 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Minimum gestation at which infant can be given BCG (Bacillus Calmette-Guerin) vaccine safely at birth is not clearly defined. Our objectives were the following: to compare Mantoux test after 6 months of BCG immunization in moderately preterm babies (31–33 weeks) vaccinated at birth and 34 weeks post conception age and to compare in above groups:(a) Interferon – gamma (IFN-γ) levels in BCG vaccinated infants who did not react to Mantoux test (b) Local BCG reaction at 6, 10, 14 weeks and 6 months (c) Complications of BCG vaccination. Interventional, randomized comparative trial. Moderately preterm infants (31–33 weeks), 90 in each group. At birth, 180 moderately preterm infants were recruited and randomly allocated into 2 groups. Two ml venous blood was drawn for estimation of IFN-γ levels. Infants were given BCG vaccine within 72 hours of birth and followed up after 2, 4, 6, 10, 14 weeks and 6 months (group 1). Infants were recruited at birth and held up till 34 weeks post conception age (group 2) and then given BCG vaccine and followed up similarly as group 1. At each visit, local BCG reaction, any local or unusual complication and anthropometric measurements were noted. At six months, Mantoux test was done and 2 ml venous blood sample was collected for IFN-γ levels post vaccination. Presence or absence of BCG local reaction, PPD conversion rates and complications were analyzed using Chi square or Fisher's exact test. IFN-γ levels were analyzed by ANOVA. In all 117 infants could be followed till 6 months after BCG immunization in 2 groups, and Mantoux test was positive in 38.4% of them. The rate of Mantoux test positivity was similar irrespective of the age of giving BCG immunization (group 1- 39.1% vs group 2- 37.5%; p > 0.05). IFN-γ levels were significantly raised at 6 months in 60% (n = 21/41) and 65% (n = 15/27) Mantoux negative infants in group 1 and group 2 respectively. The sequence and order of local BCG reaction at 2, 4, 6, 10, 14 weeks and 6 months was in the form of papule, pustule, ulcer, scab and scar. Scar was formed in 94.2% and 89.5% infants in group 1 and group 2 respectively. One infant in group 1 showed abortive reaction (0.85%). Only 3.4% of infants developed lymphadenopathy and was similar in both the groups. Moderately preterm infants (31–33 weeks) exhibited 98.3% immunogenicity after BCG immunization at birth and can be safely vaccinated without any risk of severe complications.
Introduction
Tuberculosis continues to be a major public health problem worldwide. In 2010, there were an estimated 8.8 million incident cases (equivalent to 128 cases per 100,000 population) and 12 million prevalent cases (equivalent to 178 per 100,000 population) of Tuberculosis (TB) globally.Citation1 India is a high TB burden country contributing 26% of total cases in the world and the prevalence of Multidrug resistant (MDR) -TB is <3% among new TB cases.Citation2
Bacillus Calmette-Guerin (BCG) is a live attenuated vaccine given routinely to all newborn infants in developing countries under Universal Immunization Program.Citation3 Reconstituted 0.1 ml of BCG vaccine contains 0.1–0.4 million live viable mycobacterium bovis bacilli of Danish 1331 strain. Efficacy ranging from 0 to 80%, BCG vaccine is credited to reduce the incidence of disseminated tuberculosis and tubercular meningitis in vaccinated children.Citation4,5 It has been reported that BCG vaccine confers nonspecific benefits improving survival.Citation6 When administered in the neonatal period, the BCG vaccine is said to enhance heterologous helper T lymphocyte-1 (Th-1) immune effects to the tetanus toxoid and poliovirus vaccines given 2–3 months later.Citation7
BCG vaccine induces delayed type of hypersensitivity reaction and cell mediated immunity (CMI) in the host 4–8 weeks after immunization. A sequence of changes has been described at the site of BCG vaccination in the form of papule, pustule, ulcer and scar and is considered an evidence of successful vaccination.Citation5 Failure of scar formation may indicate non-reactors or an abortive reaction.Citation8
In India approximately 30–40% infants are born low birth weight (LBW) and 7–22% as preterm.Citation9 The minimum gestation and birth weight at which infant can be safely given BCG vaccine at birth is not clearly mentioned in immunization guidelines. Therefore, many health care workers refrain from giving BCG vaccine to very preterm and very LBW infants at birth, being unsure whether the vaccination is for the normal weight full term babies only, and its safety and immunogenicity. These infants eventually remain unimmunized. It has been reported that prevalence of unvaccinated LBW infants is 1.5–3 times more than normal birth weight infants.Citation10 Failure to give BCG vaccine at birth will have negative impact on immunization coverage for LBW infants, and will render them vulnerable to infection. In fact a major opportunity of immunizing these infants on time is missed by the health personnel.Citation11
Keeping in view the limited data on the subject and potential need for developing scientific evidence on the timing of BCG vaccination in preterm and LBW infants, it would be desirable to conduct a randomized trial on the immunological response and safety of BCG immunization in moderately preterm infants (gestation age 31–33weeks) at birth.
The specific objectives of the present study were to compare Mantoux test after 6 months of BCG immunization in moderately preterm (gestation age 31–33 completed weeks) infants immunized at birth (group 1) and at 34 completed weeks post conception age (group 2), and to compare in above groups IFN-γ levels, local BCG reaction at 2, 4, 6, 10, 14 weeks and 6 months and complications of BCG immunization. Creating scientific evidence for facilitating a national policy consideration through this study was also a secondary objective.
Results
Out of 180 preterm infants we could follow 117 infants up to 6 months. Remaining 63 (35%) babies expired or were lost to follow up (). Of 117 infants who completed the study, early BCG vaccination group (group1) comprised of 69 (58.9%) infants, and the late BCG vaccination group (group 2) included 48 (41%) infants. Mean birth weight of the infants in group 1 and group 2 was 1618 ± 160 grams and 1470 ± 220 grams respectively. Group 1 comprised of 40 males and group 2, had 25 male infants; the male to female ratio was 1.4: 1 and 1.08:1 in the 2 groups respectively. Both the groups were comparable for birth weight, sex and socioeconomic status (p > 0.05).
Figure 1. Flowchart depicting the recruitment of infants of early vs. late BCG vaccination group and comparison between the immunogenicity of 2 groups. Mx: Mantoux test, CMI: Cell mediated immunity.
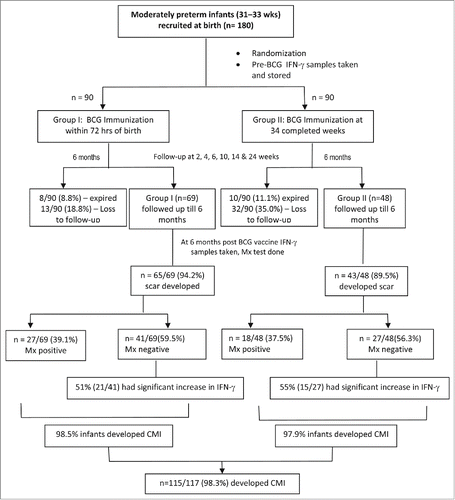
At week 2 of follow up, only 11.3% of babies in group 1 (immunized early at birth) and 3.4% of babies in group 2 (immunized late) developed local reaction in the form of papule. In group 1, only papule formed at the site of vaccination in 65% infants by 4 weeks, whereas in group 2 both papule and pustule developed in 51.6% and 9.6% infants respectively by this time. The number and state of local reaction showed increment as time from immunization increased. At 6 weeks post immunization, scar started appearing in 4.2% and 8.3% infants respectively in group 1 and 2. By 14 weeks, 97.3% (37/38) of infants in group 1 had developed a local reaction and 78.9% (30/38) infants had scar formation. At the same time 93.5% (29/31) and 70.9% (22/31) infants in group 2 developed local reaction and scar respectively. At 6 months, 94.2% infants in group 1 and 89.5% infants in group 2 had developed BCG scar (p > 0.05). Over all 92.3% (n = 108/117) moderately preterm infants developed scar after BCG immunization; in 3 infants (group 1-one, group 2- 2) either pustule or ulcer was still present at 6 months ().
Table 1. Local BCG reaction in group 1 and group 2 at each follow up
IFN-γ levels were significantly raised in almost half of the infants in both groups. The mean rise in the post BCG immunization IFN-γ levels, measured 6 months after BCG vaccine administration, was 3.33 ± 4.490pg/ml (p = 0.00) and 3.33 ± 5.819pg/ml (p = 0.005) in group 1 and group 2 respectively, from the base level of zero, taken at birth before BCG vaccination. However, the rise of IFN-γ levels in 2 groups was comparable (p > 0.05) (). Seven infants in group-1 and 6 infants in group-2 had raised pre BCG vaccination IFN-γ levels as well as increased C-reactive protein (>0.6 mg/dl). In these infants sepsis screen was positive with the presence of at least 2 of the parameters; total Leucocyte count (TLC) <5000/mm3, platelet count <100,000/mm3, toxic granule in the peripheral smear, micro Erythrocyte sedimentation rate (µESR) >15 mm in the 1st hour, evidence of pneumonia on X-ray chest and/or evidence of meningitis in the CSF examination (). They were appropriately treated as per neonatal intensive care unit (NICU) protocol and were excluded from the post BCG IFN-γ level analysis.
Table 2. IFN-γ levels in 2 groups
Table 3. Results of sepsis screen of Infants with raised pre IFN-γ levels
In group 1, 39.1% (n = 27) infants had positive Mantoux reaction (5–9 mm) whereas 37.5% (n = 18) infants in group 2 were Mantoux test positive (p = 0.473). On comparison of mean post vaccination IFN-γ level with Mantoux test induration size, no significant difference was found (p = 0.503). Mean BCG scar size was 3.6±1.3 mm and 3.3±1.3 mm in group 1 and 2 respectively which was not significantly different (p = 0.219). Similarly there was no correlation between scar size and IFN-γ levels (p = 0.29). Further the mean BCG scar size did not differ significantly with the induration size of the Mantoux test be it <5mm, 5–9mm and ≥10 mm (p = 0.192) ().
Table 4. Mantoux test reaction, mean scar size and mean post BCG IFN-γ levels at 6 months
In 4 infants the Mantoux test reading was 10 mm or more, maximum size being 13 mm. One infant belonged to group 1 and 3 infants were from group 2. They were further investigated and none of them showed evidence of tubercular disease in the form of clinical symptoms including fever, cough, and failure to gain weight, cervical lymphadenopathy, and evidence of primary complex in the chest X-ray. ().
Six (5.12%) infants failed to develop BCG scar. In 5 infants, including 2 infants in group 1 and 3 in group 2, no local reaction occurred, and it might be presumed that BCG immunization failed to revoke any response in them. However, Mantoux test was 7mm in one infant and <5 mm in remaining 4 infants. The post BCG immunization IFN-γ was raised in 3 of them; 7.5 pg/ml in 2 infants and 5 pg/ml in one baby. In one infant belonging to group 1 a papule developed at 4 weeks and persisted till 14 weeks. On examination at 6 months no local reaction was visible including there being no scar, which was consistent with the “abortive reaction.”Citation8 In this child Mantoux test induration was <5 mm, and pre and post BCG immunization IFN-γ levels were zero and 7.5pg/ml respectively.
On the basis of local BCG reaction including scar formation, positive Mantoux test and significantly raised post BCG immunization IFN-γ levels 98.5% and 97.9% infants developed cell mediated immunity to BCG immunization in group 1 and group 2 respectively; the overall immunogenicity was 98.3% (n = 115/117).
Left axillary lymphadenopathy was the only complication seen in 2 infants (3.4%) each in both groups. The maximum size of the lymph nodes was <5 mm and maximum number of enlarged lymph nodes was 3. These were firm, shotty, non-tender and non adherent to subcutaneous tissue on palpation.
Discussion
The immunogenicity of the BCG vaccine in moderately preterm infants was elicited by in-vivo and in-vitro methods. In-vivo tools were Purified protein derivative (PPD) conversion, elicited by Mantoux test, and scar formation 6 months after BCG immunization. The in-vitro assessment was done at 6 months by recording significant rise in the post BCG vaccination IFN-γ levels, from pre BCG vaccination levels at birth, especially in those infants, who failed to show immunogenicity by the in-vivo tests.
Though, a little more than one-third infants in our study showed PPD conversion by Mantoux test, available literature has reported that preterm and LBW infants show poor PPD conversion following BCG immunization.Citation19,20 Indian studies have documented tuberculin conversion to be as low as 10–20% and as high as 50–80% 3 months after BCG vaccination in preterm and LBW infants.Citation12–17 Another study by Sedaghatian et al reported only 31% tuberculin conversion in preterm babies following BCG immunization at birth.Citation19 Similarly Kaur et al in their study observed only 25% and 41.7% Mantoux test reactivity in preterm infants born at 31–33 weeks at 12 weeks and 6 months after BCG immunization respectively. They stated that tuberculin reactivity continued to increase from 3 months to 6 months, and probably further.Citation20 These infants were not given BCG and Oral Polio Vaccine (OPV) simultaneously and therefore, this could not be the cause of lower purified protein derivative (PPD) conversion rate among them as has been suggested by some workers.Citation20-23 Contrary findings have also been documented where no significant difference between the preterm and term infants on their PPD conversion rate is reported.Citation11,15,24,25 Thayyill-Sudhan et al.,Citation11 reported almost similar conversion rates to PPD in pre-term babies of 34–36 weeks (80% conversion) and 38–40 weeks post conception age (80.7% conversion). What has come out as an important finding in our study is that there is no significant difference in the proportion of infants exhibiting PPD conversion between early and late BCG immunized groups, and thus any waiting for the infants born between 31–33 weeks gestation, to reach post conception age of 34 weeks, to improve immunogenicity and safety of the BCG vaccine, is not of any consequences.
The formation of scar at the site of inoculation is taken as a marker of immunogenicity to BCG immunization. A classic reaction is described as papule 2–3 weeks after BCG immunization, which develops in to a vesicle and then to a pustule. The pustule breaks into ulcer with a waxing and waning course and leads to a scar formation, 5–7 mm in size in 6–12 weeks.Citation26 In some children abortive reaction may develop where papule/pustule disappears without any scar formation.Citation8 Similar sequential development of cutaneous local reaction was noted in all infants in our study as well, and a scar formed in 94.2% and 89.5% infants in group 1 and group 2 respectively after 6 months. Our observations on the pattern of development of local reaction and formation of scar were in conformity with the findings of other authors.Citation8,11,20,26-30 Similar sequential pattern of local reaction after BCG immunization has been described among preterm twin infants also by Gupta et al who followed 153 twin infants >34 weeks gestation for 14 weeks.Citation31. One study has reported that scar formation is less likely in very preterm (gestational age <33 weeks) male infants.Citation32 Many studies have tried to correlate presence of scar and its size with the cell mediated immunity induced by BCG immunization.Citation18,19 We did not find any significant difference in the mean scar size with the size of PPD induration (p = 0.192).
IFN-γ, an essential component of host defense against mycobacteria, responsible for the activation of macrophages and stimulation of their anti-mycobacterial properties, is increased after BCG immunization.Citation33 A significant change was found in the pre and post IFN-γ levels after BCG immunization in our study also, but no significant difference was observed in the IFN-γ levels in the early and late immunized groups. IFN-γ levels did not show any significant correlation either with the BCG scar size or size of the PPD conversion (p = 0.503). IFN-γ was not found in the pre BCG immunization blood samples in both groups. The mean post BCG IFN-γ values were lower than standard deviation and this phenomenon was seen in both groups. Though rise in the IFN-γ levels in the post BCG immunization samples signified immunogenic response to BCG vaccine yet its statistical significance might be naïve.
IFN-γ is a cytokine that may rise in sepsis and other infections. Though IFN-γ levels measured in our study were not specific for BCG antigens, but clinically the infants were free of infection; and sepsis screen including CRP were negative. The raised levels of post BCG immunization IFN-γ at 6 months could be attributed only to the BCG vaccination administered at birth. Though several workers have measured IFN-γ levels in infants and adults to test immunogenicity of BCG vaccineCitation21,34-37 yet, as per available literature, there is no study to show changes in the IFN-γ levels following BCG vaccination in moderately preterm newborn infants so far. Lalor et al and Black et al described population/ethnic/racial differences in the immune responses to IFN-γ following BCG immunization to infants in UK from those immunized in Malawi.Citation35,38
In six infants scar did not form including abortive reaction in one infant. Conventionally this might be taken as failure of BCG uptake. But IFN-γ levels were significantly raised in 2 of them and in one infant both Mantoux and IFN-γ levels were positive. The infant with abortive reaction also showed increased IFN-γ levels. Thus four out of 6 infants, where scar did not develop, exhibited immunogenicity to BCG immunization. Out of 117 infants, scar was present in 111 infants, one infant had abortive reaction and 3 infants had positive levels of IFN-γ, making 115 (98.3%) infants developing CMI after BCG immunization. The time of BCG administration, early (at birth) versus at 34 weeks post conception age, did not make any difference in the reactogenicity and immunogenicity of the BCG vaccine in moderately preterm infants (31–33 weeks). In all 98.5% and 97.9% infants in group 1 and group 2 exhibited immunogenicity of BCG vaccine based on Mantoux test, scar formation and raised IFN-γ levels. In our study the BCG immunization at birth has been observed to be safe in moderately preterm infants as only 3.4% infants had axillary lymphadenopathy without there being any difference in any group, that compared well with the findings of other studies.Citation12,15-17,38-40
Due to unclear guidelines on the BCG immunization the health care workers adopt a very cautious and subjective approach while immunizing preterm and low birth weight (LBW) infants with BCG vaccine due to apprehension of poor immunogenicity and safety and lack of robust scientific evidence in favor, especially in moderately preterm and very LBW infants. Present study amply demonstrates that BCG vaccine given within 3 days after birth to infants 31 to 33 weeks gestation is optimally immunogenic and is safe. However, the limitation of the study is that gamma interferon levels have not been studied against tubercular antigens and the loss to follow up in one group is more than anticipated.
Materials and Methods
Design: Interventional, randomized comparative trial. The institutional ethics committee approved the study.
Sample size: The number of subjects was chosen on the basis of a study by Faridi et al. (2009), entitled ‘Tuberculin conversion and leucocyte migration inhibition test after BCG vaccination in newborn infants'.Citation41 All calculations were done taking proportion of infants with positive Mantoux test at 6 months in 2 groups namely 31–33 weeks and 34–36 weeks. Taking level of significance 5% and power of study 80%, Z1−α/2 = 1.96, Z(1-β) = 0.845
The following formula was applied:
P1 = Mantoux positivity at 6 months in 31-33 weeks preterm babies
P2 = Mantoux positivity at 6 months in 34-36 weeks preterm babies
Two tailed sample was taken. Keeping in view the possibility of attrition (20%) at 6 months of follow up 90 infants were recruited in each group. We could not achieve the target sample size in our study.
Subjects
After taking informed consent from parents residing within a radius of 10 km from the study area, and only those who could be contacted whenever required, were included. Gestation was calculated by LMP (Last menstrual period) and modified Ballard's Score in all the babies. In case(s) of disparity of >2 weeks gestation between the 2 methods then modified Ballard score was taken as final.
Inclusion criteria
Infants of 31–33 completed weeks' gestation and weighing appropriate for gestational age, of both sexes were included after obtaining approval of the institution ethics committee and informed consent of the parents.
Exclusion criteria
The following babies were excluded from the study:
Infants of mothers suffering from tuberculosis or receiving anti-tubercular drugs, eclampsia, chronic infection, severe anemia (Hb<7g %), known HIV, Hepatitis B and Hepatitis C positive status.
Infants having clinical evidence of intrauterine infection, undergoing exchange transfusion, receiving blood or blood products, severe birth asphyxia (Apgar score <3 at 1 min), critically ill babies (defined as those requiring assisted ventilation or parenteral fluids above 2-thirds of their total requirement), septicemia.
Babies with congenital anomalies and or clinical suspicion of chromosomal disease.
Infants of mothers with history of smoking and alcohol were also excluded.
Randomization
Moderately preterm babies (31–33 weeks gestation) recruited at birth were randomly allocated into 2 groups by computer generated randomization numbers kept in an opaque, serially numbered and sealed envelope prepared by a person not directly related to the study.
Intervention
Immunization
1a.Group 1(Early immunization group): Infants were given BCG vaccine within 72 hours of birth and followed up after 2, 4, 6, 10, 14 weeks and 6 months.
1b.Group 2 (Late immunization group): Infants were followed up till 34 weeks post conception age and then given BCG vaccine within 3 days and followed up similarly as group 1.
1c. OPV and hepatitis B vaccine were not given to these infants along with BCG vaccine. However, vaccines under National Immunization Program were given to all infants at 6, 10, and 14 weeks of age during follow up.
Blood sampling
Two ml venous blood was taken from infants of both groups in a plain vial within 24 hours of birth and 6 months after BCG immunization; serum was separated and stored at −20°C for estimation of pre and post BCG immunization IFN-γ and CRP levels. One ml venous blood was obtained from all infants in EDTA vial for complete blood count including total leucocyte count, platelet count, micro ESR and toxic granules in the peripheral blood picture. Since acute bacterial infection could raise IFN-γ, therefore, qualitative C- reactive protein (acute phase reactant) was also done simultaneously. CRP was done by latex agglutination method in the microbiology laboratory by trained staff and a CRP level of >0.6 mg/dl was taken as positive indicator for bacterial sepsis.
Mantoux test
After 6 months of BCG immunization Mantoux test was done on all infants of both the groups.
BCG administration
BCG vaccine used was Danish 1331 Strain, prepared at Guindy, Chennai, India and was given intradermally on the left arm just above the insertion of the deltoid muscle, by a trained staff nurse, with a 26G needle with a tuberculin syringe to produce a wheal of 5 mm. The freeze dried vaccine was inspected for viability based on vaccine vial monitor (VVM) on the vial, and was reconstituted with normal saline preserved at 4–8°C and left over reconstituted vaccine was discarded after 3 hours. Standard injection safety precautions were taken.
Follow Up
All infants were followed up at 2weeks, 4(±1) weeks, 6(±2) weeks, 10(±2) weeks, 14(±2) weeks and 24(±2) weeks. At each visit local BCG reaction and any local or unusual complication were noted and mothers were counseled for exclusive breastfeeding. At each visit weight, length and head circumference of the infants were measured by standard method. At 6, 10 and 14 weeks, OPV and pentavalent vaccine (diphtheria, pertussis, tetanus, hepatitis B, hemophilus influenza) were administered. After six months of BCG immunization, Mantoux test was done, 2 ml blood sample was collected for IFN-γ levels and scar size was noted.
Method of scar measurement
Scar size was recorded by measuring the maximum transverse diameter of the scar with the help of calipers having blunted ends. The scar size was recorded in millimeters.
Method of tuberculin administration and interpretation:
Mantoux test was performed using 5 TU of PPD with Tween 80 (0.1 ml) injected intradermally with a tuberculin syringe fitted with 26 G needle on the upper one third of the flexor aspect of the left forearm to produce a wheal of at least 5 mm. The mother was counseled to report at 48 hours (±4 hr) for test reading. Maximum transverse diameter of the induration was measured by ball-pen method.Citation42 Induration less than 5 mm was taken as negative. A positive response was taken as an induration of 5–9 mm. The infant showing an induration of ≥10 mm was further investigated for tubercular infection or active tuberculosis.
Measurement of interferon gamma (IFN-γ) levels in blood samples
Measurement of IFN-γ levels in the stored serum samples was done by ELISA method (kit no. 950.000.096, batch no. 1200-52 manufactured by Gen-Probe Diaclone SAS), according to manufacturer's instructions and were interpreted by constructing a standard curve using the optical density (OD) of the 7 standards. The sensitivity, minimum detectable dose of IFN-γ using this kit, was less than 5 pg/ml.
Statistical analysis
Presence or absence of BCG local reaction, PPD conversion rates and complications were analyzed using Chi square or Fisher's exact test. BCG scar size was compared by student t-test. IFN-γ levels were compared between and within the groups by repeated measure ANOVA. Anthropometric measures were compared in both the groups over the repeated intervals by Generalized Estimating Equations (GEE). Multiple comparisons were obtained by Bonferroni correction. P-value of less than 0.05 was considered significant.
Conclusion
It can be concluded that there was no difference in the PPD conversion rate after 6 months of BCG immunization in moderately preterm infants immunized early soon after birth (39.1%) or given vaccination late at completion of 34 weeks post conception age (37.5%).
The BCG scar formed in 94.2% and 89.5% infants in 2 groups. There was significant rise in the post BCG immunization IFN-γ levels in all infants without risk of increased complications due to BCG immunization. In all 98.5% and 97.9% infants in group 1 (immunized early) and group 2 (immunized late) exhibited immunogenicity to BCG vaccine based on Mantoux test, local BCG reaction and scar formation, and raised IFN-γ levels.
Thus it can be recommended that BCG vaccine may be safely given to moderately preterm infants (31–33 weeks) at birth.
What is already known
BCG vaccine is immunogenic and can be given safely to infants beyond 34 weeks gestation.
What this study adds
BCG vaccine administered to 31–33 weeks preterm infants at birth is immunogenic and safe.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
M.M.A. Faridi conceptualized the study, supervised the work, and critically reviewed the manuscript and will stand guarantor. Megha Saroha collected the data, searched literature, helped in estimation of IFN-γ levels and prepared initial draft. Prerna Batra reviewed the literature and drafted the manuscript. Iqbal Kaur carried out IFN-γ levels and prepared manuscript. DK Dewan helped in writing final version of the manuscript with valuable suggestions. All authors approved the final draft.
References
- Desikan P, Perspective. Sputum smear microscopy in tuberculosis: Is it still relevant? Indian J Med Res 2013; 137:442-4; PMID:23640550
- Sharma SK, Mohan A. Tuberculosis: From an incurable scourage to a curable disease- journey over a millennium. Indian J Med Res 2013; 137:455-93; PMID:23640554
- Chadha VK, Jaganath PS, Kumar P. Tuberculin sensitivity among children vaccinated with BCG under universal immunization programme. Indian J Pediatr 2004; 71:1063-8; PMID:15630312; http://dx.doi.org/10.1007/BF02829815
- Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol 1993; 22:1154-8; PMID:8144299; http://dx.doi.org/10.1093/ije/22.6.1154
- Dam HG, Toman K, Hitze KL, Guld J. Present knowledge of immunization against tuberculosis. Bull World Health Organ 1976; 54:255-69; PMID:798638
- Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, Stensballe L, Diness BR, Lausch KR, Lund N, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis 2011 Jul 15; 204(2):245-52; PMID:21673035; http://dx.doi.org/10.1093/infdis/jir240
- Libraty DH, Zhang L, Woda M, Acosta LP, Obcena A, Brion JD, Capeding RZ. Neonatal BCG vaccination is associated with enhanced T-helper 1 immune responses to heterologous infant vaccines. Trials Vaccinol 2014; 3:1-5; PMID:24611083; http://dx.doi.org/10.1016/j.trivac.2013.11.004
- Faridi MMA, Krishnamurthy S. Abortive reaction and time of scar formation after BCG vaccination. Vaccine 2008; 26:89-90; http://dx.doi.org/10.1016/j.vaccine.2007.11.018
- Singh M. Nomenclature and definitions, care of newborn. New Delhi, Sagar Publications, 1996: page 1-4.
- Okan F, Karagoz S, Nuhoglu A. Bacillus Calmette-Guérin vaccination in preterm infants. Int J Tuberc Lung Dis 2006; 10:1337-41; PMID:17167949
- Thayyil-Sudhan S, Kumar A, Singh M, Paul VK, Deorari AK. Safety and effectiveness of BCG vaccination in preterm babies. Arch Dis Child Fetal Neonatal Ed 1999; 81:64-6; http://dx.doi.org/10.1136/fn.81.1.F64
- Aggarwal A, Dutta AK. Timing and dose of BCG vaccination in infants as assessed by post vaccination tuberculin sensitivity. Indian Pediatr 1995; 32:635-38; PMID:8613331
- Dabral S, Ghosh S, Taneja PN. Tuberculin conversion after BCG vaccination in neonatal period. Indian Pediatr 1969; 6:84-88; PMID:5783828
- Patel TB, Shah VP. Nature of allergy in BCG vaccinated children. Indian J Med Res 1961; 15:939
- Mehta JB, Sukhani SC, Saxena S. BCG vaccination in newborns and tuberculin conversion. Indian pediatr 1972; 9:485-9
- Das SK, Gautam KD, Mehrotra ML, Ranjan RD, Sharma JP. Timing of BCG vaccination in infants. Indian J Tuberc 1980; 27:63-4
- Thapar RK, Mehrotra ML, Rajan JD, Verma JK, Dayal RS, Parshad R. Mantoux conversion in infants after BCG vaccination in the neonatal period. Indian Pediatr 1971; 8:439-42; PMID:5131805
- Camargos P, Ribeiro Y, Teixeira A, Menezes L. Tuberculin skin reactivity alter neonatal BCG vaccination in preterm infants in Minas Gerais, Brail, 2001–2002. Arch Pediatr 2002; 9(6):629-37; PMID:12108319; http://dx.doi.org/10.1016/S0929-693X(01)00935-6
- Sedaghatian MR, Kardouni K. Tuberculin response in preterm infants after BCG vaccination at birth. Arch Dis Child 1993; 69:309-11; PMID:8215572; http://dx.doi.org/10.1136/adc.69.3_Spec_No.309
- Kaur S, Faridi MM, Agarwal KN. BCG vaccination reaction in low birth weight infants. Indian J Med Res 2002 Aug; 116:64-9
- Sartono E, Lisse IM, Terveer EM, van de Sande PJ, Whittle H, Fisker AB, Roth A, Aaby P, Yazdanbakhsh M, Benn CS. Oral polio vaccine influences the immune response to BCG vaccination. A natural experiment. PLoS One 2010; 5(5):e10328; PMID:20502641; http://dx.doi.org/10.1371/journal.pone.0010328
- Faridi MM, Srivastava S. Effect of Simultaneous Administration of Oral Polio Vaccine on Local Reaction of BCG Vaccine in Term Infants. Indian Pediatr 2015 Feb 8; 52(2):115-8; PMID:25691177; http://dx.doi.org/10.1007/s13312-015-0583-4
- Jensen KJ, Karkov HS, Lund N, Andersen A, Eriksen HB, Barbosa AG, Kantsø B, Aaby P, Benn CS. The immunological effects of oral polio vaccine provided with BCG vaccine at birth: A randomised trial. Vaccine 2014 Oct 14; 32(45):5949-56. Epub 2014 Sep 16; PMID:25223267; http://dx.doi.org/10.1016/j.vaccine.2014.08.062
- Dawodu AH. Tuberculin conversion following BCG vaccination in preterm infants. Acta Paediatr Scand 1985; 74:564-7; PMID:4024927; http://dx.doi.org/10.1111/j.1651-2227.1985.tb11030.x
- Negrete-Esqueda L, Vargas-Origel A. Response to Bacillus Calmette-Guérin vaccine in full-term and preterm infants. Am J Perinatol 2007; 24(3):183-9; PMID:17372860; http://dx.doi.org/10.1055/s-2007-970080
- Yewale V, Choudhary P, Thacker N. IAP Guidebook on Immunization, 2009–2011. Mumbai: IAP; 2011:51-2.
- Surekha RH, Vijaylaxmi V, Kumar S, Lakhmi KA, Sumanlatha G, Murthy K. CMI in children with scar failure following BCG vaccination. Indian Pediatr 1998;35:123-127; PMID:9707854
- Agarwal RK, Kapur D, Kumari S. Development of BCG scar in relation to age and nutritional status. Indian Pediatr 1990; 27:291-3; PMID:2351451
- Vijayalakshmi V, Devi PS, Murthy KJ, Rao DV, Jain SN. Cell mediated immune responses in BCG vaccinated children. Indian Pediatr 1993; 30:899-903; PMID:8132282
- Fine PE, Ponnighaus JM, Marine N. The distribution and implications of BCG scars in the northern Malawi. Bull World Health Organ 1989; 67:35-42; PMID:2706726
- Gupta P, Faridi MM, Shah D, Dev G. BCG reaction in twin newborns: effect of zygocity and chorionicity. IndainPediatr 2008;45(4):271-77
- Sedaghatian MR, Hashem F, Hossain MM. BacilleCalmette-Guérin vaccination in pre-term infants. Int J Tuberc Lung Dis 1998; 2: 679-82; PMID:9712284
- Orme I. Beyond BCG. The potential for a more effective TB vaccine. Mol Med Today 1997;5:487-92; http://dx.doi.org/10.1016/S1357-4310(99)01594-4
- Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomized controlled studies Lancet 2002; 359:1393-401.
- Weir RE, Fine PE, Floyd S. Comparison of IFN-gamma responses to mycobacterial antigens as markers of response to BCG vaccination. Tuberculosis (Edinb) 2008; 88:31-8; PMID:18277396; http://dx.doi.org/10.1016/j.tube.2007.10.001
- Hussain S, Afzal N, Javaid K, Ullah MI, Ahmad T, Saleem-Uz-Zaman. Level of interferon gamma in the blood of tuberculosis patients. Iran J Immunol 2010; 4:240-6
- Lalor MK, Ben-Smith A, Gorak-Stolinska P, Weir RE, Floyd S, Blitz R, Mvula H, Newport MJ, Branson K, McGrath N, et al. Population differences in immune responses to Bacille Calmette Guerin vaccination in infancy. J Infect Disease 2009; 199:795-800; PMID:19434928; http://dx.doi.org/10.1086/597069
- Mussi-Pinhata MM, Goncalves AL, Foss NT. BCG vaccination of full term infants with chronic intrauterine malnutrition: Influence of immunization age on development of post vaccination delayed tuberculin hypersensitivity. Bull WHO 1993;71:41-8; PMID:8440036
- Stogman W. BCG vaccination. Wein Med Wochenschr 1991; 141:265-70
- Casonava JL, Blanche S, Emile JF, Jouanguy E, Lamhamedi S, Altare F, Stéphan JL, Bernaudin F, Bordigoni P, Turck D, et al. Idiopathic Disseminated BCG infection: A French National Retrospective Study. Pediatrics 1996; 98:774-8; PMID:8885960
- Faridi M, Kaur S, Krishnamurthy S, Kumar P. Tuberculin conversion and leucocyte migration inhibition test after BCG vaccination in newborn infants. Hum Vaccine 2009; 5:690-5; http://dx.doi.org/10.4161/hv.5.10.9317
- Pouchot J, Grasland A, Collet C, Coste J, Esdaile JM, Vinceneux P. Reliability of tuberculin skin test measurement. Ann Intern Med 1997; 126:210-4; PMID:9027271; http://dx.doi.org/10.7326/0003-4819-126-3-199702010-00005
