Abstract
Dengue is a mosquito-borne viral disease that is endemic in India. We evaluated the immunogenicity and safety of recombinant, live-attenuated, tetravalent dengue vaccine (CYD-TDV) in Indian adults. In this observer-blind, randomized, placebo-controlled, Phase II study, adults aged 18–45 years were randomized 2:1 to receive CYD-TDV or placebo at 0, 6 and 12 months in sub-cutaneous administration. Immunogenicity was assessed using a 50% plaque reduction neutralization test (PRNT50) at baseline and 28 days after each study injection. 189 participants were enrolled (CYD-TDV [n = 128]; placebo, [n = 61]). At baseline, seropositivity rates for dengue serotypes 1, 2, 3 and 4 ranged from 77.0% to 86.9%. Seropositivity rates for each serotype increased after each CYD-TDV injection with a more pronounced increase after the first injection. In the CYD-TDV group, geometric mean titres (GMTs) were 2.38 to 6.11-fold higher after the third injection compared with baseline but remained similar to baseline in the placebo group. In the CYD-TDV group, the GMTs were 1.66 to 4.95-fold higher and 9.23 to 24.6-fold higher after the third injection compared with baseline in those who were dengue seropositive and dengue seronegative, respectively. Pain was the most commonly reported solicited injection site reaction after the first injection in both the CYD-TDV (6.3%) and placebo groups (4.9%), but occurred less frequently after subsequent injections. No serious adverse events were vaccine-related, no immediate unsolicited adverse events, and no virologically-confirmed cases of dengue, were reported during the study. The immunogenicity and safety of CYD-TDV was satisfactory in both dengue seropositive and seronegative Indian adults.
Introduction
Dengue is a mosquito-borne viral disease that is endemic in tropical and subtropical countries and represents a global pandemic threat.Citation1 The virus is endemic in India, with several known hyper-endemic sub-regions.Citation2,3 Dengue is a notifiable disease, that is of significant public health concern in India due to annual outbreaks and all 4 dengue virus serotypes circulate in the country. In 2013, more than 75,000 cases and 167 deaths were reported to the Indian national surveillance program.Citation2-6 However, dengue is believed to be significantly under-reported in India and the average annual number of cases has been estimated to vary widely up to 33 million apparent cases annually (which would represent 34% of the global total).Citation7-9 In addition, the economic burden of dengue illness in India is considerable with annual medical and non-medical and indirect costs estimated at US$1.11 billion.Citation8
There is no specific treatment for dengue and current control measures have involved promoting the principles of integrated vector management and deploying locally-adapted vector control measures, but they have in general failed to reduce the occurrence of dengue epidemics.Citation1 Vaccination is acknowledged as a potentially effective strategy for the control of dengue, but there is no registered vaccine currently available.
A recombinant, yellow fever-17D–dengue virus, live-attenuated, tetravalent dengue vaccine (CYD-TDV) is in late phase of development for the control of dengue disease with a 3 dose-schedule regimen, with vaccinations given 6 months apart via the subcutaneous route.Citation10,11 Two landmark phase III studies with CYD-TDV, in children aged 2–14 years in 5 countries in Asia and those aged 9–16 years in 5 countries Latin America, have shown that, over the 25 month active surveillance period, the vaccine was efficacious against virologically-confirmed dengue and severe disease and there were fewer hospitalizations in the vaccine group compared to control.Citation12,13 In the Latin American study CYD-TDV vaccination was associated with overall efficacy of 60.8% against symptomatic dengue and efficacy against hospitalization was 80.3% over the 25 month active surveillance period.Citation13 Similarly, in the Asian study, CYD-TDV vaccination was associated with overall efficacy against symptomatic dengue of 56.5%, prevented 80% of cases of dengue hemorrhagic fever (during the 13 month period after the third dose), and led to a clinically important reduction in the risk of hospitalization due to dengue (during the 25 month active surveillance period).Citation12 The favorable vaccine safety profile observed during the 25 month active surveillance period was consistent with that documented in prior studies in Asia.Citation10,11,14-16 As a first step before evaluating the vaccine in children in India, we conducted a clinical trial in Indian adults. Here we describe the immunogenicity and safety profiles of CYD-TDV in healthy Indian adults (ClinicalTrials.gov NCT01550289 and CTRI/2012/03/002518).
Results
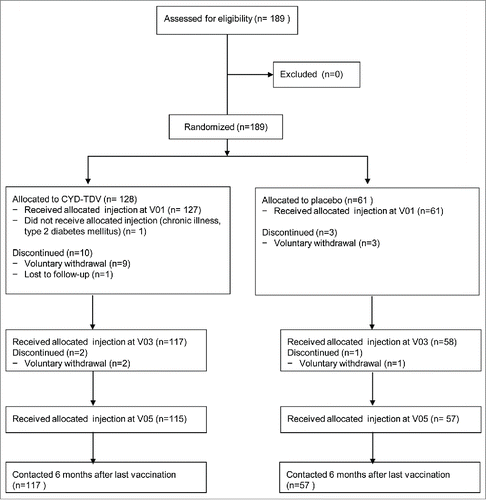
A total of 189 participants were enrolled and randomized (128 in the CYD-TDV group and 61 in the placebo group) with a 2:1 ratio between 27 March 2012 and 28 June 2012. Among the 128 participants randomized in the CYD-TDV group, 127 (99.2%) received the first injection and one participant was excluded due to chronic type 2 diabetes mellitus and did not receive study injection. One hundred and 17 participants (91.4%) received the second injection, and 115 (89.8%) received the third injection. A total of 17 participants (9.0%) discontinued the study, but none were withdrawn from the study due to an SAE or AE. Overall, 172 participants completed the study (115 in the CYD-TDV group and 57 in the placebo group). The flow of participants through the study is shown in
The demographics and baseline characteristics are summarized in . All of the participants were of Asian ethnicity with an overall mean (±SD) age of 29.5 ± 6.9 years at enrolment. However, there was a higher proportion of male participants (151/187 [80.7%]) than female participants (36/187 [19.3%]) in the FAS and this was reflected in the composition of both vaccination groups ().
Table 1. Demographic characteristics and seropositivity rates for each dengue virus serotype of randomized participants at baseline (FAS)
Immunogenicity
At baseline, the percentages of participants who were seropositive (PRNT50 antibody titer ≥10 1/dil) for each dengue serotype were high and similar in both groups. In the CYD-TDV group, the seropositivity rate ranged from 77.0% (97/126) for serotype 4 to 84.9% (107/126) for serotype 3; in the placebo group, the seropositivity rate ranged from 80.3% (49/61) for serotypes 1 and 4 to 86.9% (53/61) for serotypes 2 and 3. The percentages of participants in the CYD-TDV and placebo groups who were seropositive at baseline and after the third injection for at least one dengue virus serotype are presented in and
Figure 2. Seropositivity rates (PRNT50 antibody titer ≥10 1/dil [95% CI]) against one, 2, 3 or 4 dengue virus serotypes at baseline and 28 days after each study injection given at 0, 6 and 12 months (FAS).
![Figure 2. Seropositivity rates (PRNT50 antibody titer ≥10 1/dil [95% CI]) against one, 2, 3 or 4 dengue virus serotypes at baseline and 28 days after each study injection given at 0, 6 and 12 months (FAS).](/cms/asset/a23a41cc-7183-496c-bf82-54afd013c321/khvi_a_1076598_f0002_c.gif)
Balanced immune responses against all 4 serotypes were noted in the CYD-TDV group. Seropositivity rates for each dengue virus serotype increased after each injection with a more pronounced increase after the first injection (). GMTs in the CYD-TDV and placebo groups at baseline and after the first, second and third injections are presented in . In the CYD-TDV group, GMTs were 2.38 to 6.11-fold higher after the third dengue injection compared with baseline. In the placebo group, GMTs remained similar to baseline after each injection with increases from baseline from 1.05 to 1.44-fold 28 days after the third injection.
Figure 3. Serotype-specific seropositivity rate (PRNT50 titer ≥10 1/dil [95% CI]) at baseline and 28 days after each study injection given at 0, 6 and 12 months (FAS).
![Figure 3. Serotype-specific seropositivity rate (PRNT50 titer ≥10 1/dil [95% CI]) at baseline and 28 days after each study injection given at 0, 6 and 12 months (FAS).](/cms/asset/99e63801-31da-46bf-b106-bd46a44c77dc/khvi_a_1076598_f0003_c.gif)
Figure 4. Geometric mean titres (GMTs [1/dil]) and 95% CIs for antibodies against each dengue virus serotype at baseline and 28 days after the 3 study injections given at 0, 6 and 12 months (FAS).
![Figure 4. Geometric mean titres (GMTs [1/dil]) and 95% CIs for antibodies against each dengue virus serotype at baseline and 28 days after the 3 study injections given at 0, 6 and 12 months (FAS).](/cms/asset/a991c67a-3325-4d61-823d-65052d6f31c5/khvi_a_1076598_f0004_b.gif)
GMTs after each CYD-TDV injection are summarized by baseline dengue serostatus in (data for the placebo-injected group are not shown). The GMTs in the dengue seropositive and dengue seronegative groups were 1.66 to 4.95-fold higher and 9.23 to 24.6-fold higher, respectively, after the third injection compared with baseline. In contrast, in the placebo groups, GMTs remained similar to baseline after each injection in both the dengue seropositive and seronegative groups but, overall, were higher in dengue seropositive participants than dengue seronegative participants 28 days after the third injection.
Safety and Reactogenicity
A total of 188 participants (127 in the CYD-TDV group and 61 in the placebo group) received at least one injection and were included in the SAS (). One participant in the CYD-TDV group (0.8%) and one participant in the placebo group (1.6%) experienced at least one SAE (megaloblastic anemia and viral upper respiratory tract infection, respectively). Both participants recovered and neither of these events were considered related to study vaccination.
Table 2. Solicited and unsolicited adverse events and adverse reactions after any injection (SAS).
No immediate unsolicited AEs were reported during the study. However, solicited injection site reactions and solicited systemic reactions were reported by higher percentages of participants in the CYD-TDV group (9.5% and 19.0%, respectively) compared to the placebo group (4.9% and 6.6%, respectively) (). Pain, which appeared within 3 days of the first injection and resolved spontaneously, was the only reported solicited injection site reaction after the first injection in both the CYD-TDV group (8/126 [6.3%]) and the placebo group (3/61 [4.9%]). Most of the solicited injection site pain was of Grade 1 intensity. Two participants in the CYD-TDV group (1.6%) experienced Grade 2 injection site pain. No action was taken and both reactions resolved spontaneously. No Grade 3 solicited injection site reactions were reported after the first injection. Similarly, pain was the only reported solicited injection site reaction after the second injection in the CYD-TDV and placebo groups (2/117 [1.7%] vs 2/58 [3.4%], respectively) and after the third injection in the CYD-TDV group (3/115 [2.6%]). Erythema and swelling were not reported within 7 days after any injection.
Unsolicited AEs within 28 days after any injection were also reported by a slightly higher proportion of participants in the CYD-TDV group (9.4%) compared to the placebo group (6.6%) (). There were no immediate unsolicited AEs in either the CYD-TDV or placebo group. All unsolicited AEs were considered as non-serious in both groups and did not lead to participant discontinuation. There were no unsolicited adverse reactions and no deaths reported during the study and no virologically-confirmed cases of dengue. In the CYD-TDV group, 3/127 (2.4%) participants reported at least one non-serious AESI, suggestive of an allergic reaction (Grade 1 pruritus on the day of the first injection which resolved spontaneously on the same day (n = 2) and Grade 1 papular rash on the day of the first injection which resolved spontaneously 2 days after vaccination (n = 1)). None of these 3 participants had a medical history of allergy and for all of them the suspected allergen was unknown and these AESIs were considered as not related to study vaccination. No AESIs were observed in the placebo group and no other occurrences of these AESIs were observed after the second and third injections.
Discussion
This was the first clinical trial of CYD-TDV in India, a country which in 2010 was estimated to have contributed approximately 34% of the global total of dengue infections.Citation9 The high dengue seropositivity rates (80–87% against all 4 serotypes) at enrolment in this study in healthy Indian adults confirm the high degree of endemicity of dengue disease in India.
Overall, CYD-TDV administered at 0, 6 and 12 months produced a balanced humoral immune response against all 4 dengue serotypes in Indian adults regardless of baseline dengue immune status and displayed a satisfactory safety profile, comparable to that observed in similar trials elsewhere in Asia.Citation10,15,16 The majority of participants completed the study and no safety issues with CYD-TDV were reported during vaccine administration and subsequent 6 month safety follow-up. The safety results during the 6 month observation period Indian adults are consistent with the previous phase I and II studies.Citation10,11 and the long term safety follow-up is ongoing in the Asia and Latin America efficacy trials to assess severe disease due to subsequent heterologous dengue infections.Citation17-20 Data published recently from the Philippines study by Capeding et al.Citation21 shows the tetravalent dengue vaccine appears to have good safety within the observation period and persistence of antibodies over 5 years after the 3-dose schedule.
GMTs for neutralizing antibodies against all 4 dengue serotypes in this study in Indian adults increased irrespective of baseline dengue serostatus, but the GMTs were highest in those who were dengue seropositive at baseline in line with previous studies.Citation10,14,15,22 Moreover, GMTs in this study were comparable with or seemed rather higher than those reported for other Asian countries where dengue endemicity is high e.g. Vietnam (adults and children).Citation15 and the Philippines (adults).Citation10
Phase III lots of CYD-TDV were used in this study. The safety profile of CYD-TDV does not appear to be adversely affected by previous dengue exposure. Reactogenicity with CYD-TDV was comparable to the placebo injection and, in terms of solicited reactions and unsolicited AEs tended to be lower in both CYD-TDV and placebo groups after the second and third injection compared to the first injection. The trend toward a lower incidence of AEs with subsequent injections has also been observed in previous trials with both Phase II lots of CYD-TDV Citation15,16 and Phase III lots.Citation14,24 (CYD17 study, manuscript in preparation). Moreover, there was a lack of vaccine-related SAEs in line with the findings of previous studies in high endemic regions.Citation10-12,14,15,23
In conclusion, the overall immunogenicity and safety profile of CYD-TDV administered at 0, 6, 12 months was satisfactory in both dengue seropositive and dengue seronegative Indian adults.
Patients and Methods
Study design and participants
This was a multi-center, observer-blind, randomized, placebo-controlled, Phase II study of CYD-TDV in healthy adult participants in India (endemic population). The study was conducted at centers in New Delhi, Pune, Ludhiana, Bangalore and Kolkata between 27 March 2012 and 10 June 2013. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Conference on the Harmonization-Good Clinical Practice. Written informed consent was obtained from all participants before study entry. An independent data monitoring committee (IDMC) oversaw the conduct of the study and was involved in the review of all deaths, related serious adverse events (SAEs) and virologically-confirmed dengue cases.
Individuals aged 18 to 45 years in good health based on medical history and physical examination, able to attend all study visits and to comply with study procedures, were eligible for participation in this study. Participants were ineligible if they: had febrile illness (temperature ≥38.0°C) or moderate or severe acute illness/infection on the day of vaccination; had known seropositivity for human immu-nodeficiency virus (HIV), hepatitis B, and hepatitis C; had known or suspected congenital or acquired immunodeficiency, or receipt of immunosuppressive therapy within the previous 6 months, or long-term systemic corticosteroid therapy (prednisone or equivalent for >2 consecutive weeks within the previous months); had receipt of blood or blood-derived products in the previous 3 months that might interfere with assessment of immune responses; were in receipt of (or planned receipt of) any vaccine in the 4 weeks preceding or following any trial vaccination. Pregnant or lactating women were also ineligible for inclusion and all female participants were required to be of non-child-bearing potential (i.e., post-menopausal for at least 1 year, surgically sterile, or using an effective method of contraception or were abstinence from at least 4 weeks prior to the first vaccination and until at least 4 weeks after the last vaccination). Women who became pregnant during the study were not to be vaccinated further. Contraindications for the second or third CYD-TDV doses included: evidence of anaphylactic or other significant allergic reaction to the previous study vaccination; an ongoing clinical adverse event (AE) related to the previous study vaccination or SAE following the previous study vaccination.
Participants were randomized 2:1 at enrolment by study site personnel via an interactive voice response system/interactive web response system (IVRS/IWRS), using a permuted block method with block size of 9 and stratification by site. A double randomization system was used, such that the participant treatment allocation was separated from dose dispensing. Each dose had both a code number and a dose number. The code number was used by the IVRS while the dose number was entered in the participant's eCRF. The unique dose numbers were defined according to a random list to ensure that dose numbers could not be used to distinguish between treatment groups.
Participants received a 3-dose primary series of subcutaneous injections of CYD-TDV or an inactive placebo (0.5 mL NaCl 0.9% solution) in the deltoid region of the upper arm at visits 0, 6, and 12 months, followed by a 6-month safety follow-up. Observers, investigators, the sponsor and participants were all blinded to the allocation of study injections. Phase III lots of CYD-TDV (Sanofi Pasteur S.A., France) were supplied as powder and solvent for suspension for subcutaneous injection. Each 0.5 mL injection of reconstituted vaccine contained 4.5–6.0 log10 cell-culture infectious dose 50% (CCID50) of each live, attenuated, recombinant dengue serotype 1, 2, 3, 4 virus. The solvent consisted of NaCl 0.4%.
Immunogenicity
Blood samples were taken for determination of antibody responses at baseline and 28 days after each study injection. Serum levels of neutralizing antibodies against each of the 4 parental dengue strains of CYD-TDV were determined using a 50% plaque reduction neutralization test (PRNT50) as described elsewhere.Citation15 The lower limit of quantitation (LLOQ) of the assay was 10 (1/dilution [dil]). Geometric mean titres (GMTs) were calculated.
Safety and reactogenicity
Participants were kept under observation for 30 minutes after each trial injection to assess the occurrence of any immediate AEs. AEs monitored throughout the study included: unsolicited systemic AEs within 30 minutes of each injection; solicited injection site reactions (tenderness, redness, and swelling) up to 7 days after each injection; solicited systemic reactions (headache, fever, malaise, myalgia and asthenia) up to 14 days after each injection; unsolicited AEs up to 28 days after each vaccination; SAEs throughout the trial; and adverse events of special interest (AESIs) considered to be relevant for the monitoring of the safety profile of CYD-TDV. AESIs included: hypersensitivity/allergic reactions within 7 days; serious viscerotropic disease and serious neurotropic disease within 30 days; and serious dengue disease at any time during the study. AE data after the initial 30-minute observation period were collected using diary cards with the specific solicited adverse reactions and an open field for unsolicited AEs. AEs were classified according to the following scale: Grade 1, no interference with activity; Grade 2, some interference with activity; and Grade 3, significant (prevents daily activity). Investigators assessed the causal relationship of each unsolicited systematic AE and vaccination and the participants rated the intensity of solicited and unsolicited AEs on their diary cards. SAEs were defined as events that were life-threatening or resulted in death, required in-patient hospitalization or prolonged existing hospitalization, resulted in persistent or significant disability or incapacity, resulted in a congenital anomaly or birth defect, or were regarded as an important medical event. Participants were followed up at 6 months after the last injection for information on AEs occurring since the last visit.
Detection of symptomatic cases
Participants with suspected dengue, defined as febrile episodes (temperature ≥38°C) on at least 2 consecutive days, were assessed for dengue infection. The following tests were performed to virologically confirm any potential dengue case: dengue reverse transcriptase polymerase chain reactions (RT-PCRs), dengue non-structural protein (NS) 1 antigen (Ag) enzyme-linked immunosorbent assay (ELISA) and immunoglobulin (Ig) M/IgG ELISA. If a sample was positive for the wild type (WT) dengue RT-PCR and/or the NS1 assay was positive, then it was classified as a virologically-confirmed dengue infection.
Statistical Methods and Analysis Populations
Analyses were descriptive and no hypotheses were tested. The sample size was set to 126 participants for the CYD-TDV group to provide a 95% probability of observing an event that had a true incidence of 2.38 % in this group. For the main parameters, 95% confidence intervals (CI) of point estimates were calculated using the normal approximation for quantitative data and the exact binomial distribution (Clopper-Pearson method) for proportions. Analyses were performed on all available data, with no replacement of missing data. The full analysis set (FAS) for immunogenicity included all participants who received at least one dose of CYD-TDV or placebo and had at least one valid serology result after study injection. The safety analysis set (SAS) comprised of participants who received at least one dose of CYD-TDV or placebo injection.
Disclosure of Potential Conflicts of Interest
APD, SA, JC, AN and SG received funds to their institutes from Sanofi Pasteur to support their work in the CYD47 trial. TAW, AB and JM are employees of Sanofi Pasteur.
Acknowledgments
Dengue testing was carried out at Global Clinical Immunology (GCI; Sanofi Pasteur, Swiftwater, Pennsylvania, USA). The authors would like to thank the participants who enrolled in the trial. Editorial assistance with the preparation of the manuscript was provided by a professional medical writer, Simon Lancaster of inScience Communications, Springer Healthcare, funded by Sanofi Pasteur.
Funding
This study was sponsored by Sanofi Pasteur. The study sponsor participated in the trial design and managed all operational aspects of the study, including monitoring data collection, statistical analyses, and writing of the report.
References
- World Health Organization. Global strategy for dengue prevention and control: 2012–2020. Available from: http://www.who.int/denguecontrol/9789241504034/en/ (Accessed 7 July 2014) 2012
- National Vector Borne Disease Control Programme. Dengue cases and deaths in the country since 2008. Available at: http://nvbdcp.gov.in/den-cd.html (Accessed 7 July 2014) 2014
- Chakravarti A, Arora R, Luxemburger C. Fifty years of dengue in India. Trans R Soc Trop Med Hyg 2012; 106:273-82.; PMID:22357401; http://dx.doi.org/10.1016/j.trstmh.2011.12.007
- Government of India. Guidelines for Clinical Management of Dengue Fever, Dengue Haemorrhagic Fever and Dengue Shock Syndrome. Available at: http://www.nvbdcp.gov.in/Doc/Clinical%20Guidelines.pdf (Accessed 7 July 2014) 2008
- Garg P, Nagpal J, Khairnar P, Seneviratne SL. Economic burden of dengue infections in India. Trans R Soc Trop Med Hyg 2008; 102:570-7; PMID:18402995; http://dx.doi.org/10.1016/j.trstmh.2008.02.015
- Rai MA, Khan H. Dengue: Indian subcontinent in the line of fire. J Clin Virol 2007; 38:269-70; PMID:17254843; http://dx.doi.org/10.1016/j.jcv.2006.12.010
- Kakkar M. Dengue fever is massively under-reported in India, hampering our response. BMJ 2012; 345:e8574; PMID:23255584; http://dx.doi.org/10.1136/bmj.e8574
- Shepard DS, Halasa YA, Tyagi BK, Adhish SV, Nandan D, Karthiga KS, Chellaswamy V, Gaba M, Arora NK, INCLEN Study Group. Economic and disease burden of dengue illness in india. Am J Trop Med Hyg 2014;91:1235-42; PMID:25294616; http://dx.doi.org/10.4269/ajtmh.14-0002
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature 2013; 496:504-7; PMID:23563266; http://dx.doi.org/10.1038/nature12060
- Capeding RZ, Luna IA, Bomasang E, Lupisan S, Lang J, Forrat R, Wartel A, Crevat D. Live-attenuated, tetravalent dengue vaccine in children, adolescents and adults in a dengue endemic country: randomized controlled phase I trial in the Philippines. Vaccine 2011; 29:3863-72; PMID:21477675; http://dx.doi.org/10.1016/j.vaccine.2011.03.057
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 2012; 380:1559-67; PMID:22975340; http://dx.doi.org/10.1016/S0140-6736(12)61428-7
- Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384:1358-65; PMID:25018116; http://dx.doi.org/10.1016/S0140-6736(14)61060-6
- Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramirez JO, Carrasquilla G, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 2015; 372:113-23; PMID:25365753; http://dx.doi.org/10.1056/NEJMoa1411037
- Hss AS, Koh MT, Tan KK, Chan LG, Zhou L, Bouckenooghe A, Crevat D, Hutagalung Y. Safety and immunogenicity of a tetravalent dengue vaccine in healthy children aged 2-11 years in Malaysia: a randomized, placebo-controlled, Phase III study. Vaccine 2013; 31:5814-21; PMID:24135573; http://dx.doi.org/10.1016/j.vaccine.2013.10.013
- Tran NH, Luong CQ, Vu TQH, Lang J, Vu QD, Bouckenooghe A, Wartel TA. Safety and immunogenicity of recombinant, live attenuated tetravalent dengue vaccine (CYD- TDV) in healthy Vietnamese adults and children. J Vaccines Vaccin 2012; 3:162
- Leo YS, Wilder-Smith A, Archuleta S, Shek LP, Chong CY, Leong HN, Low CY, Oh ML, Bouckenooghe A, Wartel TA, et al. Immunogenicity and safety of recombinant tetravalent dengue vaccine (CYD-TDV) in individuals aged 2-45 y: Phase II randomized controlled trial in Singapore. Hum Vaccin Immunother 2012; 8:1259-71; PMID:22894958; http://dx.doi.org/10.4161/hv.21224
- Anderson KB, Gibbons RV, Cummings DA, Nisalak A, Green S, Libraty DH, Jarman RG, Srikiatkhachorn A, Mammen MP, Darunee B, et al. A shorter time interval between first and second dengue infections is associated with protection from clinical illness in a school-based cohort in Thailand. J Infect Dis 2014; 209:360-8; PMID:23964110; http://dx.doi.org/10.1093/infdis/jit436
- Bhoomiboonchoo P, Nisalak A, Chansatiporn N, Yoon IK, Kalayanarooj S, Thipayamongkolgul M, Endy T, Rothman AL, Green S, Srikiatkhachorn A, et al. Sequential dengue virus infections detected in active and passive surveillance programs in Thailand, 1994-2010. BMC Public Health 2015; 15:250; PMID:25886528; http://dx.doi.org/10.1186/s12889-015-1590-z
- Guzman MG, Kouri G, Valdes L, Bravo J, Vazquez S, Halstead SB. Enhanced severity of secondary dengue-2 infections: death rates in 1981 and 1997 Cuban outbreaks. Rev Panam Salud Publica 2002; 11:223-7; PMID:12049030; http://dx.doi.org/10.1590/S1020-49892002000400003
- Halstead SB. Identifying protective dengue vaccines: guide to mastering an empirical process. Vaccine 2013; 31:4501-7; PMID:23896423; http://dx.doi.org/10.1016/j.vaccine.2013.06.079
- Capeding MR, Laot TM, Boaz M, Wartel TA, Crevat D. Immunogenicity and safety of a tetravalent dengue vaccine during a five-year follow-up period. Trials in Vaccinology 2015; 4:19-23; http://dx.doi.org/10.1016/j.trivac.2015.03.002
- Qiao M, Shaw D, Forrat R, Wartel-Tram A, Lang J. Priming effect of dengue and yellow fever vaccination on the immunogenicity, infectivity, and safety of a tetravalent dengue vaccine in humans. Am J T rop Med Hyg 2011; 85:724-31; http://dx.doi.org/10.4269/ajtmh.2011.10-0436
- Dayan GH, Garbes P, Noriega F, Izoton de Sadovsky AD, Rodrigues PM, Giuberti C, Dietze R. Immunogenicity and Safety of a Recombinant Tetravalent Dengue Vaccine in Children and Adolescents Ages 9-16 Years in Brazil. Am J Trop Med Hyg 2013; 89:1058-65; PMID:24189367; http://dx.doi.org/10.4269/ajtmh.13-0304
- Torresi J, Heron LG, Qiao M, Marjason J, Chambonneau L, Bouckenooghe A, Boaz M, van der Vliet D, Wallace D, Hutagalung Y, Nissen MD, Richmond PC. Lot-to-lot consistency of a tetravalent dengue vaccine in healthy adults in Australia: a randomised study. 2015 Aug 13. pii: S0264-410X(15)01122-6; http://dx.doi.org/10.1016/j.vaccine.2015.08.008

![Figure 5. Geometric mean titres (GMTs [1/dil]) and 95% CIs for antibodies against each dengue virus serotype at baseline and 28 days after the 3 study injections given at 0, 6 and 12 months in the CYD-TDV group by baseline dengue serostatus (FAS) (data for the placebo-injected group are not shown).](/cms/asset/fdc9227f-a498-4bfe-a4d1-c8b0889e59e5/khvi_a_1076598_f0005_b.gif)