Abstract
The highly pathogenic avian influenza (HPAI) H5N1 virus remains a threat to public health because of its continued spread in poultry in some countries and its ability to infect humans with high mortality rate, calling for the development of effective and safe vaccines against H5N1 infection. Here, we constructed 4 candidate vaccines by fusing H5N1 hemagglutinin 1 (HA1) with foldon (HA1-Fd), human IgG Fc (HA1-Fc), foldon and Fc (HA1-FdFc) or His-tag (HA1-His). We then compared their ability to induce mucosal immune responses and neutralizing antibodies in the presence or absence of Poly(I:C) and CpG adjuvants via the intranasal route. Without an adjuvant, HA1-FdFc could elicit appreciable humoral immune responses and local mucosal IgA antibodies in immunized mice, while other vaccine candidates only induced background immune responses. In the presence of Poly(I:C) and CpG, both HA1-Fd and HA1-Fc elicited much higher levels of serum IgG and local mucosal IgA antibodies than HA1-His. Poly(I:C) and CpG could also augment the neutralizing antibody responses induced by these 4 vaccine candidates in the order of HA1-FdFc > HA1-Fc > HA1-Fd > HA1-His. These results suggest that both Fd and Fc potentiate the immunogenicity of the recombinant HA1 protein and that Poly(I:C) and CpG serve as efficient mucosal adjuvants in promoting efficacy of these vaccine candidates to induce strong systemic and local antibody responses and potent neutralizing antibodies, providing a useful strategy to develop effective and safe mucosal H5N1 vaccines.
Introduction
The highly pathogenic avian influenza (HPAI) H5N1 is an influenza A virus transmitted between birds and humans. Since the first outbreak of H5N1 in Hong Kong,Citation1 the virus has spread to the Middle East, Africa and Europe. From January 2003 to March 2015, a total of 784 H5N1-infected human cases with 429 deaths were reported to the WHO (~55% mortality rate) (http://www.who.int/influenza/human_animal_interface/EN_GIP_20150303cumulativeNumberH5N1cases.pdf). Consequently, development of novel strategies is urgently needed to prevent further spread of H5N1, among which vaccination is considered as one of the most effective intervention tools to control virus infection
Influenza virus hemagglutinin (HA) is a homotrimeric membrane glycoprotein on the viral surface. HA monomers are initially synthesized as precursors, followed by cleavage of each precursor polypeptide by host proteases into 2 smaller polypeptides, HA1 and HA2 ().Citation2 The design of recombinant HA-based vaccines against H5N1 is based on the role of HA in inducing highly potent neutralizing antibody responses.Citation3-5
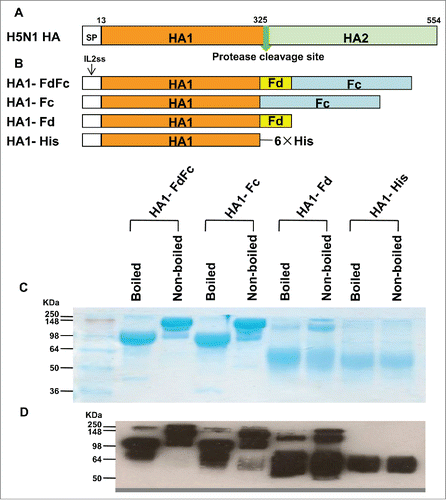
Figure 1. Schematic structures of HA protein of H5N1 and construction and characterization of recombinant HA1-FdFc, HA1-Fc, HA1-Fd, and HA1-His proteins. (A) Schematic structures of HA protein of A/Anhui/1/2005 (H5N1 HA). The HA protein consists of signal peptide (SP, residues 1-12), HA1 (residues 13-325) and HA2 (residues 326-554) fragments. (B) Construction of HA1-FdFc, HA1-Fc, HA1-Fd, and HA1-His protein fragments. The four fragments were constructed by fusing HA1 fragment with Fd and Fc (HA1-FdFc), Fc (HA1-Fc), Fd (HA1-Fd), or His6, respectively. IL2ss, a signal peptide, induces expressed proteins to culture supernatants. The purified protein samples were detected by SDS-PAGE, following by staining with Coomassie Blue (C), or Western blot using HA1-specific HA-7 mAb (D).
Currently, intramuscular (i.m.) and subcutaneous (s.c.) injections, as traditional vaccination methods, have been extensively utilized in clinical vaccine research.Citation6 However, vaccines immunized through these pathways fail to elicit sufficient mucosal immune responses and are therefore not providing effective protection against infections at the mucosal surface,Citation7,8 the place where is most easily attached by influenza virus. In contrast, mucosal vaccines immunized through the intranasal (i.n.) pathway are expected to induce strong local and mucosal immune responses. Intranasal vaccination is painless and easy to use, particularly suitable for children.Citation9
Adjuvant is essential for enhancing immune responses to vaccine antigens and therefore plays a vital role in the development of effective subunit mucosal vaccines. CpG-containing oligodeoxynucleotides (CpG ODN), polyinosinic-polycytidylic acid (Poly(I:C)), cholera holotoxin, the cholera toxin B subunit (CTB), the Escherichia coli–derived heat-labile enterotoxin, and lactosylceramide have all been reported as mucosal adjuvants.Citation10 Among them, CpG ODN, a short synthetic DNA sequence, activates the immune system by inducing toll-like receptor 9 (TLR9), while Poly(I:C), a synthetic dsRNA, is a TLR3 agonist.Citation11 By their well-defined mechanisms of action, CpG and poly (I:C) are frequently used as adjuvants in vaccine development.Citation11,12
Foldon (Fd) is a natural trimerization domain, derived from native T4 bacteriophage fibritin.Citation13,14 It is reported that the stabilization of Fd-based trimeric structures is better than others, such as IQ (a trimeric GCN4 motif: RMKQIEDKIEEIESKQKKIENEIARIKK) and IZ (a trimeric motif: IKKEIEAIKKEQEAIKKKIEAIEK),Citation13 and that Human IgG1 Fc, an immunoenhancer, may significantly improve immunogenicity of subunit vaccines.Citation4,15 Our previous study has shown that recombinant proteins containing HA1 of H5N1 fused with Fc with or without Fd (HA1-Fc and HA1-FdFc) induced neutralizing antibodies via traditional subcutaneous route.Citation3
In the present study, we constructed 2 additional proteins by fusing HA1 of H5N1 with or without Fd (HA1-Fd, HA1-His) and compared the mucosal immune responses and neutralizing activity induced by the 4 recombinant proteins expressing HA1 of H5N1 in the presence or absence of Poly(I:C) and CpG adjuvants. Moreover, we adopted intranasal pathway as a mouse vaccination method, which is an attractive alternative to injected vaccination, as explained above.
Results
Characterization of the 4 recombinant proteins
The HA1 of A/Anhui/1/2005(H5N1) (AH/1) was fused with foldon (HA1-Fd), human IgG1 Fc (HA1-Fc), foldon and Fc (HA1-FdFc), or His-tag (HA1-His) to form the recombinant vaccine candidates, according to the previously described methods ().Citation3,4 All 4 recombinant proteins were expressed in the culture supernatant of transfected 293T cells, followed by purification of the proteins using protein A columns, and analyzed by SDS-PAGE and Western-blot.Citation4,15 As shown in , the molecular weight of nonboiled HA1-Fc and HA1-FdFc proteins is about one-fold higher than that of the boiled samples, suggesting that the boiled proteins are in monomeric, while the nonboiled proteins are mainly in dimeric form. The molecular weight of the major band of the nonboiled HA1-Fd is about 3-fold higher than that of the major band of boiled sample, indicating that most nonboiled HA1 is in trimeric form, whereas the boiled HA1 is mainly in monomeric form. However, both the nonboiled and boiled HA1-His protein remained in monomeric form. The above results confirm that the purified HA1s fused with Fc and/or Fd maintain dimeric or trimeric structures, respectively, indicating consistency with the results of our previous report.Citation3,4,15 Western analysis showed that all 4 proteins could be recognized by H5N1 HA1-specific monoclonal antibody (mAb) developed in our laboratory ().Citation16
Intranasal immunization of H5N1 HA1 proteins fused with Fc and/or Fd induced strong humoral immune responses in immunized mice
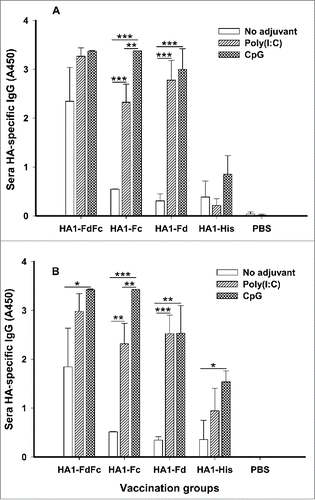
To evaluate the humoral antibody responses induced by the 4 recombinant protein candidates, mice were immunized, and sera were collected 10 days post-last vaccination to detect HA1-specific IgG by ELISA (). As shown in , in the presence of Poly(I:C) and CpG adjuvants, HA1-FdFc, HA1-Fc and HA1-Fd proteins induced stronger HA1-specific IgG antibody response than HA1-His protein when the plates were coated with respective HA1 fusion proteins () or HA1-His protein (), indicating the high specificity of induced immune responses to H5N1 HA1. In addition, stronger IgG antibodies were induced by HA1-FdFc than HA1-Fc or HA1-Fd for both adjuvants, except for the groups of HA1-Fc plus CpG (). Significant differences were observed between Poly(I:C) and CpG groups for HA1-Fc (). Surprisingly, in the absence of adjuvant, HA1-FdFc protein was still able to elicit stronger IgG antibody response than the other proteins, indicating its potential to serve as a good nonadjuvant, mucosal vaccine candidate (). No IgG antibody response was detectable in the mouse sera of PBS control ()
Figure 2. Mouse vaccination procedure and sample collection. Groups of mice were i.n. immunized with HA1-FdFc, HA1-Fc, HA1-Fd, and HA1-His protein, respectively, or PBS, in the presence of Poly (I:C) or CpG adjuvant, or without adjuvant. Mice were immunized 3 times at 3-week intervals. Ten days later, after the last vaccination, mouse sera and lung wash were collected to detect IgG, IgA, and neutralizing antibodies.
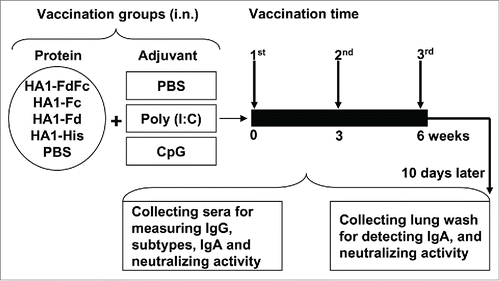
Figure 3. Detection of IgG antibody responses by ELISA in mice immunized with HA1 fusion proteins plus Poly(I:C) or CpG adjuvant. PBS with or without adjuvants was used as the negative control. ELISA plates were coated with HA1-FdFc, HA1-Fc, HA1-Fd, or HA1-His protein, respectively (A), or full-length HA1-His protein (B), and IgG antibody was detected using mouse sera (1:3,200) from 10 days post-last vaccination. The data are presented as A450 ± SD of 4 mice per group. The *, ** and *** indicate the significant difference with P < 0.05, 0.01 and 0.001, respectively, between the groups with or without adjuvants.
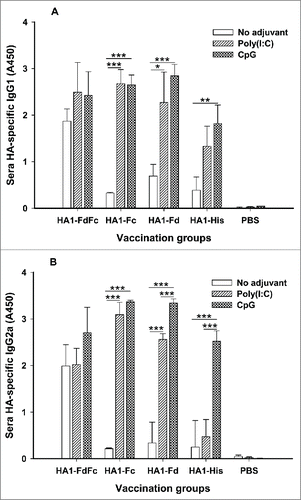
IgG1 and IgG2a subtypes induced by HA1 fusion proteins were then investigated in the mouse sera collected at 10 days post-last vaccination. In the presence of Poly(I:C) and CpG adjuvants, HA1-FdFc, HA1-Fc and HA1-Fd elicited similarly high levels of HA1-specific IgG1 (), and IgG2a induced by either HA1-Fc or HA1-Fd plus CpG was also higher than the other groups (). In addition, significant differences were revealed between Poly(I:C) and CpG groups for HA1-Fd-induced IgG1 () or HA1-Fc-, HA1-Fd-, and HA1-His-induced IgG2a, respectively (). No IgG1 or IgG2a antibody response was found in the mouse sera of PBS control (). Similar to IgG, HA1-FdFc protein, but not the other proteins, also induced strong IgG1 and IgG2a antibodies in the absence of adjuvants ()
Figure 4. Detection of IgG1 and IgG2a subtype antibody responses by ELISA in mice immunized with HA1 fusion proteins plus Poly(I:C) or CpG adjuvant. PBS with or without adjuvants was used as the negative control. The ability of IgG1 (A) and IgG2a (B) antibodies to bind H5N1 HA1 protein was detected using sera from 10 days post-last vaccination. The data are presented as A450 ± SD of 4 mice per group. The *, ** and *** indicate the significant difference with P < 0.05, 0.01 and 0.001, respectively, between the groups with or without adjuvants.
The above data suggested that H5N1 HA1 protein plus Fc and Fd has adjuvanticity in inducing humoral immune responses and that HA1 fusion proteins with adjuvants were able to induce strong antibody responses via the mucosal route
Intranasal immunization of H5N1 HA1 proteins fused with Fc and/or Fd induced strong mucosal immune responses in immunized mice
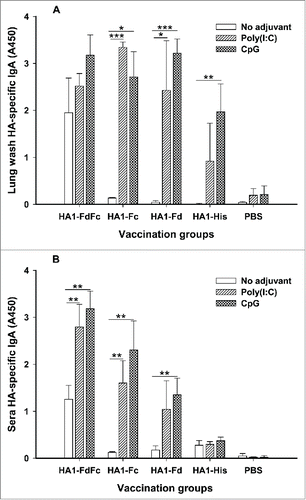
To elucidate the mucosal immune responses induced by HA1 fusion proteins, mouse lung washes and sera from 10 days post-last immunization were tested for IgA antibody. As shown in , in the presence of Poly(I:C) and CpG adjuvants, HA1-FdFc, HA1-Fc and HA1-Fd induced strong HA1-specific IgA antibody response in the lung wash. In general, CpG promoted HA1 fusion proteins, particularly HA1-FdFc and HA1-Fd, to elicit higher, or significantly higher, IgA antibody than Poly(I:C), while the IgA induced by Poly(I:C) was significantly higher than CpG for HA1-Fc. Analysis of serum IgA revealed that HA1-Fc, particularly HA-FdFc, elicited significantly higher IgA in the presence of CpG than Poly(I:C) (). Compared with other proteins, HA1-FdFc alone without adjuvants was able to induce IgA antibody response in both lung wash and sera (), suggesting that H5N1 HA1 protein plus Fc and Fd has adjuvanticity in inducing mucosal immune responses. On the contrary, low, to no, IgA antibody was detected by HA1-Fc, HA1-Fd and HA1-His proteins without adjuvants (), indicating that mucosal adjuvants Poly(I:C) and, particularly, CpG, play an important role in inducing mucosal IgA antibody responses for these proteins. As expected, only background level of IgA was detected in mouse lung wash and sera of PBS control (). The above data confirmed the ability of H5N1 HA1 fusion proteins to induce strong mucosal immune responses through the intranasal pathway
Figure 5. Detection of IgA antibody responses by ELISA in mice immunized with HA1 fusion proteins plus Poly(I:C) or CpG adjuvant. PBS with or without adjuvants was used as the negative control. The ability of IgA to bind H5N1 HA1 protein was detected using mouse lung wash (A) and sera (B) from 10 days post-last vaccination. The data are presented as A450 ± SD of 4 mice per group. The *, ** and *** indicate the significant difference with P < 0.05, 0.01 and 0.001, respectively, between the groups with or without adjuvants.
Intranasal immunization of H5N1 HA1 proteins fused with Fc and/or Fd induced strong neutralizing antibodies in immunized mice
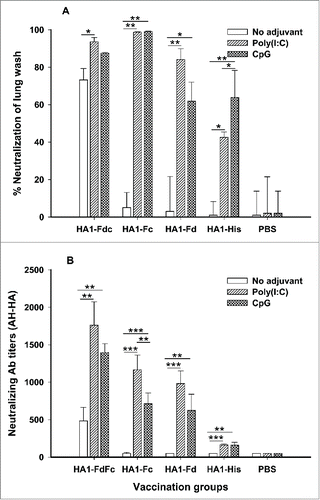
To elucidate neutralizing potential induced by these HA1 fusion proteins, mouse sera and lung wash from 10 days post-last immunization were tested for neutralization against H5N1 pseudovirus. As indicated in , HA1-FdFc and HA1-Fc plus Poly(I:C) and CpG adjuvants induced similarly high levels of neutralizing antibodies in lung wash, inhibiting over 80% of pseudovirus entry into target cells. While HA1-FdFc alone without adjuvant showed 72% inhibitory activity in lung wash (), the rate of inhibition induced by HA1-Fd plus Poly(I:C) was 84% in lung wash (). Further evaluation of the neutralizing activity of these proteins in vaccinated mouse sera revealed that proteins with Fd and/or Fc were able to induce a significantly higher level of neutralizing antibodies than proteins with His6 tag against homologous (AH-HA) H5N1 pseudovirus (). Surprisingly, proteins plus Poly(I:C) and CpG adjuvants induced a significantly higher level of neutralizing antibodies than proteins without adjuvants (). Among them, proteins plus Poly(I:C) adjuvants induced the highest level of neutralizing antibody titers. As expected, no neutralizing activity was detected in the PBS control group (). These results demonstrated the ability of H5N1 HA1 fusion proteins to induce strong neutralizing antibodies through the intranasal pathway.
Figure 6. Comparison of neutralizing antibodies in lung wash (A) and sera (B) of mice immunized with HA1 fusion proteins plus Poly(I:C) or CpG adjuvant. PBS with or without adjuvants was used as the negative control. The lung wash (1:1,000) and sera from 10 days post-last immunization were pooled in each group and tested for neutralizing antibodies. The data of panel A are presented as mean percentages of inhibition of duplicated wells. The data are presented as mean ± SD of duplicated wells. The *, ** and *** indicate the significant difference with P < 0.05, 0.01 and 0.001, respectively.
Discussion
The continuous spread and unpredictability of HPAI H5N1 demonstrate the need to develop effective influenza vaccines, among which viral HA protein is an important vaccine target based on its crucial role in receptor binding and membrane fusion.Citation3,4 Recombinant protein-based subunit vaccines are considered safer than other vaccine types, such as inactivated virus-based vaccine, VLP-based vaccine and viral vector-based vaccine,Citation17,18 making them essential for further development as a prophylactic against H5N1 infection. As a rule, however, subunit vaccines themselves cannot induce strong immune responses, requiring the utilization of appropriate adjuvants to improve their efficacy. As a mucosal pathogen, influenza virus infects humans mainly through the mucosal route.Citation15 Thus, pairing a suitable mucosal adjuvant with a rationally designed mucosal subunit vaccines should increase the efficiency of H5N1 influenza vaccines
The importance of Fc and Fd as fusion partners of subunit vaccines has been extensively studied. For example, Fc of human IgG could be used as a motif for coexpression with viral proteins to form conformational structures, thereby increasing the immunogenicity of proposed proteins.Citation3,4,19-21 By helping fusion proteins with correct folding, Fc promotes their binding to antigen-presenting cells (APCs) or Fc receptor-expressing cells.Citation22,23 At the same time, Fd, a trimeric motif, can enhance the immunogenicity and improve the antiviral activity of recombinant proteins.Citation21,24
Our previous studies have shown that subcutaneously immunized full-length or partial-length H5N1 HA1 proteins fused with Fc and/or Fd induced strong immune responses, neutralizing activity and cross-protection against divergent H5N1 viruses,Citation3,4,15 suggesting the importance of such HA1-based subunit vaccines in the prevention of H5N1 infection. Studies have also demonstrated that Poly(I:C) and CpG enhanced mucosal and humoral immune responses in vaccinated mice, and these TLR-based adjuvants can function on APCs that are associated with innate and adaptive immunity.Citation25,26 Therefore, this study aimed to investigate the possibility of inducing mucosal immunity using recombinant Fc- and Fd-fused full-length H5N1 HA1 proteins as model antigens and Poly(I:C) and CpG as adjuvants administered through the mucosal route
Our data showed that HA1 proteins fused with Fc and/or Fd did, indeed, result in the formation of conformational structures and did induce strong HA1-specific IgG antibody responses in immunized mice, while the monomeric HA1-His without fusion with Fc and Fc had no such ability, suggesting the importance of conformational structures in the induction of strong humoral immune responses. These results agree with other studies showing that proteins able to maintain native trimeric or oligomeric structures of HA could induce stronger immune responses than monomers.Citation27-29 In addition, mucosal immunization of HA1-FdFc and HA1-Fc proteins plus Poly(I:C) and CpG adjuvants induced high level of IgA antibodies, particularly in the vaccinated mouse lung wash, suggesting that Poly(I:C) and CpG, as mucosal adjuvants, worked very well with H5N1 HA1 fusion proteins
Our results also revealed that sera and lung wash from immunized HA1 proteins with Fc and/or Fd plus adjuvants efficiently inhibited H5N1 pseudovirus entry into target cells, demonstrating the ability of these proteins to elicit strong neutralizing antibodies through the mucosal route. While HA1 with Fd and Fc without adjuvant showed inhibitory activity in these samples, HA1 fused with Fc and/or Fd with Poly(I:C) and CpG adjuvants could significantly improve this inhibitory activity, suggesting that HA1-FdFc itself has adjuvanticity, but that adjuvant still plays an important role in promoting the efficacy of HA1-based subunit H5N1 vaccines
In summary, this study identified promising recombinant H5N1 mucosal subunit vaccines, demonstrated their ability to induce strong local mucosal and humoral systemic immune responses, and elucidated the mechanism of mouse antisera in the inhibition of virus infection. It also proved the importance of mucosal adjuvants in the promotion of the aforementioned immune responses and neutralizing antibodies via the mucosal route. Taken together, this study will provide useful guidance for the development of novel, effective, and safe H5N1 subunit vaccines
Materials and Methods
Ethics statement
Six- to 8-week-old female BALB/c mice were used in the study. All animal studies were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the American Veterinary Medical Association (AVMA). The animal protocol was approved by the Committee on the Ethics of Animal Experiments of the New York Blood Center (Approval number: 322.04)
Construction, expression and purification of recombinant HA1 proteins
The construction, expression and purification of recombinant HA1-Fd and HA1-His proteins were performed as previously described.Citation3 Briefly, the genes encoding full-length HA1 containing residues 13-325 of A/Anhui/1/2005(H5N1)(AH/1) (GenBank: ABD28180) fused with Fd were amplified by PCR using a recombinant HA1 plasmid fused with Fd and human IgG1 Fc (HA1-FdFc) as the template. A 6× Histidine (His) tag was added at the C-termini of HA1 (HA1-His) for easy purification of the proteins.Citation3 The amplified gene fragments were digested and inserted into the pFUSE-hIgG1-Fc2 expression vector (hereinafter named Fc, InvivoGen, San Diego, CA), followed by transfecting recombinant plasmids into 293T cells using the calcium phosphate method. Culture medium was replaced by fresh Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Grand Island, NY) 10 h later, and supernatant was collected 72 h post-transfection. The recombinant proteins were purified by Protein affinity chromatography (GE Healthcare, Piscataway, NJ) for proteins with Fc, or Ni-NTA Superflow (Qiagen, Valencia, CA) for proteins with His tag, according to the manufacturers' instructions. Constructed recombinant fragments were shown in
SDS-PAGE and Western blot
The purified HA1 proteins were analyzed by SDS-PAGE and Western blot as previously described.Citation3 Briefly, purified proteins (10 µg) were either nonboiled or boiled at 95°C for 5 min, separated by 10% Tris-Glycine SDS-PAGE gels, and then stained with Coomassie Blue or transferred to nitrocellulose membranes. After blocking overnight at 4°C, the blots were incubated with HA-7 mAb (1:3,000)Citation16 for 1 h at room temperature. After three washes, the blots were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:5,000, Invitrogen) for 1 h at room temperature. Signals were visualized with ECL Western blot substrate reagents and Amersham Hyperfilm (GE Healthcare)
Mouse vaccination and sample collection
Mice were i.n. immunized with the aforementioned HA1 proteins for 3 times at 3-week intervals (10 µg/200 µl/mouse) in the presence or absence of Poly(I:C) and CpG adjuvants. Control mice were i.n. injected with the same amount of PBS with or without Poly(I:C) and CpG, respectively (). Sera were collected at 10 days post-last vaccination to measure HA-specific IgG, subtypes and neutralizing antibodies. Mice were then sacrificed, and lung wash was collected to detect IgA and neutralizing antibodies
ELISA
Antibody responses were evaluated by ELISA in collected mouse sera as previously described.Citation30 Briefly, 96-well ELISA plates were precoated with respective HA1 fusion proteins or full-length HA1-His protein overnight at 4°C and blocked with 2% non-fat milk at 37°C for 2 h. Serially diluted mouse sera or lung wash (1:1,000) were added to the plates and incubated at 37°C for 1 h, followed by 4 washes. Bound antibodies were incubated with HRP-conjugated goat anti-mouse IgG (1:2,000, Invitrogen), anti-mouse IgG1 (1:2,000, Bethyl Laboratories, Montgomery, TX), anti-mouse IgG2a (1:2,000, Invitrogen) or anti-mouse IgA (1:2,000, Invitrogen) at 37°C for 1 h. The reaction was visualized by substrate 3,3′,5,5′-Tetramethylbenzidine (TMB) (Invitrogen) and stopped by 1 N H2SO4. The absorbance at 450 nm (A450) was measured by ELISA plate reader (Tecan, San Jose, CA, USA)
Pseudovirus neutralization Assay
Neutralizing activity in collected mouse sera and lung wash was performed by pseudovirus neutralization assay as previously described.Citation31 Briefly, 293T cells were cotransfected with a plasmid encoding Env-defective, luciferase-expressing HIV-1 genome (pNL4-3.luc.RE) and the plasmid encoding HA of homologous AH/1 (AH-HA). Exogenous bacterial neuraminidase (NA) (Sigma) was added 24 and 48 h later, and supernatants were harvested 72 h post-transfection for single-cycle infection. The pseudovirus-containing supernatants were incubated with serially diluted mouse sera or lung wash at 37°C for 1 h before adding to 293T cells. Fresh medium was added 24 h later, followed by lysing cells 72 h later using cell lysis buffer (Promega, Madison, WI) and transferring the lysates into 96-well luminometer plates. Luciferase substrate (Promega) was added to the plates, and relative luciferase activity was determined in an Ultra 384 luminometer (Tecan). The neutralizing antibody was calculated and presented as percentages of inhibition of virus infection
Disclosure of Potential Conflicts of Interest
The authors declared no conflict of interest.
Funding
This study was supported by the grants from the National Institute of Allergy And Infectious Diseases of the National Institutes of Health (R21AI111152) and CAST, HK, Macau, Taiwan Collaborative Programs (201200007673).
References
- Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 1998; 279:393-6; PMID:9430591; http://dx.doi.org/10.1126/science.279.5349.393
- Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, Wilson IA. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science 2006; 312:404-10; PMID:16543414; http://dx.doi.org/10.1126/science.1124513
- Du L, Leung VH, Zhang X, Zhou J, Chen M, He W, Zhang HY, Chan CC, Poon VK, Zhao G, et al. A recombinant vaccine of H5N1 HA1 fused with foldon and human IgG Fc induced complete cross-clade protection against divergent H5N1 viruses. PLoS One 2011; 6:e16555; PMID:21304591; http://dx.doi.org/10.1371/journal.pone.0016555
- Du L, Zhao G, Sun S, Zhang X, Zhou X, Guo Y, Li Y, Zhou Y, Jiang S. A critical HA1 neutralizing domain of H5N1 influenza in an optimal conformation induces strong cross-protection. PLoS One 2013; 8:e53568; PMID:23320093; http://dx.doi.org/10.1371/journal.pone.0053568
- Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Wilson IA, García-Sastre A, Moran TM, Palese P. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci USA 2010; 107:18979-84; PMID:20956293; http://dx.doi.org/10.1073/pnas.1013387107
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 2006; 6:148-58; PMID:16491139; http://dx.doi.org/10.1038/nri1777
- Levine MM. Immunization against bacterial diseases of the intestine. J Pediatr Gastroenterol Nutr 2000; 31:336-55; PMID:11045827; http://dx.doi.org/10.1097/00005176-200010000-00003
- Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol 1997; 51:311-40; PMID:9343353; http://dx.doi.org/10.1146/annurev.micro.51.1.311
- Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, Bernstein DI, Hayden FG, Kotloff K, Zangwill K, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med 1998; 338:1405-12; PMID:9580647; http://dx.doi.org/10.1056/NEJM199805143382002
- Wegmann F, Gartlan KH, Harandi AM, Brinckmann SA, Coccia M, Hillson WR, Kok WL, Cole S, Ho LP, Lambe T, et al. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat Biotechnol 2012; 30:883-8; PMID:22922673; http://dx.doi.org/10.1038/nbt.2344
- Chuai X, Chen H, Wang W, Deng Y, Wen B, Ruan L, Tan W. Poly(I:C)/alum mixed adjuvant priming enhances HBV subunit vaccine-induced immunity in mice when combined with recombinant adenoviral-based HBV vaccine boosting. PLoS One 2013; 8:e54126; PMID:23335993; http://dx.doi.org/10.1371/journal.pone.0054126
- Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011; 470:543-7; PMID:21350488; http://dx.doi.org/10.1038/nature09737
- Yang X, Lee J, Mahony EM, Kwong PD, Wyatt R, Sodroski J. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J Virol 2002; 76:4634-42; PMID:11932429; http://dx.doi.org/10.1128/JVI.76.9.4634-4642.2002
- Papanikolopoulou K, Forge V, Goeltz P, Mitraki A. Formation of highly stable chimeric trimers by fusion of an adenovirus fiber shaft fragment with the foldon domain of bacteriophage t4 fibritin. J Biol Chem 2004; 279:8991-8; PMID:14699113; http://dx.doi.org/10.1074/jbc.M311791200
- Li Y, Du L, Qiu H, Zhao G, Wang L, Zhou Y, Jiang S, Gao J. A recombinant protein containing highly conserved hemagglutinin residues 81-122 of influenza H5N1 induces strong humoral and mucosal immune responses. Biosci Trends 2013; 7:129-37; PMID:23836036
- Du L, Jin L, Zhao G, Sun S, Li J, Yu H, Li Y, Zheng BJ, Liddington RC, Zhou Y, et al. Identification and structural characterization of a broadly neutralizing antibody targeting a novel conserved epitope on the influenza virus H5N1 hemagglutinin. J Virol 2013; 87:2215-25; PMID:23221567; http://dx.doi.org/10.1128/JVI.02344-12
- Zhang N, Jiang S, Du L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Rev Vaccines 2014; 13:761-74; PMID:24766432; http://dx.doi.org/10.1586/14760584.2014.912134
- Zhang N, Tang J, Lu L, Jiang S, Du L. Receptor-binding domain-based subunit vaccines against MERS-CoV. Virus Res 2014; 202:151-9; PMID:25445336
- He Y, Zhou Y, Liu S, Kou Z, Li W, Farzan M, Jiang S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun 2004; 324:773-81; PMID:15474494; http://dx.doi.org/10.1016/j.bbrc.2004.09.106
- He Y, Lu H, Siddiqui P, Zhou Y, Jiang S. Receptor-binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation-dependent epitopes that induce highly potent neutralizing antibodies. J Immunol 2005; 174:4908-15; PMID:15814718; http://dx.doi.org/10.4049/jimmunol.174.8.4908
- Chen X, Lu L, Qi Z, Lu H, Wang J, Yu X, Chen Y, Jiang S. Novel recombinant engineered gp41 N-terminal heptad repeat trimers and their potential as anti-HIV-1 therapeutics or microbicides. J Biol Chem 2010; 285:25506-15; PMID:20538590; http://dx.doi.org/10.1074/jbc.M110.101170
- Chen H, Xu X, Jones IM. Immunogenicity of the outer domain of a HIV-1 clade C gp120. Retrovirology 2007; 4:33; PMID:17509143; http://dx.doi.org/10.1186/1742-4690-4-33
- Martyn JC, Cardin AJ, Wines BD, Cendron A, Li S, Mackenzie J, Powell M, Gowans EJ. Surface display of IgG Fc on baculovirus vectors enhances binding to antigen-presenting cells and cell lines expressing Fc receptors. Arch Virol 2009; 154:1129-38; PMID:19557497; http://dx.doi.org/10.1007/s00705-009-0423-8
- Meier S, Guthe S, Kiefhaber T, Grzesiek S. Foldon, the natural trimerization domain of T4 fibritin, dissociates into a monomeric A-state form containing a stable β-hairpin: atomic details of trimer dissociation and local β-hairpin stability from residual dipolar couplings. J Mol Biol 2004; 344:1051-69; PMID:15544812; http://dx.doi.org/10.1016/j.jmb.2004.09.079
- Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine 2011; 29:3341-55; PMID:20713100; http://dx.doi.org/10.1016/j.vaccine.2010.08.002
- Beutler BA. TLRs and innate immunity. Blood 2009; 113:1399-407; PMID:18757776; http://dx.doi.org/10.1182/blood-2008-07-019307
- Weldon WC, Wang BZ, Martin MP, Koutsonanos DG, Skountzou I, Compans RW. Enhanced immunogenicity of stabilized trimeric soluble influenza hemagglutinin. PLoS One 2010; 5:e12466; PMID:20824188; http://dx.doi.org/10.1371/journal.pone.0012466
- Loureiro S, Ren J, Phapugrangkul P, Colaco CA, Bailey CR, Shelton H, Molesti E, Temperton NJ, Barclay WS, Jones IM. Adjuvant-free immunization with hemagglutinin-Fc fusion proteins as an approach to influenza vaccines. J Virol 2011; 85:3010-4; PMID:21191017; http://dx.doi.org/10.1128/JVI.01241-10
- Wei CJ, Xu L, Kong WP, Shi W, Canis K, Stevens J, Yang ZY, Dell A, Haslam SM, Wilson IA, et al. Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian H5N1 influenza virus. J Virol 2008; 82:6200-8; PMID:18417563; http://dx.doi.org/10.1128/JVI.00187-08
- Du L, Zhao G, He Y, Guo Y, Zheng BJ, Jiang S, Zhou Y. Receptor-binding domain of SARS-CoV spike protein induces long-term protective immunity in an animal model. Vaccine 2007; 25:2832-8; PMID:17092615; http://dx.doi.org/10.1016/j.vaccine.2006.10.031
- Du L, Zhao G, Zhang X, Liu Z, Yu H, Zheng BJ, Zhou Y, Jiang S. Development of a safe and convenient neutralization assay for rapid screening of influenza HA-specific neutralizing monoclonal antibodies. Biochem Biophys Res Commun 2010; 397(3):580-5; PMID:20617558; http://dx.doi.org/10.1016/j.bbrc.2010.05.161
