Abstract
Pneumonia is the fourth-leading cause of death globally, and Streptococcus pneumoniae is the most important causative pathogen. Because the incidence of pneumococcal diseases is likely to increase with the aging society, we should determine an optimal strategy for pneumococcal vaccination. While consensus indicates that 23-valent pneumococcal polysaccharide vaccine prevents invasive pneumococcal diseases (IPD), its effects on community-acquired pneumonia (CAP) remain controversial. Recently, a 13-valent pneumococcal conjugate vaccine (PCV13) was released. The latest clinical study (CAPiTA study) showed that PCV13 reduced vaccine-type CAP and IPD. Based on these results, the Advisory Committee on Immunization Practices recommended initial vaccination with PCV13 for the elderly. Scientific evidence regarding immunosenescence is needed to determine a more ideal vaccination strategy for the elderly with impaired innate and adaptive immunity. Continuing research on the cost effectiveness of new vaccine strategies considering constantly changing epidemiology is also warranted.
Abbreviations
| CAP | = | community-acquired pneumonia |
| HAP | = | hospital-acquired pneumonia |
| VAP | = | ventilator-associated pneumonia |
| IPD | = | invasive pneumococcal diseases |
| PPV23 | = | 23-valent pneumococcal polysaccharide vaccine |
| PCV13 | = | 13-valent pneumococcal conjugate vaccine |
| CAPiTA | = | Community-Acquired Pneumonia Immunization Trial in Adults |
| ACIP | = | Advisory Committee on Immunization Practices |
| OI | = | opsonisation index |
| AID | = | activation-induced cytidine deaminase |
| PCV7 | = | 7-valent pneumococcal conjugate vaccine |
| IL | = | interleukin. |
Introduction
Pneumonia is the fourth-leading cause of death globally, according to the World Health Reports 2014 by the World Health Organization.Citation1 In developed countries, such as Japan, more than 95% of deaths from pneumonia occur in the elderly (≥65 years old). Therefore, faced with the increasing aging population in both developed and developing countries, it is becoming more important to prevent pneumonia.Citation2
In terms of clinical settings, pneumonia can be classified into several categories such as community-acquired pneumonia (CAP), hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), or healthcare-associated pneumonia.Citation3,4 In addition, based on etiology, pneumonia could be categorized as aspiration pneumonia or post-influenza pneumonia.Citation5,6 Streptococcus pneumoniae (S. pneumoniae) is the leading bacterial cause of CAP, aspiration pneumonia, and post-influenza pneumonia Citation7,8 and also one of the major causative bacteria of HAP and VAP.Citation9 Therefore, to reduce the disease burden of pneumonia in the aging society, infections with S. pneumonia should be addressed; the introduction of pneumococcal vaccination decreases the relative occurrence of pneumococcal infections, compared with other causative bacteria.Citation10,11 In this review, we aimed to review new evidence for pneumococcal vaccines from both basic and clinical points of view to build an optimal vaccine strategy.
Serotype of Streptococcus Pneumoniae
The morphological characteristics of S. pneumoniae include a polysaccharide capsule that surrounds a diplococci of S. pneumoniae.Citation12 There are more than 90 serotypes of S. pneumonia based on the type of capsule, on which the antigenicity also chiefly depends.Citation13 Among the various pathogenic factors including neuraminidase and pneumolysin, the capsule is the most important barrier by which binding of the host's complement to the surface of the bacteria is inhibited, allowing escape from opsonisation.Citation14,15 Based on this feature, different S. pneumoniae serotypes have different pathogenicities, including colony forming ability, severity, invasiveness, and drug resistance profile.Citation16,17 For instance, serotype 3 is one of the most invasive serotypes since it has the thickest capsule of all S. pneumoniae serotypes.Citation18,19
Invasive pneumococcal diseases, non-invasive pneumococcal diseases, and pneumococcal pneumonia
In invasive pneumococcal diseases (IPDs), S. pneumoniae is detected in sterile sites such as cerebrospinal fluid, pleural fluid, joint fluid, and the blood ().Citation20 Although bacteraemia occurs in only 10–30% of pneumococcal pneumonias, bacteraemic pneumonia is the most common IPD owing to the clinical frequency. On the other hand, non-IPD usually includes non-bacteraemic pneumococcal pneumonia, bronchitis, sinusitis, and otitis media.
Figure 1. Schematic spectrum of pneumococcal disease Pneumococcal diseases consist of 2 groups: invasive pneumococcal disease (IPD) and non-IPD. IPD is a pneumococcal disease in which Streptococcus pneumoniae is detected in a sterile space. IPD consists of bacteraemic pneumonia, pleuritis, meningitis, arthritis, and bacteraemia, and non-IPD consists of non-bacteraemic pneumonia, otitis media, sinusitis, and bronchitis. Although bacteraemia occurs in only 10–30% of pneumococcal pneumonia cases, bacteraemic pneumonia is the most common IPD owing to the clinical frequency of pneumococcal pneumonia. In clinical settings, pleuritis usually occurrs with pneumonia.
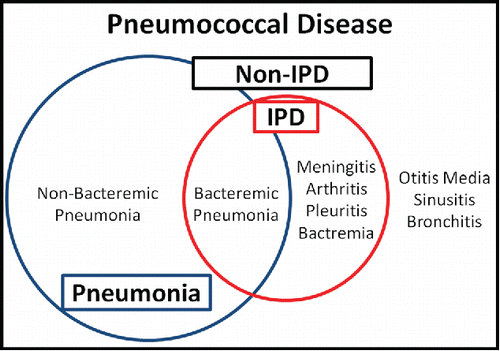
It is important to understand the differences between IPDs and non-IPDs. First, the distribution of S. pneumoniae serotypes is epidemiologically different as with the case of pathogenicities.Citation21-23 Moreover, the geographical serotype distribution could change chronologically with the vaccine introduction and its gradual spread, even in the same region.Citation16 Therefore, assessing the epidemiological distribution of both IPD and non-IPD is the important initial action at the time of introducing pneumococcal vaccination. Second, the disease severity is different; despite a considerably lower incidence of IPD than pneumococcal pneumonia, the prognosis is poorer, and the aftereffects result in greater patient burden.Citation24 In this context, both pneumococcal pneumonia and IPD are clinically meaningful.
Characteristics of the 23-valent pneumococcal polysaccharide vaccine (PPV23) and 13-valent pneumococcal conjugate vaccine (PCV13)
The 23-valent pneumococcal polysaccharide vaccine (PPV23) is an inactivated vaccine that is refined from polysaccharide capsules of 23 types of S. pneumoniae.Citation25 The polysaccharide antigen of capsules induces only B-cell immunity, not T-cell immunity, resulting in a short lasting immune response and lack of memory immunity ().Citation26 The activity of the serotype-specific antibody and opsonisation decreases approximately 5 y after vaccination; therefore, re-vaccination is recommended in many countries and regions.Citation27 However, booster effects might be limited because of hyporesponsiveness associated with a depletion of the memory B-cell population.Citation28
Table 1. Comparison between 23-valent pneumococcal polysaccharide vaccine (PPV23) and 13-valent pneumococcal conjugate vaccine (PCV13)
However, this phenomenon is controversial because antigen-specific opsonisation is sufficiently evoked even by revaccination with PPV23, which is more closely related to immunogenicity rather than antigen-specific IgG levels.Citation29 Regarding more data on hyporesponsiveness of PPV23, Hammitt et al. studied the immunogenicity of PPV23 among adults aged 55–74 y who were administered up to 4 doses of PPV23. The IgG titer levels and opsonisation index (OI) were measured 30 d after vaccination.Citation30 Hyporesponsiveness was not observed with repeated PPV23 vaccination. Also, Musher et al. also reported that IgG concentrations still exceeded vaccine-naïve levels 10 y after revaccination and that the second and third doses were potently immunogenic.Citation31 Available data for immunogenicity following the 13-valent pneumococcal conjugate vaccine (PCV13) vaccination in adults are limited at the present because of a brief history since the introduction. Therefore, we should keep updating with data for a long-term immunogenicity for those who receive the PPV23 revaccination and forthcoming head-to-head data comparing immunogenicity between PPV23 and PCV13.
PCV13 is also an inactivated vaccine as PPV23, but it is capable of activating both B-cell and T-cell immune responses through the action of diphtheria toxoid binding to the polysaccharide leading to the induction of a sufficient immune reaction even in infants who have immature B-cell immunity.Citation32,33 For this reason, PCV13 is licensed in many countries for infants.Citation34 In addition, efficacy has been demonstrated in high-risk adults because of the evoked T-cell immune response and enhanced memory response with a booster vaccination.Citation35-37 Thus, it has been reported that in individuals with HIV, chronic obstructive pulmonary disease, or renal transplantation, PCV13 evokes better immunogenicity than PPV23 in a subset of serotypes.Citation38-41
PCV13 also reportedly reduces nasopharyngeal carriage of the vaccine type of pneumococci, especially in children.Citation42 The incidence of pneumococcal disease in adults is reportedly reduced in some regions owing to herd immunity with PCV13, since nasopharyngeal carriage of pneumococci in adults can be transmitted from children.Citation43,44
Regarding serotype coverage, PPV23 contains polysaccharides of pneumococcal serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, and 23F, while PCV13 contains polysaccharides of pneumococcal serotypes 1, 3, 4, 5, 6A, 7F, 6B, 9V, 14, 18C, 19A, 19F, and 23F. Therefore, PPV23 has wider coverage than PCV13, although serotype 6A is covered only by PCV13. The most recent domestic reports from Japan of the serotype distribution of CAP and IPD indicate that the proportions of CAP serotypes covered by PPV23 and PCV13 are 62.7% and 49.3%, respectively, and the proportions of IPD serotypes covered by PPV23 and PCV13 are 69.6% and 48.0%, respectively. The pneumococcal serotype distribution have shifted from vaccine-type to non-vaccine-type serotypes since the introduction of PCVs.Citation45,46 The more PPV23 and PCV13 become prevalent both in infants and adults, the further the coverage of vaccine-type serotypes is expected to decrease in the future. Therefore, we should monitor epidemiological information on serotype transition.
Cochrane review of PPV23 and other clinical study on PPV23
The effectiveness of PPV23 for prevention of IPD in adults is widely accepted.Citation47 The Cochrane review published in 2013 consistently showed strong evidence that PPV23 is effective for preventing IPD.Citation48 A meta-analysis including 11 randomized controlled trials found that PPV23 was effective against IPD (odds ratio [OR] 0.26, 95% confidence interval [CI] 0.14–0.45, I2 statistic = 0%) with no statistical heterogeneity. Generally, I2 statistic values of 25%, 50%, and 75% indicate low, moderate, and high heterogeneity, respectively.
Regarding the effect of PPV23 on CAP, the Cochrane review does not provide compelling evidence to support the routine use of PPV23 for the prevention of all-cause pneumonia or mortality. Although efficacy against all-cause pneumonia was shown in low-income countries (OR 0.54, 95% CI 0.43–0.67, I2 statistic = 19%), it was not demonstrated in the general population (OR 0.71, 95% CI 0.45–1.12, I2 statistic = 93%) or adults with chronic illness (OR 0.93, 95% CI 0.73–1.19, I2 statistic = 10%) in high-income countries. Furthermore, PPV23 was not associated with substantial reductions in all-cause mortality (OR 0.90, 95% CI 0.74–1.09; random-effects model, I2 statistic = 69%). As indicated by the high I2 statistic values, there was obvious heterogeneity in those randomized clinical trials.
Although not included in the Cochrane review, the efficacy of PPV23 was supported in the CAPAMIS study, which was a population-based prospective cohort study involving 27,204 individuals aged >60 years in Spain.Citation49 Among a total of 76,033 person-years, 29,065 (38%) person-years were assigned to immunized subjects. Although PPV23 did not appear to be effective in primary analyses, PPV23 vaccination administered within 5 y was associated with reduced risks for bacteraemic pneumococcal CAP, non-bacteraemic pneumococcal CAP, overall CAP, and all-cause CAP by analyses adjusted for multiple variables. Noteworthy, they proved a protective effect of recent PPV23 vaccination by focusing on the subgroup vaccinated within previous 5 y. This implies a protective effect of PPV23 last for about 5 y from the epidemiological aspect.
CAPiTA study
In 2011, PCV13 was licensed for older adults under the United States Food and Drug Administration's accelerated approval program.Citation50 The Netherlands-based Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA) was designed to meet regulatory commitments under the accelerated approval program and verify the clinical benefit of PCV13 Citation51 in the first episode of vaccine-type CAP in adults ≥65 years of age. This clinical trial excluded users of immunosuppressants and participants with potential nursing and healthcare-associated pneumonia. Reductions in vaccine-type IPD (75.00%; P = 0.0005), vaccine-type pneumococcal CAP (45.56%; P = 0.0006), and non-bacteraemic/non-invasive vaccine-type pneumococcal CAP (45.00%; P = 0.0067) were observed.Citation52 However, the CAPiTA study did not demonstrate clinical efficacy of PCV13 for non-vaccine-type pneumococcal CAP. The safety profile of PCV13 in the CAPiTA study was consistent with studies previously conducted in adults.Citation53-55
Advisory Committee on Immunization Practices (ACIP) recommendations
The CAPiTA study demonstrated clinical efficacy of PCV13 for vaccine-type pneumococcal CAP, which was not supported as compelling evidence by the Cochrane review. From this study, the Advisory Committee on Immunization Practices (ACIP) announced recommendation in the Morbidity and Mortality Weekly Report issued in September 2014 that all adults ≥65 years of age should receive initially PCV13 followed subsequently PPSV23 in 6–12 months.Citation56 This 2-step vaccination approach aims to maximize both the efficacy of pneumococcal vaccination in terms of immunogenicity developing acquired memory T-cell function by initial PCV13 and wider serotype coverage by subsequent PPV23. As a theoretical background for initial vaccination with PCV13, some clinical trials have demonstrated booster effects with PPV23 inoculation after PCV13 but not with PCV13 inoculation after PPV23. For example, elderly people receiving PPV23 one year after an initial 7-valent pneumococcal conjugate vaccine (PCV7) vaccination had 3-fold lower IgG and OI values than those who received PCV7/PCV7 or PCV7/PPV23 vaccinations.Citation57
Notably, review of the ACIP recommendation is planned for 2018 owing to potential changes in the epidemiological situation.Citation56 In particular, recent epidemiological studies have reported that IPD due to non-PCV13 serotypes has increased not only among infants but also among the elderly despite a dramatic reduction in IPD due to PCV-13 serotypes.Citation58-61 Not only in IPD, the relative increase in non-bacteraemic pneumococcal pneumonia has been also observed among adults in some regions as ‘serotype replacement’.Citation62 The distribution of pneumococcal diseases due to non-PCV13 is expected to increase more resulting from its direct effect and herd immunity effect; therefore, we should objectively re-evaluate the efficacy and cost-effectiveness based on the timely epidemiological data.
Immunosenescence of pneumococcal vaccinations
Because the importance of pneumonia prevention is increasing with the aging population and the higher immunologic risk in elderly people, an optimal strategy for pneumococcal vaccination considering maximal immunogenicity is needed, for which we need to have a good understanding of the effects of aging on the fundamental immunological functions.Citation63 Recent translational studies of immunological senescence, which is also called ‘immunosenescence’, have focused on both adaptive and innate immunity.Citation64-66
Aging negatively affects T-cell immunity partly resulting from atrophy of both the thymic cortex and medulla, where T-cells are generated.Citation67 In particular, the age-dependent reduction in the number of mature naïve T-cells causes elderly people to be more vulnerable to never encountered pathogens.Citation68 In aged mouse models, antigen-inexperienced T-cells are functionally hampered and difficult to prime.Citation69 Besides naïve T-cells, senescence results in decreased affinity of antigen-specific memory T-cells to antigens at the peripheral level, such as lymph nodes and the inflammation site.Citation70,71 Although memory T-cells survive longer, proliferation and cytokine production are impaired during the recall phase.Citation69
Senescence also dampens the function of B-cells primarily because of reduced interleukin (IL)-7 production, decreased activation-induced cytidine deaminase (AID) activity, and hampered transcriptional factor E47 activity.Citation72-75 IL-7 is a cytokine that promotes the differentiation from pre-B-cells to B-cells and their migration from the bone marrow to the blood stream.Citation76 Aging diminishes IL-7 production by depressing the function of bone marrow stroma cells.Citation77 AID is an essential enzyme for the class switch of antibodies and causes higher affinity against antigens by modulating somatic hypermutation.Citation78 Therefore, it is noted that impaired AID function owing to senescence results in immaturation of humoral immunity. In terms of innate immunity in the elderly, the reduction in the phagocytic capacity of neutrophils and macrophages,Citation79 the impairment of up-regulation of major histocompatibility complex class II, and defects in the expression of toll-like receptors are known.Citation80,81 Chronic inflammation in aging also decreases the ability to recognize danger signals provoked by vaccination.Citation82 Furthermore, capacity of complements and natural killer-cells, which play a pivotal role in the protection against pneumococcal infection, have been also reported to be reduced with senescence.Citation81,83-86
Taken together, aging results in insufficient immunogenicity in response to vaccination. In turn, it leads to increased susceptibility to pneumococcal infections. Moreover, age-dependent impaired function of IgGs, complements, and neutrophils Citation87 would fail to coordinate sufficient opsonisation which is one of the most important immunological strategies against S. pneumoniae.
Evaluation of vaccine efficacy and the vaccination strategy for the elderly
Although pneumococcal pneumonia-related mortality or a pneumococcal pneumonia event would be desirable primary endpoint in clinical trials, a large sample size is required such as in the CAPiTA study to evaluate these endpoints.Citation52 However, it is not feasible to gather a large sample size from the perspectives of costs and efforts. Therefore, surrogate markers are considered more realistic, although time point of antibody level measurement after vaccination also remains controversial.Citation88 In clinical trials of pneumococcal vaccines, IgG and OI are usually used as surrogate markers to evaluate vaccine efficacy.Citation89 Generally, OI, reflecting the functional ability of IgG to opsonize S. pneumoniae has been considered to be appropriate indicator for evaluating vaccine efficacy, rather than quantitative IgG measurement.
In our evaluation of serotype-specific IgG antibodies and serotype-specific OIs between PPV23 and PCV7 for pneumococcus vaccine-naïve elderly people (≥80 years of age),Citation90 both PPV23 and PCV7 elicited increases in IgG and OI. Meanwhile, PCV7 was more potent than PPV23 in terms of its immunogenicity against 4 serotypes out of the 7 included in PCV7 (serotypes 4, 9V, 18C, and 23F). The safety of these preparations in these elderly individuals was also demonstrated, with no serious adverse effects observed in either group. A greater functional immune response with PCV13 than PPV23 as reported in the current study has also been reported regarding the majority of serotypes covered by PCV13 among adults aged >50 years.Citation53,54,91,92
Based on these clinical findings, PCV13 could elicit more potent immunogenicity than PPV23 among the elderly. Furthermore, previous studies of successive immunization with PPV23 and PCV13 have already demonstrated that PCV13 vaccination followed by PPV23 is advantageous from an immunological point of view, as recommended by the ACIP.Citation55,57,93 Concretely, initial immunization by PCV13 cause T-cell priming and subsequent PPV23 vaccination results in a booster effect even though PPV23 does not elicit T-cell activation. On the other hand, since initial immunization by PPV23 does not elicit T-cell priming, a booster effect of subsequent PCV13 vaccination is estimated to be weaker.
Gap between immunological efficacy and vaccine strategy
As we have previously discussed regarding immunological efficacy, PCV13 vaccination alone or PCV13 vaccination followed by PPV23 elicits more potent immunogenicity than PPV23. This T cell mediated immune response is more dispensable for elderly people, who are under immunosenescence, in order to evoke sufficient immunogenicity.
In addition to this immunological theory, vaccine strategies need to consider the regional health care system, cost effectiveness, and serotype epidemiology. Vaccination against childhood diseases are considered to be cost effective, but some vaccinations for the elderly are still under debate. Citation94-97 For instance, the Joint Committee of the Japanese Respiratory Society and Japanese Association for Infectious Diseases do not recommend the routine use of PCV13 for adults due to the lack of currently available evidence regarding cost effectiveness in Japan, despite the existence of the evidence in other countries.Citation98-100 In Japan, the gap between immunological efficacy and domestic vaccine strategies should be remembered, and clinical study in high quality to fill this gap is strongly warranted.
Prerequisite for decision-making of pneumococcal vaccination policy –cost effectiveness
A discussion of the cost effectiveness of the 2 pneumococcal vaccines is essential, especially for making decisions regarding their introduction. This notion is supported by the United States Institute of Medicine, which stated that health economic deliberation plays an essential role in the prioritization of future vaccine strategies.Citation101 Regarding pneumococcal vaccines, there have been some previous studies favoring the cost-effectiveness of PCV13; however, cost-effectiveness studies directly comparing PPV23 and PCV13 in the elderly are still limited.Citation98,102 In general, the outcome of cost-effectiveness studies depends on several factors, such as the estimation of the vaccine effectiveness, proportion of people who are vaccinated, serotype epidemiology, estimation of the change in serotype distribution, baseline probability of infection, and medical expenses in the specific region.Citation99 From this standpoint, the present results could not be fully applied to every region. Furthermore, these cost-effectiveness studies were sensitive to the assumption that PCV13, but not PPV23, is effective against non-bacteraemic pneumococcal pneumonia, which is different from the results of the CAPAMIS study. As supported in the CAPAMIS study or the clinical trials conducted in Japan, if PPV23 is somewhat effective against non-bacteraemic pneumococcal pneumonia, these outcomes showing superiority of PCV13 in terms of cost-effectiveness could differ considerably.Citation49,103,104
When making decisions regarding vaccination policy, we should ideally calculate the cost-effectiveness for each region as well as referring to previous studies. However, some regions and countries do not have accurate epidemiological data, which is needed for studies regarding efficacy and cost-effectiveness of the vaccines. Even in Japan, data regarding the precise pneumococcal serotype distribution has been unavailable until quite recently. This shortage of epidemiological data has been an obstacle for vaccine introduction.Citation105 In addition, tight budgets for immunization have hindered vaccine introduction, especially in low- and middle-income countries. To overcome these social problems, future collaborative program among international partners will play an important role as a new way of decision-making.Citation106
Conclusions
With the global aging society, to take appropriate measures regarding pneumococcal diseases in the elderly becomes more important. To implement effective vaccination strategies, a comprehensive understanding of immunosenescence and translation of additional basic findings and clinical evidence to clinical practice are warranted. To this end, a multidisciplinary research approach focusing on cost effectiveness and epidemiology is particularly essential.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- World Health Statistics 2014. Available from: http://www.who.int/gho/publications/world_health_statistics/en/
- Beard JR, Bloom DE. Towards a comprehensive public health response to population ageing. Lancet 2015; 385:658-61; PMID:25468151; http://dx.doi.org/10.1016/S0140-6736(14)61461-6
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, et al. Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44 Suppl 2:S27-72; PMID:17278083; http://dx.doi.org/10.1086/511159
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388-416; PMID:15699079; http://dx.doi.org/10.1164/rccm.200405-644ST
- DiBardino DM, Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care 2015; 30:40-8; PMID:25129577; http://dx.doi.org/10.1016/j.jcrc.2014.07.011
- Viasus D, Marinescu C, Villoslada A, Cordero E, Galvez-Acebal J, Farinas MC, Gracia-Ahufinger I, Fernandez-Navarro A, Niubo J, Ortega L, et al. Community-acquired pneumonia during the first post-pandemic influenza season: a prospective, multicentre cohort study. J Infect 2013; 67:185-93; PMID:23747416; http://dx.doi.org/10.1016/j.jinf.2013.05.006
- Bartlett JG. Diagnostic tests for agents of community-acquired pneumonia. Clin Infect Dis 2011; 52 Suppl 4:S296-304; PMID:21460288; http://dx.doi.org/10.1093/cid/cir045
- McMorrow ML, Wemakoy EO, Tshilobo JK, Emukule GO, Mott JA, Njuguna H, Waiboci L, Heraud JM, Rajatonirina S, Razanajatovo NH, et al. Severe acute respiratory illness deaths in sub-saharan Africa and the role of influenza: a case series from 8 countries. J Infect Dis 2015; PMID:25712970; http://dx.doi.org/10.1093/infdis/jiv100
- Barbier F, Andremont A, Wolff M, Bouadma L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med 2013; 19:216-28; PMID:23524477; http://dx.doi.org/10.1097/MCP.0b013e32835f27be
- Ciruela P, Martinez A, Izquierdo C, Hernandez S, Broner S, Munoz-Almagro C, Dominguez A. Epidemiology of vaccine-preventable invasive diseases in Catalonia in the era of conjugate vaccines. Hum Vaccin Immunother 2013; 9:681-91; PMID:23303166; http://dx.doi.org/10.4161/hv.23266
- Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369:155-63; PMID:23841730
- Yother J. Capsules of Streptococcus pneumoniae and other bacteria: paradigms for polysaccharide biosynthesis and regulation. Annu Rev Microbiol 2011; 65:563-81; PMID:21721938; http://dx.doi.org/10.1146/annurev.micro.62.081307.162944
- Chiba N, Kobayashi R, Hasegawa K, Morozumi M, Nakayama E, Tajima T, Iwata S, Ubukata K. Antibiotic susceptibility according to genotype of penicillin-binding protein and macrolide resistance genes, and serotype of Streptococcus pneumoniae isolates from community-acquired pneumonia in children. J Antimicrob Chemother 2005; 56:756-60; PMID:16131518; http://dx.doi.org/10.1093/jac/dki302
- Garcia-Suarez Mdel M, Vazquez F, Mendez FJ. Streptococcus pneumoniae virulence factors and their clinical impact: an update. Enferm Infecc Microbiol Clin 2006; 24:512-7; PMID:16987470; http://dx.doi.org/10.1157/13092469
- Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun 2010; 78:704-15; PMID:19948837; http://dx.doi.org/10.1128/IAI.00881-09
- Chiba N, Morozumi M, Shouji M, Wajima T, Iwata S, Ubukata K. Changes in capsule and drug resistance of Pneumococci after introduction of PCV7, Japan, 2010-2013. Emerg Infect Dis 2014; 20:1132-9; PMID:24960150
- Grabenstein JD, Musey LK. Differences in serious clinical outcomes of infection caused by specific pneumococcal serotypes among adults. Vaccine 2014; 32:2399-405; PMID:24637174; http://dx.doi.org/10.1016/j.vaccine.2014.02.096
- Weinberger DM, Harboe ZB, Sanders EA, Ndiritu M, Klugman KP, Ruckinger S, Dagan R, Adegbola R, Cutts F, Johnson HL, et al. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin Infect Dis 2010; 51:692-9; PMID:20715907; http://dx.doi.org/10.1086/655828
- Ahl J, Littorin N, Forsgren A, Odenholt I, Resman F, Riesbeck K. High incidence of septic shock caused by Streptococcus pneumoniae serotype 3–a retrospective epidemiological study. BMC Infect Dis 2013; 13:492; PMID:24148181; http://dx.doi.org/10.1186/1471-2334-13-492
- Hung IF, Tantawichien T, Tsai YH, Patil S, Zotomayor R. Regional epidemiology of invasive pneumococcal disease in Asian adults: epidemiology, disease burden, serotype distribution, and antimicrobial resistance patterns and prevention. Int J Infect Dis 2013; 17:e364-73; PMID:23416209; http://dx.doi.org/10.1016/j.ijid.2013.01.004
- Skoczynska A, Kuch A, Sadowy E, Wasko I, Markowska M, Ronkiewicz P, Matynia B, Bojarska A, Wasiak K, Golebiewska A, et al. Recent trends in epidemiology of invasive pneumococcal disease in Poland. Eur J Clin Microbiol Infect Dis 2015; 34:779-87; PMID:25475124; http://dx.doi.org/10.1007/s10096-014-2283-8
- Keck JW, Wenger JD, Bruden DL, Rudolph KM, Hurlburt DA, Hennessy TW, Bruce MG. PCV7-induced changes in pneumococcal carriage and invasive disease burden in Alaskan children. Vaccine 2014; 32:6478-84; PMID:25269095; http://dx.doi.org/10.1016/j.vaccine.2014.09.037
- Chiba N, Morozumi M, Sunaoshi K, Takahashi S, Takano M, Komori T, Sunakawa K, Ubukata K. Serotype and antibiotic resistance of isolates from patients with invasive pneumococcal disease in Japan. Epidemiol Infect 2010; 138:61-8; PMID:19538821; http://dx.doi.org/10.1017/S0950268809990239
- Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect 2014; 20 Suppl 5:45-51; PMID:24313448; http://dx.doi.org/10.1111/1469-0691.12461
- Fedson DS, Guppy MJ. Pneumococcal vaccination of older adults: conjugate or polysaccharide? Hum Vaccin Immunother 2013; 9:1382-4; PMID:23732892; http://dx.doi.org/10.4161/hv.24692
- Aliberti S, Mantero M, Mirsaeidi M, Blasi F. The role of vaccination in preventing pneumococcal disease in adults. Clin Microbiol Infect 2014; 20 Suppl 5:52-8; PMID:24410778; http://dx.doi.org/10.1111/1469-0691.12518
- Grabenstein JD, Manoff SB. Pneumococcal polysaccharide 23-valent vaccine: long-term persistence of circulating antibody and immunogenicity and safety after revaccination in adults. Vaccine 2012; 30:4435-44; PMID:22542818; http://dx.doi.org/10.1016/j.vaccine.2012.04.052
- O'Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis 2007; 7:597-606; PMID:17714673; http://dx.doi.org/10.1016/S1473-3099(07)70210-4
- Ohshima N, Nagai H, Matsui H, Akashi S, Makino T, Akeda Y, Oishi K. Sustained functional serotype-specific antibody after primary and secondary vaccinations with a pneumococcal polysaccharide vaccine in elderly patients with chronic lung disease. Vaccine 2014; 32:1181-6; PMID:24120483; http://dx.doi.org/10.1016/j.vaccine.2013.09.060
- Hammitt LL, Bulkow LR, Singleton RJ, Nuorti JP, Hummel KB, Miernyk KM, Zanis C, Whaley M, Romero-Steiner S, Butler JC, et al. Repeat revaccination with 23-valent pneumococcal polysaccharide vaccine among adults aged 55-74 years living in Alaska: no evidence of hyporesponsiveness. Vaccine 2011; 29:2287-95; PMID:21255685; http://dx.doi.org/10.1016/j.vaccine.2011.01.029
- Musher DM, Manoff SB, McFetridge RD, Liss CL, Marchese RD, Raab J, Rueda AM, Walker ML, Hoover PA. Antibody persistence ten years after first and second doses of 23-valent pneumococcal polysaccharide vaccine, and immunogenicity and safety of second and third doses in older adults. Hum Vaccin 2011; 7:919-28; PMID:21860256; http://dx.doi.org/10.4161/hv.7.9.15996
- Oishi K, Tamura K, Akeda Y. Global control of pneumococcal infections by pneumococcal vaccines. Trop Med Health 2014; 42:83-6; PMID:25425955; http://dx.doi.org/10.2149/tmh.2014-S11
- Poolman JT, Peeters CC, van den Dobbelsteen GP. The history of pneumococcal conjugate vaccine development: dose selection. Expert Rev Vaccines 2013; 12:1379-94; PMID:24195479; http://dx.doi.org/10.1586/14760584.2013.852475
- Ruiz-Aragon J, Marquez Pelaez S, Molina-Linde JM, Grande-Tejada AM. Safety and immunogenicity of 13-valent pneumococcal conjugate vaccine in infants: a meta-analysis. Vaccine 2013; 31:5349-58; PMID:24055349; http://dx.doi.org/10.1016/j.vaccine.2013.09.008
- Martikainen JA, Soini EJ, Laine J, Ahman H, Postila V, Klemets P. Economic impact of 13-valent pneumococcal conjugate vaccine in Finnish adults >/=50 years with underlying chronic medical conditions. J Eval Clin Pract 2014; 20:333-41; PMID:24813690; http://dx.doi.org/10.1111/jep.12131
- Vila-Corcoles A, Ochoa-Gondar O. Preventing pneumococcal disease in the elderly: recent advances in vaccines and implications for clinical practice. Drugs Aging 2013; 30:263-76; PMID:23420119; http://dx.doi.org/10.1007/s40266-013-0060-5
- Lynch JP 3rd, Zhanel GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med 2009; 30:189-209; PMID:19296419; http://dx.doi.org/10.1055/s-0029-1202938
- French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, Mwaiponya M, Zijlstra EE, Molyneux ME, Gilks CF. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812-22; PMID:20200385; http://dx.doi.org/10.1056/NEJMoa0903029
- Dransfield MT, Harnden S, Burton RL, Albert RK, Bailey WC, Casaburi R, Connett J, Cooper JA, Criner GJ, Curtis JL, et al. Long-term comparative immunogenicity of protein conjugate and free polysaccharide pneumococcal vaccines in chronic obstructive pulmonary disease. Clin Infect Dis 2012; 55:e35-44; PMID:22652582; http://dx.doi.org/10.1093/cid/cis513
- Dransfield MT, Nahm MH, Han MK, Harnden S, Criner GJ, Martinez FJ, Scanlon PD, Woodruff PG, Washko GR, Connett JE, et al. Superior immune response to protein-conjugate versus free pneumococcal polysaccharide vaccine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 180:499-505; PMID:19556517; http://dx.doi.org/10.1164/rccm.200903-0488OC
- Tobudic S, Plunger V, Sunder-Plassmann G, Riegersperger M, Burgmann H. Randomized, single blind, controlled trial to evaluate the prime-boost strategy for pneumococcal vaccination in renal transplant recipients. PLoS One 2012; 7:e46133; PMID:23029408; http://dx.doi.org/10.1371/journal.pone.0046133
- Usuf E, Bottomley C, Adegbola RA, Hall A. Pneumococcal carriage in sub-Saharan Africa–a systematic review. PLoS One 2014; 9:e85001; PMID:24465464; http://dx.doi.org/10.1371/journal.pone.0085001
- Roca A, Hill PC, Townend J, Egere U, Antonio M, Bojang A, Akisanya A, Litchfield T, Nsekpong DE, Oluwalana C, et al. Effects of community-wide vaccination with PCV-7 on pneumococcal nasopharyngeal carriage in the Gambia: a cluster-randomized trial. PLoS Med 2011; 8:e1001107; PMID:22028630; http://dx.doi.org/10.1371/journal.pmed.1001107
- von Gottberg A, de Gouveia L, Tempia S, Quan V, Meiring S, von Mollendorf C, Madhi SA, Zell ER, Verani JR, O'Brien KL, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med 2014; 371:1889-99; PMID:25386897; http://dx.doi.org/10.1056/NEJMoa1401914
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962-73; PMID:21492929; http://dx.doi.org/10.1016/S0140-6736(10)62225-8
- Elberse KE, van der Heide HG, Witteveen S, van de Pol I, Schot CS, van der Ende A, Berbers GA, Schouls LM. Changes in the composition of the pneumococcal population and in IPD incidence in The Netherlands after the implementation of the 7-valent pneumococcal conjugate vaccine. Vaccine 2012; 30:7644-51; PMID:22521844; http://dx.doi.org/10.1016/j.vaccine.2012.04.021
- Ogilvie I, Khoury AE, Cui Y, Dasbach E, Grabenstein JD, Goetghebeur M. Cost-effectiveness of pneumococcal polysaccharide vaccination in adults: a systematic review of conclusions and assumptions. Vaccine 2009; 27:4891-904; PMID:19520205; http://dx.doi.org/10.1016/j.vaccine.2009.05.061
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2013; 1:CD000422; PMID:23440780
- Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, Gomez-Bertomeu F, Figuerola-Massana E, Raga-Luria X, Hospital-Guardiola I. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged >/= 60 years: 3 years of follow-up in the CAPAMIS study. Clin Infect Dis 2014; 58:909-17; PMID:24532544; http://dx.doi.org/10.1093/cid/ciu002
- Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR Morb Mortal Wkly Rep 2012; 61:394-5; PMID:22647745
- Hak E, Grobbee DE, Sanders EA, Verheij TJ, Bolkenbaas M, Huijts SM, Gruber WC, Tansey S, McDonough A, Thoma B, et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. Neth J Med 2008; 66:378-83; PMID:18990781
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114-25; PMID:25785969; http://dx.doi.org/10.1056/NEJMoa1408544
- Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2013; 31:3585-93; PMID:23688527; http://dx.doi.org/10.1016/j.vaccine.2013.05.010
- Jackson LA, Gurtman A, van Cleeff M, Jansen KU, Jayawardene D, Devlin C, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine 2013; 31:3577-84; PMID:23688526; http://dx.doi.org/10.1016/j.vaccine.2013.04.085
- Greenberg RN, Gurtman A, Frenck RW, Strout C, Jansen KU, Trammel J, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults 60-64 years of age. Vaccine 2014; 32:2364-74; PMID:24606865; http://dx.doi.org/10.1016/j.vaccine.2014.02.002
- Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822-5; PMID:25233284
- de Roux A, Schmole-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N, Baker SA, Razmpour A, Emini EA, Fernsten PD, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008; 46:1015-23; PMID:18444818; http://dx.doi.org/10.1086/529142
- Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis 2015; 15:535-43; PMID:25801458; http://dx.doi.org/10.1016/S1473-3099(15)70044-7
- Demczuk WH, Martin I, Griffith A, Lefebvre B, McGeer A, Shane A, Zhanel GG, Tyrrell GJ, Gilmour MW. Serotype distribution of invasive Streptococcus pneumoniae in Canada during the introduction of the 13-valent pneumococcal conjugate vaccine, 2010. Can J Microbiol 2012; 58:1008-17; PMID:22827750; http://dx.doi.org/10.1139/w2012-073
- Chang Q, Stevenson AE, Croucher NJ, Lee GM, Pelton SI, Lipsitch M, Finkelstein JA, Hanage WP. Stability of the pneumococcal population structure in Massachusetts as PCV13 was introduced. BMC Infect Dis 2015; 15:68; PMID:25887323; http://dx.doi.org/10.1186/s12879-015-0797-z
- Munson S, Raluy-Callado M, Lambrelli D, Wasiak R, Eriksson D, Gray S. Clinical burden of pneumonia, meningitis and septicemia in Norway 2 years after 7-valent pneumococcal conjugate vaccine introduction. Scand J Public Health 2015; PMID:25979727
- Horacio AN, Lopes JP, Ramirez M, Melo-Cristino J. Non-invasive pneumococcal pneumonia in Portugal–serotype distribution and antimicrobial resistance. PLoS One 2014; 9:e103092; PMID:25075961; http://dx.doi.org/10.1371/journal.pone.0103092
- Jackson LA, Janoff EN. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin Infect Dis 2008; 47:1328-38; PMID:18844484; http://dx.doi.org/10.1086/592691
- Black S, De Gregorio E, Rappuoli R. Developing vaccines for an aging population. Sci Transl Med 2015; 7:281ps8; PMID:25834107; http://dx.doi.org/10.1126/scitranslmed.aaa0722
- Poland GA, Ovsyannikova IG, Kennedy RB, Lambert ND, Kirkland JL. A systems biology approach to the effect of aging, immunosenescence and vaccine response. Curr Opin Immunol 2014; 29:62-8; PMID:24820347; http://dx.doi.org/10.1016/j.coi.2014.04.005
- Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol 2013; 14:428-36; PMID:23598398; http://dx.doi.org/10.1038/ni.2588
- Aspinall R, Mitchell W. Reversal of age-associated thymic atrophy: treatments, delivery, and side effects. Exp Gerontol 2008; 43:700-5; PMID:18562142; http://dx.doi.org/10.1016/j.exger.2008.04.014
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol 2004; 5:133-9; PMID:14749784; http://dx.doi.org/10.1038/ni1033
- Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, Craft J. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol 2004; 173:673-81; PMID:15210831; http://dx.doi.org/10.4049/jimmunol.173.1.673
- Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol 2005; 17:468-75; PMID:16098723; http://dx.doi.org/10.1016/j.coi.2005.07.020
- Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells – a reflection of T cell differentiation, cell senescence and host environment. Semin Immunol 2012; 24:365-72; PMID:22560928; http://dx.doi.org/10.1016/j.smim.2012.04.003
- Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol 2003; 171:2326-30; PMID:12928378; http://dx.doi.org/10.4049/jimmunol.171.5.2326
- Labrie JE 3rd, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med 2004; 200:411-23; PMID:15314072; http://dx.doi.org/10.1084/jem.20040845
- Frasca D, Riley RL, Blomberg BB. Effect of age on the immunoglobulin class switch. Crit Rev Immunol 2004; 24:297-320; PMID:15663361; http://dx.doi.org/10.1615/CritRevImmunol.v24.i5.10
- Cancro MP, Hao Y, Scholz JL, Riley RL, Frasca D, Dunn-Walters DK, Blomberg BB. B cells and aging: molecules and mechanisms. Trends Immunol 2009; 30:313-8; PMID:19540810; http://dx.doi.org/10.1016/j.it.2009.04.005
- Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J Immunol 1997; 158:1598-609; PMID:9029095
- Tsuboi I, Morimoto K, Hirabayashi Y, Li GX, Aizawa S, Mori KJ, Kanno J, Inoue T. Senescent B lymphopoiesis is balanced in suppressive homeostasis: decrease in interleukin-7 and transforming growth factor-beta levels in stromal cells of senescence-accelerated mice. Exp Biol Med (Maywood) 2004; 229:494-502; PMID:15169968
- Keim C, Kazadi D, Rothschild G, Basu U. Regulation of AID, the B-cell genome mutator. Genes Dev 2013; 27:1-17; PMID:23307864; http://dx.doi.org/10.1101/gad.200014.112
- Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol 2008; 43:718-28; PMID:18586079; http://dx.doi.org/10.1016/j.exger.2008.05.016
- van Duin D, Shaw AC. Toll-like receptors in older adults. J Am Geriatr Soc 2007; 55:1438-44; PMID:17767688; http://dx.doi.org/10.1111/j.1532-5415.2007.01300.x
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol 2002; 169:4697-701; PMID:12391175; http://dx.doi.org/10.4049/jimmunol.169.9.4697
- Franceschi C, Olivieri F, Marchegiani F, Cardelli M, Cavallone L, Capri M, Salvioli S, Valensin S, De Benedictis G, Di Iorio A, et al. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: the lesson of centenarians. Mech Ageing Dev 2005; 126:351-61; PMID:15621218; http://dx.doi.org/10.1016/j.mad.2004.08.028
- Butcher SK, Chahal H, Nayak L, Sinclair A, Henriquez NV, Sapey E, O'Mahony D, Lord JM. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol 2001; 70:881-6; PMID:11739550
- Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol 2004; 76:291-9; PMID:15039467; http://dx.doi.org/10.1189/jlb.1103592
- Solana R, Mariani E. NK and NK/T cells in human senescence. Vaccine 2000; 18:1613-20; PMID:10689137; http://dx.doi.org/10.1016/S0264-410X(99)00495-8
- Chelvarajan RL, Collins SM, Van Willigen JM, Bondada S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J Leukoc Biol 2005; 77:503-12; PMID:15629885; http://dx.doi.org/10.1189/jlb.0804449
- Simell B, Vuorela A, Ekstrom N, Palmu A, Reunanen A, Meri S, Kayhty H, Vakevainen M. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine 2011; 29:1929-34; PMID:21236231; http://dx.doi.org/10.1016/j.vaccine.2010.12.121
- Iyer AS, Ohtola JA, Westerink MA. Age-related immune response to pneumococcal polysaccharide vaccination: lessons for the clinic. Expert Rev Vaccines 2015; 14:85-97; PMID:25269650; http://dx.doi.org/10.1586/14760584.2015.963058
- Chen M, Ssali F, Mulungi M, Awio P, Yoshimine H, Kuroki R, Furumoto A, Tanimura S, Kityo C, Nagatake T, et al. Induction of opsonophagocytic killing activity with pneumococcal conjugate vaccine in human immunodeficiency virus-infected Ugandan adults. Vaccine 2008; 26:4962-8; PMID:18639599; http://dx.doi.org/10.1016/j.vaccine.2008.06.093
- Namkoong H, Funatsu Y, Oishi K, Akeda Y, Hiraoka R, Takeshita K, Asami T, Yagi K, Kimizuka Y, Ishii M, et al. Comparison of the immunogenicity and safety of polysaccharide and protein-conjugated pneumococcal vaccines among the elderly aged 80 years or older in Japan: an open-labeled randomized study. Vaccine 2015; 33:327-32; PMID:25448102; http://dx.doi.org/10.1016/j.vaccine.2014.11.023
- Miernyk KM, Butler JC, Bulkow LR, Singleton RJ, Hennessy TW, Dentinger CM, Peters HV, Knutsen B, Hickel J, Parkinson AJ. Immunogenicity and reactogenicity of pneumococcal polysaccharide and conjugate vaccines in alaska native adults 55-70 years of age. Clin Infect Dis 2009; 49:241-8; PMID:19522655; http://dx.doi.org/10.1086/599824
- Goldblatt D, Southern J, Andrews N, Ashton L, Burbidge P, Woodgate S, Pebody R, Miller E. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50-80 years. Clin Infect Dis 2009; 49:1318-25; PMID:19814624; http://dx.doi.org/10.1086/606046
- Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine 2013; 31:3594-602; PMID:23688525; http://dx.doi.org/10.1016/j.vaccine.2013.04.084
- Assaad U, El-Masri I, Porhomayon J, El-Solh AA. Pneumonia immunization in older adults: review of vaccine effectiveness and strategies. Clin Interv Aging 2012; 7:453-61; PMID:23152675
- Honkanen PO, Keistinen T, Miettinen L, Herva E, Sankilampi U, Laara E, Leinonen M, Kivela SL, Makela PH. Incremental effectiveness of pneumococcal vaccine on simultaneously administered influenza vaccine in preventing pneumonia and pneumococcal pneumonia among persons aged 65 years or older. Vaccine 1999; 17:2493-500; PMID:10418894; http://dx.doi.org/10.1016/S0264-410X(99)00069-9
- Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, Thompson WW. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348:1747-55; PMID:12724480; http://dx.doi.org/10.1056/NEJMoa022678
- Johnstone J, Eurich DT, Minhas JK, Marrie TJ, Majumdar SR. Impact of the pneumococcal vaccine on long-term morbidity and mortality of adults at high risk for pneumonia. Clin Infect Dis 2010; 51:15-22; PMID:20504233; http://dx.doi.org/10.1086/653114
- Ordonez JE, Orozco JJ. Cost-effectiveness analysis of pneumococcal conjugate vaccine 13-valent in older adults in Colombia. BMC Infect Dis 2014; 14:172; PMID:24679135; http://dx.doi.org/10.1186/1471-2334-14-172
- Jiang Y, Gauthier A, Keeping S, Carroll S. Cost-effectiveness of vaccinating the elderly and at-risk adults with the 23-valent pneumococcal polysaccharide vaccine or 13-valent pneumococcal conjugate vaccine in the UK. Expert Rev Pharmacoecon Outcomes Res 2014; 14:913-27; PMID:25189087; http://dx.doi.org/10.1586/14737167.2014.950232
- Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine 2013; 31:6011-21; PMID:24148572; http://dx.doi.org/10.1016/j.vaccine.2013.10.024
- Black S. The role of health economic analyses in vaccine decision making. Vaccine 2013; 31:6046-9; PMID:23968768; http://dx.doi.org/10.1016/j.vaccine.2013.08.008
- Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA 2012; 307:804-12; PMID:22357831
- Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, D'Alessandro-Gabazza C, Nakayama S, Nishikubo K, Noguchi T, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ 2010; 340:c1004; PMID:20211953; http://dx.doi.org/10.1136/bmj.c1004
- Kawakami K, Ohkusa Y, Kuroki R, Tanaka T, Koyama K, Harada Y, Iwanaga K, Yamaryo T, Oishi K. Effectiveness of pneumococcal polysaccharide vaccine against pneumonia and cost analysis for the elderly who receive seasonal influenza vaccine in Japan. Vaccine 2010; 28:7063-9; PMID:20723631; http://dx.doi.org/10.1016/j.vaccine.2010.08.010
- Kularatna S, Wijesinghe PR, Abeysinghe MR, Karunaratne K, Ekanayake L. Burden of invasive pneumococcal disease (IPD) in Sri-Lanka: deriving a reasonable measure for vaccine introduction decision making. Vaccine 2015; 33:3122-8; PMID:25976543; http://dx.doi.org/10.1016/j.vaccine.2015.04.093
- Blau J, Hoestlandt C, D Clark A, Baxter L, Felix Garcia AG, Mounaud B, Mosina L. Strengthening national decision-making on immunization by building capacity for economic evaluation: implementing ProVac in Europe. Vaccine 2015; 33 Suppl 1:A34-9; PMID:25919171; http://dx.doi.org/10.1016/j.vaccine.2014.12.073
