Abstract
Since cancer vaccines do not always elicit beneficial effects in treated patients, identification of biomarkers for predicting clinical outcomes would be highly desirable. We previously reported that abnormal granulocytes present in peripheral blood mononuclear cells (PBMC) may contribute to poor prognosis in advanced prostate cancer patients receiving personalized peptide vaccination (PPV). In the current study, we examined whether soluble factors derived from granulocytes, such as matrix metalloproteinase 9 (MMP-9), myeloperoxidase (MPO), and arginase 1 (ARG1), and inhibitory cytokine TGFβ in pre-vaccination plasma were useful for predicting prognosis after PPV in advanced cancer patients. In biliary tract cancer (n=25), multivariate Cox regression analysis demonstrated that patients with higher plasma MMP-9 levels had a significantly worse overall survival (OS) [hazard ratio (HR) = 4.637, 95% confidence interval (CI) = 1.670 - 12.877, P = 0.003], whereas MPO, ARG1, or TGFβ levels were not correlated with OS. Similarly, patients with higher MMP-9 levels showed worse prognosis than those with lower MMP-9 levels in other types of advanced cancers, including non-small cell lung cancer (n=32, P = 0.037 by log-rank test), and pancreatic cancer (n=41, P = 0.042 by log-rank test). Taken together, plasma MMP-9 levels before vaccination might be potentially useful as a biomarker for selecting advanced cancer patients who would benefit from PPV.
Introduction
Although recent advances in chemotherapies and/or targeted therapies have helped to improve clinical outcomes in patients with various types of advanced cancers, the prognosis of cancer patients at a refractory stage still remains very poor.Citation1-3 Therefore, the development of new therapeutic approaches, including cancer vaccines, would be highly desirable.Citation4-7 Although many clinical trials of cancer vaccines were conducted for advanced cancer patients, most of them have failed to demonstrate any meaningful therapeutic benefit over existing treatments.Citation4-7 Since current cancer vaccines do not elicit beneficial effects in all of the treated patients, identification of biomarkers for predicting clinical outcomes would be highly desirable.Citation8,9
We have developed a new approach of peptide-based vaccination, named “personalized peptide vaccination (PPV),” in which vaccine antigens are individually selected and administered based on pre-existing host immunity before vaccination.Citation10,11 We have shown promising results of PPV in various types of advanced cancers.Citation11-16 Recently, we have reported that abnormal granulocyte signature, which was assessed by DNA microarray analysis in peripheral blood mononuclear cells (PBMC), may contribute to decreased overall survival (OS) in advanced castration-resistant prostate cancer (CRPC) patients receiving PPV.Citation17 The patients with higher mRNA expression of granulocyte-related genes, such as matrix metalloproteinase 9 (MMP-9), myeloperoxidase (MPO), and arginase 1 (ARG1), showed poorer prognosis than those with lower mRNA expression.Citation17 In the current study, we further examined whether assessment of soluble factors derived from these granulocyte-related genes, MMP-9, MPO, and ARG1, and inhibitory cytokine TGFβ in pre-vaccination plasma were useful for predicting prognosis after PPV in patients with advanced cancers, including biliary tract cancer (BTC), non-small cell lung cancer (NSCLC), and pancreatic cancer (PC). Our results suggested that analysis of plasma MMP-9 would be informative for selecting cancer patients, who would likely benefit from PPV.
Results
Prognostic significance of MMP-9, MPO, ARG1, and TGFβ in advanced BTC patients undergoing PPV
We previously analyzed pre-vaccination clinical findings or laboratory data, including complete blood counts, serum biochemistry tests, cytokines, and inflammation markers, to identify potential prognostic biomarkers in advanced BTC patients undergoing PPV (n = 25).Citation14 The multivariate Cox regression analysis demonstrated that lower IL-6 and higher albumin levels before vaccination and greater numbers of peptides selected for vaccination were significantly favorable factors for OS.Citation14 In the current study, we further assessed prognostic significance of other soluble factors, including MMP-9, MPO, ARG1, and TGFβ, in pre-vaccination blood samples from the same cohort of BTC patients (n = 25). The levels of MMP-9 were highly correlated with those of MPO (Spearman rank correlation coefficient, 0.6954; p = 0.0001), but no correlations were observed between other factors.
As shown in , univariate Cox regression analysis showed that the levels of MMP-9 (P = 0.003) and MPO (P = 0.049), but not ARG1 (P = 0.641) or TGFβ (P = 0.239), in pre-vaccination blood samples were significantly associated with OS. After dividing the patients into 2 subgroups according to the median value of these factors, the survival curves were estimated by the Kaplan-Meier method with the log-rank test. The patients with higher MMP-9 (P = 0.021) or MPO (P = 0.018) levels in the pre-vaccination samples showed worse prognosis than those with lower levels (). However, there were no statistical differences in OS between higher and lower subsets of plasma ARG1 (P = 0.898) or TGFβ (P = 0.489) levels ().
Figure 1. Prognostic significance of plasma MMP-9, MPO, ARG1, and TGFβ in advanced BTC patients treated with PPV. To examine the prognostic significance of MMP-9, MPO, ARG1, and TGFβ in pre-vaccination plasma from advanced BTC patients treated with PPV (n = 25), curves for OS were estimated by the Kaplan-Meier method, and differences between survival curves were statistically analyzed using the log-rank test. Censored patients are shown as vertical bars. Patients treated with PPV were divided into 2 subgroups according to the median values of plasma MMP-9 (A), MPO (B), ARG1 (C), and TGFβ (D).
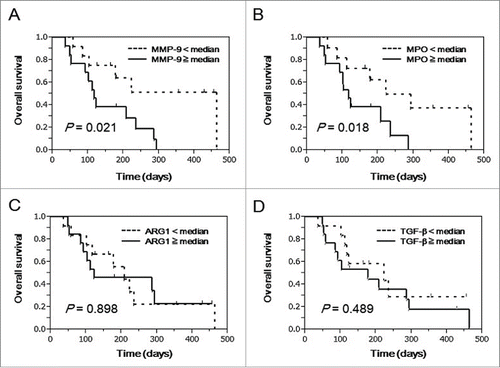
Table 1. Univariate and multivariate analyses with pre-vaccination clinical findings and laboratory data in BTC (n = 25)
Furthermore, multivariate Cox regression analysis was performed to precisely define the clinical significance of MMP-9 and MPO by adjusting for possible confounding factors. In addition to MMP-9 and MPO, only the factors with prognostic association in the univariate analysis, including hemoglobin, albumin, IL-6, CRP, and the numbers of peptides selected for vaccination (P = 0.039, P = 0.008, P = 0.002, P = 0.004, and P = 0.039, respectively), were used for the multivariate analysis (). Patients with higher MMP-9 and IL-6 levels in pre-vaccination plasma and those with smaller numbers of antigen peptides selected for vaccination showed a significantly worse OS [hazard ratio (HR) = 4.637, 95% confidence interval (CI) = 1.670 – 12.877, P = 0.003; HR = 1.186, 95% CI = 1.058 – 1.327, P = 0.003; HR = 0.326, 95% CI = 0.124 – 0.856, P = 0.023; respectively] ().
Prognostic significance of MMP-9, MPO, ARG1, and TGFβ in advanced NSCLC patients undergoing PPV
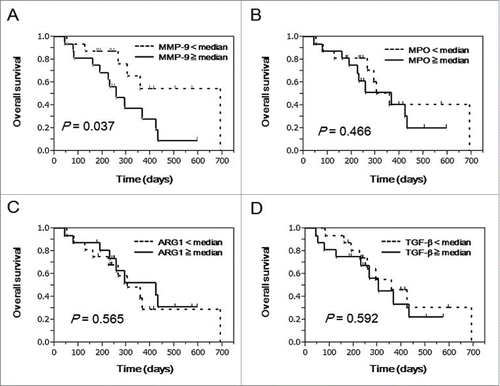
We next assessed the prognostic significance of MPO, MMP-9, ARG1, and TGFβ in advanced NSCLC patients undergoing PPV (n = 32).Citation15 Patients were divided into 2 subgroups according to the median value of these factors. The survival curves were estimated by the Kaplan-Meier method, and differences in survival functions were compared using the log-rank test. The patients with higher MMP-9 levels in the pre-vaccination plasma showed worse prognosis than those with lower levels (P = 0.037) (). However, there were no statistical differences in OS between higher and lower groups of MPO (P = 0.466), ARG1 (P = 0.565) or TGFβ (P = 0.592) levels ()
Figure 2. Prognostic significance of plasma MMP-9, MPO, ARG1, and TGFβ in advanced NSCLC patients treated with PPV. To examine the prognostic significance of MMP-9, MPO, ARG1, and TGFβ in pre-vaccination plasma from advanced NSCLC patients treated with PPV (n = 32), curves for OS were estimated by the Kaplan-Meier method, and differences between survival curves were statistically analyzed using the log-rank test. Censored patients are shown as vertical bars. Patients treated with PPV were divided into 2 subgroups according to the median values of plasma MMP-9 (A), MPO (B), ARG1 (C), and TGFβ (D).
Prognostic significance of MMP-9 and MPO in advanced PC patients undergoing PPV
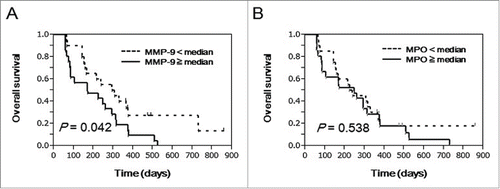
We also assessed the prognostic significance of MPO and MMP-9 in advanced PC patients undergoing PPV (n = 41).Citation16 The patients with higher MMP-9 levels in the pre-vaccination plasma showed worse prognosis than those with lower levels (P = 0.042) (). However, there was no statistical difference in OS between higher and lower groups of MPO (P = 0.538) ()
Figure 3. Prognostic significance of plasma MMP-9 and MPO in advanced PC patients treated with PPV. To examine the prognostic significance of MMP-9 and MPO in pre-vaccination plasma from advanced PC patients treated with PPV (n = 41), curves for OS were estimated by the Kaplan-Meier method, and differences between survival curves were statistically analyzed using the log-rank test. Censored patients are shown as vertical bars. Patients treated with PPV were divided into 2 subgroups according to the median values of plasma MMP-9 (A) and MPO (B).
Discussion
Since not all patients show clinical benefits from cancer immunotherapies, it would be critical to identify prognostic or predictive biomarkers for patients receiving such therapies. In previous clinical trials, several post-vaccination biomarkers, including CTL responses, Th1 responses, delayed-type hypersensitivity (DTH), autoimmunity, and anti-peptide humoral responses, have been reported to be associated with clinical responses.Citation8,9,18-20 However, there are currently no validated pre-vaccination predictive biomarkers in widespread use. Previously, we analyzed gene expression profiles by DNA microarray in pre-vaccination PBMC from CRPC patients treated with PPV, and demonstrated that granulocyte-related gene signature in PBMC, which included MMP-9, MPO and ARG1, might be related to poorer prognosis in the vaccinated patients.Citation17 In the current study, we addressed the prognostic significance of granulocyte-related soluble factors, including MMP-9, MPO, and ARG1, in pre-vaccination plasma.
The current study demonstrated that higher MMP-9 levels in pre-vaccination plasma were significantly associated with unfavorable OS in all types of cancers tested, including BTC, NSCLC, and PC, suggesting that measurement of plasma MMP-9 levels might be potentially useful for selecting cancer patients, who would benefit from PPV. Nevertheless, since plasma MMP-9 has been reported to be increased in patients with various types of cancers,Citation21-23 it is possible that MMP-9 might has only prognostic, but not predictive, utility in cancer patients undergoing PPV. To more clearly assess the relationship between plasma MMP-9 levels and OS and to elucidate prognostic vs. predictive relevance of this biomarker, further studies should be conducted in future randomized, controlled clinical trials with or without PPV.
MMP-9 is one of the metalloproteinases, which play important roles in cancer progression.Citation24-26 MMP-9 degrades a variety of extracellular matrix proteins, such as type IV collagen and gelatin, and accelerates tumor invasion and metastasis. MMP-9 also activates chemokines and/or growth factors for assisting tumor growth.Citation24-26 In addition, MMP-9 is implicated in the release and mobilization of vascular endothelial growth factor (VEGF), which facilitates angiogenesis and potentiates neovascularization in tumor tissues.Citation24-26 Since VEGF has also been reported to contribute to various immunosuppressive mechanisms, such as induction of regulatory T cells and myeloid-derived suppressor cells (MDSC), blockade of dendritic cell (DC) maturation, Th2 polarization and production of immunosuppressive cytokines, and blockade of intratumor T-cell infiltration,Citation25,26 it is possible that VEGF induction mediated by MMP-9 might be related to impaired immune responses and poorer patient survival after PPV. Therefore, VEGF-targeted therapy, such as anti-VEGF antibody and tyrosine kinase inhibitors of VEGF receptor, might be beneficial for treating the cancer patients with higher MMP-9 expression, who might show increased VEGF level and activity.
In the current study, plasma MMP-9 levels were significantly associated with OS in all types of cancers tested. However, plasma MPO levels were significantly associated with OS only in BTC, but not in NSCLC or PC. In addition, plasma ARG1 showed no prognostic significance in cancers tested. Although all of these soluble factors are reported to be produced by neutrophils, including granulocytic MDSC,Citation17,27,28 the prognostic significance seems to be different among them. This discreapancy might be explained by the fact that the plasma concentrations of each factor might be substantially affected by other sources of production and secretion.Citation24,26,28
The current study has several shortcomings and limitations. First, this is a small study with a limited number of patients, all of whom received PPV. Therefore, clinical value of MMP-9 measurement in pre-vaccination plasma remains to be confirmed in future larger-scale, prospective trials conducted in patients with or without receiving PPV. Second, the mechanism underlying the correlation between increase in MMP-9 and poorer prognosis after PPV remains to be clarified. We proposed a potential explanation associated with VEGF, but could not provide any evidence for supporting it. Although it would be quite interesting to measure the values of VEGF in blood from the vaccinated patients, we could not do in this study. Since VEGF has been reported to be especially unstable after cryopreservation,Citation29 it should be quantitated using fresh blood samples that have never been frozen. Nevertheless, fresh blood samples that have never been frozen and can be used for measurement of VEGF levels were unavailable from the vaccinated patients in this study. It would be quite important to clarify the association between MMP-9 and VEGF levels by using fresh blood samples from vaccinated patients in future studies.
In summary, the current study demonstrated that plasma MMP-9 levels may be a potentially prognostic factor for PPV. Further prospective clinical trials of PPV would be warranted to investigate whether evaluation of plasma MMP-9 levels before vaccination is useful for selecting patients who would benefit from PPV in cancer patients. In addition, it would also be interesting to examine any potential utility of MMP-9 as a prognostic marker in patients receiving other cancer vaccines. Since cancer vaccines generally take effect by the immune-related mechanisms similar to those of PPV, it might be possible that higher MMP-9 levels in pre-vaccination plasma might also be related to poorer prognosis even in patients with other cancer vaccines.
Patients and Methods
Patients
This is a retrospective analysis with peripheral blood samples from the patients with BTC (n = 25), NSCLC (n = 32), and PC (n = 41), who were enrolled in phase II clinical trials for PPV.Citation14-16 These studies were approved by the Kurume University Ethical Committee, and were registered in the UMIN Clinical Trials Registry (UMIN000002907, UMIN000001839, and UMIN000001881). Patients were eligible for inclusion in the current study if they had a histological diagnosis of BTC, NSCLC, or PC, and showed positive humoral responses to at least 2 of the 31 different vaccine candidate peptides. Other inclusion criteria and exclusion criteria and study protocols were reported previously.Citation14-16 After a full explanation of the protocol, written informed consent was obtained from all patients before enrollment.
Clinical protocol
All patients with BTC, NSCLC, and PC were treated by the same clinical protocol, as follows. Thirty-one peptides were employed for vaccination [12 peptides for HLA-A2, 16 peptides for HLA-A24, 9 peptides for HLA-A3 supertypes (-A3, -A11, -A31, and -A33), and 4 peptides for HLA-A26].Citation14-16 Vaccine peptides adequate to individual patients were selected in consideration of the pre-existing host immunity before vaccination, as assessed by the results of HLA typing and the titers of IgG specific to each of the 31 different vaccine candidates. A maximum of 4 peptides (3 mg/each peptide), which were selected, were subcutaneously administrated with incomplete Freund's adjuvant (Montanide ISA51; Seppic, Paris, France) once a week for 6 consecutive weeks as the 1st cycle. After the 1st cycle of vaccinations, up to 4 antigen peptides that were re-selected according to the titers of peptide-specific IgG were administered biweekly for 6 times. After the 2nd cycle of vaccinations, up to 4 antigen peptides that were re-selected every 6 times of vaccinations were administered every 4 weeks.
Measurement of soluble factors in plasma
Plasma samples were obtained from the enrolled patients before vaccination. To detect the levels of MMP-9, MPO, and TGFβ in plasma, a bead-based multiplex assay (Luminex® 200™ system; Luminex Corporation, Austin, TX) was used. The analyte kit used for the measurement was obtained from Millipore (Billerica, MA). The levels of ARG1, CRP, and IL-6 in plasma were examined by using an ELISA kit from BioVendor (Candler, MN), R&D systems (Minneapolis, MN), and eBioscience (San Diego, CA), respectively. Frozen plasma samples were thawed, diluted, and assayed in duplicate in accordance with the manufacturer's instructions. The mean of duplicate samples was used for statistical analysis. Complete blood counts and serum biochemistry tests were also performed before vaccinations.
Statistical Analysis
OS time was calculated from the first day of peptide vaccination until the date of death or the last date when the patient was known to be alive. Association between pre-vaccination clinical findings or laboratory data and OS were evaluated by univariate and multivariate analyses with the Cox proportional hazards regression model in BTC patients undergoing PPV. Curves for OS were also estimated by the Kaplan-Meier method, and differences in survival functions were compared using the log-rank test in BTC, NSCLC, and PC patients undergoing PPV. The strength of relationship between soluble factors was evaluated by Spearman's rank correlation coefficient. All tests were 2-sided, and differences at P < 0.05 were considered to be statistically significant. All of the statistical analyses were conducted using the JMP version 10 or SAS version 9.3 software package (SAS Institute Inc.., Cary, NC).
Disclosure of Potential Conflicts of Interest
Akira Yamada is a Board member of the Green Peptide Co., Ltd. Kyogo Itoh and Akira Yamada have stock of the Green Peptide Co., Ltd. Kyogo Itoh received research fund from Taiho Pharmaceutical Co., Ltd. No potential conflicts of interests were declared by the other authors.
Funding
This study was supported by a research program of the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct), Ministry of Education, Culture, Sports, Science and Technology of Japan; a research program of the Regional Innovation Cluster Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan; and Kurozumi Medical Foundation.
References
- Hezel AF, Deshpande V, Zhu AX. Genetics of biliary tract cancers and emerging targeted therapies. J Clin Oncol 2010; 28:3531-40; PMID:20547994; http://dx.doi.org/10.1200/JCO.2009.27.4787
- Johnson DH, Schiller JH, Bunn PA. Recent clinical advances in lung cancer management. J Clin Oncol 2014; 32:973-82; PMID:24567433; http://dx.doi.org/10.1200/JCO.2013.53.1228
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371:1039-49; PMID:25207767; http://dx.doi.org/10.1056/NEJMra1404198
- Sasada T, Komatsu N, Suekane S, Yamada A, Noguchi M, Itoh K. Overcoming the hurdles of randomised clinical trials of therapeutic cancer vaccines. Eur J Cancer 2010; 46:1514-9; PMID:20413296; http://dx.doi.org/10.1016/j.ejca.2010.03.013
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480-9; PMID:22193102; http://dx.doi.org/10.1038/nature10673
- Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol 2011; 29:4828-36; PMID:22042955; http://dx.doi.org/10.1200/JCO.2011.38.0899
- Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst 2012; 104:599-613; PMID:22395641; http://dx.doi.org/10.1093/jnci/djs033
- Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother 2011; 60:433-42; PMID:21221967; http://dx.doi.org/10.1007/s00262-010-0960-8
- Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 2010; 102:1388-97; PMID:20826737; http://dx.doi.org/10.1093/jnci/djq310
- Itoh K, Yamada A. Personalized peptide vaccines: a new therapeutic modality for cancer. Cancer Sci 2006; 97:970-6; PMID:16984371; http://dx.doi.org/10.1111/j.1349-7006.2006.00272.x
- Sasada T, Yamada A, Noguchi M, Itoh K. Personalized peptide vaccine for treatment of advanced cancer. Curr Med Chem 2014; 21:2332-45; PMID:24524766; http://dx.doi.org/10.2174/0929867321666140205132936
- Terasaki M, Shibui S, Narita Y, Fujimaki T, Aoki T, Kajiwara K, Sawamura Y, Kurisu K, Mineta T, Yamada A, et al. Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen-A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol 2011; 29:337-44; PMID:21149665; http://dx.doi.org/10.1200/JCO.2010.29.7499
- Noguchi M, Kakuma T, Uemura H, Nasu Y, Kumon H, Hirao Y, Moriya F, Suekane S, Matsuoka K, Komatsu N, et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother 2010; 59:1001-9; PMID:20146063; http://dx.doi.org/10.1007/s00262-010-0822-4
- Yoshitomi M, Yutani S, Matsueda S, Ioji T, Komatsu N, Shichijo S, Yamada A, Itoh K, Sasada T, Kinoshita H. Personalized peptide vaccination for advanced biliary tract cancer: IL-6, nutritional status and pre-existing antigen-specific immunity as possible biomarkers for patient prognosis. Exp Ther Med 2012; 3:463-9; PMID:22969912
- Yoshiyama K, Terazaki Y, Matsueda S, Shichijo S, Noguchi M, Yamada A, Mine T, Ioji T, Itoh K, Shirouzu K, et al. Personalized peptide vaccination in patients with refractory non-small cell lung cancer. Int J Oncol 2012; 40:1492-500; PMID:22307435
- Yutani S, Komatsu N, Yoshitomi M, Matsueda S, Yonemoto K, Mine T, Noguchi M, Ishihara Y, Yamada A, Itoh K, et al. A phase II study of a personalized peptide vaccination for chemotherapy-resistant advanced pancreatic cancer patients. Oncol Rep 2013; 30:1094-100; PMID:23784011
- Komatsu N, Matsueda S, Tashiro K, Ioji T, Shichijo S, Noguchi M, Yamada A, Doi A, Suekane S, Moriya F, et al. Gene expression profiles in peripheral blood as a biomarker in cancer patients receiving peptide vaccination. Cancer 2012; 118:3208-21; PMID:22071976; http://dx.doi.org/10.1002/cncr.26636
- Amos SM, Duong CP, Westwood JA, Ritchie DS, Junghans RP, Darcy PK, Kershaw MH. Autoimmunity associated with immunotherapy of cancer. Blood 2011; 118:499-509; PMID:21531979; http://dx.doi.org/10.1182/blood-2011-01-325266
- López MN, Pereda C, Segal G, Muñoz L, Aguilera R, González FE, Escobar A, Ginesta A, Reyes D, González R, et al. Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor β-expressing T cells. J Clin Oncol 2009; 27:945-52; PMID:19139436; http://dx.doi.org/10.1200/JCO.2008.18.0794
- Noguchi M, Mine T, Komatsu N, Suekane S, Moriya F, Matsuoka K, Yutani S, Shichijo S, Yamada A, Toh U, et al. Assessment of immunological biomarkers in patients with advanced cancer treated by personalized peptide vaccination. Cancer Biol Ther 2011; 10:1266-79; http://dx.doi.org/10.4161/cbt.10.12.13448
- Endo K, Maehara Y, Baba H, Yamamoto M, Tomisaki S, Watanabe A, Kakeji Y, Sugimachi K. Elevated levels of serum and plasma metalloproteinases in patients with gastric cancer. Anticancer Res 1997; 17:2253-8; PMID:9216697
- Iizasa T, Fujisawa T, Suzuki M, Motohashi S, Yasufuku K, Yasukawa T, Baba M, Shiba M. Elevated levels of circulating plasma matrix metalloproteinase 9 in non-small cell lung cancer patients. Clin Cancer Res 1999; 5:149-53; PMID:9918213
- Tutton MG, George ML, Eccles SA, Burton S, Swift RI, Abulafi AM. Use of plasma MMP-2 and MMP-9 levels as a surrogate for tumour expression in colorectal cancer patients. Int J Cancer 2003; 107:541-50; PMID:14520690; http://dx.doi.org/10.1002/ijc.11436
- Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev 2007; 26:717-24; PMID:17717634; http://dx.doi.org/10.1007/s10555-007-9089-4
- Tartour E, Pere H, Maillere B, Terme M, Merillon N, Taieb J, Sandoval F, Quintin-Colonna F, Lacerda K, Karadimou A, et al. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev 2011; 30:83-95; PMID:21249423; http://dx.doi.org/10.1007/s10555-011-9281-4
- Farina AR, Mackay AR. Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression. Cancers (Basel) 2014; 6:240-96; PMID:24473089; http://dx.doi.org/10.3390/cancers6010240
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004; 6:409-21; PMID:15488763; http://dx.doi.org/10.1016/j.ccr.2004.08.031
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 2005; 5:641-54; PMID:16056256; http://dx.doi.org/10.1038/nri1668
- Kisand K, Kerna I, Kumm J, Jonsson H, Tamm A. Impact of cryopreservation on serum concentration of matrix metalloproteinases (MMP)-7, TIMP-1, vascular growth factors (VEGF) and VEGF-R2 in Biobank samples. Clin Chem Lab Med 2011 Feb; 49(2):229-35
