQ, Xhima K, O'Reilly M, Huang Y, McLaurin J, Hynynen K, et al. Amyloid-beta plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol 2013; 248:16-29; PMID:23707300; http://dx.doi.org/10.1016/j.expneurol.2013.05.008
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 2000; 6:916-9; PMID:10932230; http://dx.doi.org/10.1038/78682
- Hsu PH, Wei KC, Huang CY, Wen CJ, Yen TC, Liu CL, Lin YT, Chen JC, Shen CR, Liu HL. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PloS One 2013; 8:e57682; PMID:23460893; http://dx.doi.org/10.1371/journal.pone.0057682
- Leinenga G, Gotz J. Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer's disease mouse model. Sci Transl Med 2015; 7:278ra33; PMID:25761889; http://dx.doi.org/10.1126/scitranslmed.aaa2512
- Barba D, Saris SC, Holder C, Rosenberg SA, Oldfield EH. Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J Neurosurg 1989; 70:175-82; PMID:2643685; http://dx.doi.org/10.3171/jns.1989.70.2.0175
- Barua NU, Lowis SP, Woolley M, O'Sullivan S, Harrison R, Gill SS. Robot-guided convection-enhanced delivery of carboplatin for advanced brainstem glioma. Acta Neurochir 2013; 155:1459-65; PMID:23595829; http://dx.doi.org/10.1007/s00701-013-1700-6
- Gately MK, Desai BB, Wolitzky AG, Quinn PM, Dwyer CM, Podlaski FJ, Familletti PC, Sinigaglia F, Chizonnite R, Gubler U, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). Journal of immunology 1991; 147:874-82; PMID:1713608
- Morini M, Albini A, Lorusso G, Moelling K, Lu B, Cilli M, Ferrini S, Noonan DM. Prevention of angiogenesis by naked DNA IL-12 gene transfer: angioprevention by immunogene therapy. Gene Ther 2004; 11:284-91; PMID:14737088; http://dx.doi.org/10.1038/sj.gt.3302175
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 1993; 260:547-9; PMID:8097338; http://dx.doi.org/10.1126/science.8097338
- Cooper NR, Bradt BM, O'Barr S, Yu JX. Focal inflammation in the brain: role in Alzheimer's disease. Immunol Res 2000; 21:159-65; PMID:10852113; http://dx.doi.org/10.1385/IR:21:2-3:159
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/10.1056/NEJMoa1200694
