Abstract
Several studies have reported poor immune responses to conventional influenza vaccines in HIV-infected individuals. This study sought to elicit more potent immunogenicity in HIV-infected adults using an intradermal vaccine compared with a conventional intramuscular vaccine. This multicenter, randomized, controlled, open-label study was conducted at 3 university hospitals during the 2011/2012 pre-influenza season. Three vaccines were used in HIV-infected adults aged 18 – 60 years: an inactivated intramuscular vaccine (Agrippal), a reduced-content intradermal vaccine (IDflu9μg) and a standard-content intradermal vaccine (IDflu15μg). Serum hemagglutination-inhibiting (HI) antibodies and INF-γ ELISpot assay were measured at the time of vaccination and 1 month after vaccination. Adverse events were recorded for 7 d. A total of 28 Agrippal, 30 IDflu9μg, and 28 IDflu15μg volunteers were included in this analysis. One month after vaccination, the GMTs and differences in INF-γ ELISpot assay results were similar among the 3 groups. Seroprotection rates, seroconversion rates and mean fold increases (MFI) among the 3 groups were also similar, at approximately 80%, 50–60% and 2.5 – 10.0, respectively. All three vaccines satisfied the CHMP criteria for the A/H1N1 and A/H3N2 strains, but not those for the B strain. In univariate analysis, no demographic or clinical factors, including age, CD4+ T-cell counts, HIV viral load, ART status and vaccine type, were related to failure to achieve seroprotection. The three vaccines were all well-tolerated and all reported reactions were mild to moderate. However, there was a tendency toward a higher incidence of local and systemic reactions in the intradermal vaccine groups. The intradermal vaccine did not result in higher immunogenicity compared to the conventional intramuscular vaccine, even with increased antigen dose.
Introduction
Human immunodeficiency virus (HIV)-infected individuals have both lower humoral and cell-mediated immunity. As a consequence, they are considered to be at risk for influenza infection and its complications. Although well-designed epidemiologic studies have been limited, several small studies have demonstrated that HIV-infected individuals are more likely to have severe or prolonged influenza infections.Citation1,2 For this reason, annual influenza immunization is recommended for patients with HIV, and an inactivated intramuscular vaccine is routinely administered.
Several studies have shown poor immune responses to conventional influenza vaccines in HIV-infected individuals compared with healthy uninfected individuals.Citation3-5 CD4+ T-cell count and HIV viral load have generally been considered to be the main factors that contribute to poor immunogenicity.Citation6,7 Although widespread use of antiretroviral therapy (ART) has restored the immune deficit in these patients, influenza vaccination has still resulted in suboptimal immunogenicity in HIV-infected individuals.Citation8,9 To overcome this hypo-responsiveness, several strategies, including use of adjuvants, boosters or high doses of vaccine, have been proposed. However, the results remain controversial and additional data are needed.
An intradermal influenza vaccine has recently been introduced. With brand name IDflu® (Sanofi Pasteur, Lyon, France), this is delivered using the Micro-Injection System that consists of a pre-filled syringe with a micro-needle (1.5 mm) and a needle shielding system. It is available as a 9 μg HA formulation for adults 18–59 y of age and as a 15 μg HA formulation for adults ≥ 60 y of age. Intradermal vaccines elicit comparable or superior immune reactions compared with conventional vaccines in healthy adults.Citation10,11 To this end, we conducted this study expecting that an intradermal vaccine would elicit more potent immunogenicity in HIV-infected adults, compared with a conventional intramuscular vaccine.
Results
Study participant characteristics
A total of 90 participants were enrolled (Agrippal, n=30; IDflu9μg, n = 30; and IDflu15 μg, n=30). Because four participants did not complete a second visit, data were available for 86 participants (Agrippal, n = 28; IDflu9μg, n = 30; and IDflu15 μg, n = 28). They declined the second visit for no definite reasons. shows the baseline characteristics of these 86 participants. Males were more common than females, and the median age was 40. The majority were on ART with a mean CD4+ count of 483 and HIV RNA levels <50 copies/mL. No statistical differences between the baseline characteristics of participants were found except for age and ART status. The IDflu9μg group was younger and had a lower ART status than did the other groups. (p<0.001 and p=0.023, respectively).
Table 1. Characteristics of HIV-1 infected patients vaccinated with 2011–2012 conventional intramuscular, intradermal (IDflu9μg, IDflu15μg) influenza vaccine
Safety
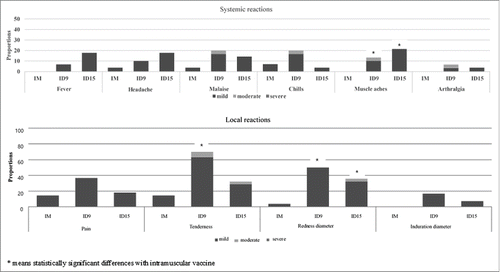
The overall incidences of local and systemic reactions are reported in Figure 1. The three vaccines were well-tolerated and all reactions were of mild to moderate grade. There was a tendency toward a higher incidence of local and systemic reactions in the intradermal vaccine groups. However, the reactions were not significantly different, except for the occurrence of local tenderness, redness diameter and muscle aches. Tenderness was more common in the IDflu9μg group than in the intramuscular vaccine and IDflu15μg groups (p<0.001); however, most events were mild (63.3% in IDflu9μg group and 28.6% in IDflu15μg group) (Fig. 1) Redness diameter was larger in both the IDflu9μg and IDflu15μg groups than in the intramuscular vaccine group (p < 0.001). Muscle aches were more common in the IDflu15μg group (p = 0.024). No unsolicited reactions were reported in any of the 3 groups.
Immunogenicity
The results of the HI assay for the 3 strains are presented in . Pre-vaccination GMTs were similar for the 3 virus strains among the 3 groups. The proportion of pre-vaccination antibody titers ≥40 did not differ. A significant increase in HI titers against all 3 strains was observed 1 month after vaccination (p < 0.001 for all strains). However, post-vaccination GMTs, seroprotection rates, seroconversion rates and MFI were similar among the 3 groups. All three vaccines satisfied all of the criteria of the CHMP recommendations for the A/H1N1 and A/H3N2 strains, but not for the B strain.
Table 2. Antibody responses as measured with the Hemagglutination-Inhibition (HI) assay according to vaccine group
INF-γ ELISpot assays were performed for 6 participants in each group. The demographic features, including gender, age, BMI, smoking history, comorbidity, AIDS-defining events, duration of HIV infection, CD4+ T-cell counts, HIV viral load, and ART status, did not differ between the 3 groups (data not shown). The data obtained are shown in . Pre-vaccination and post-vaccination INF-γ production was not different among the 3 vaccine groups, despite changes in HA concentration. The three groups all had significant increases in INF-γ production after vaccination (all p values < 0.001). However, these increases were not significantly different among the 3 groups.
Table 3. INF-γ ELISpot values in response to 2010–2011 conventional intramuscular, intradermal (IDflu9μg, IDflu15μg) influenza vaccine
Factors associated with protection
Univariate analysis was performed to identify risk factors related to failure to achieve seroprotection 1 month after vaccination (). No factors, including age, CD4+ T-cell counts and HIV viral loads, were related to seroprotection.
Table 4. Univariate analysis of demographic and clinical factors associated with failing to achieve seroprotection at 1 month after influenza vaccination for each viral strain
Discussion
Various strategies have been investigated as possible ways to increase the immunogenicity of influenza vaccination in HIV-infected individuals. The use of booster doses, high-dose vaccinations and adjuvants has been previously investigated.Citation12-15 Although adjuvanted vaccines have consistently shown additional effects on immune response, the results of other methods are conflicting. Recently, an intradermal vaccine was introduced and its possible utility in high-risk individuals has been actively investigated. This approach is potentially advantageous because it leads to improved immunogenicity due to the large number of immunostimulatory cells, such as macrophages and dendritic cells, in the dermis.Citation16
The aim of this study was to evaluate the immunogenicity of the 9-μg and 15-μg intradermal vaccines compared with the intramuscular vaccine in HIV-infected adults. Contrary to our expectations, immunogenicity profiles were not improved in the intradermal vaccine groups. These results suggest that neither the intradermal route nor the use of standard 15μg dose influenced or improved immune reactions in HIV-infected individuals. To our knowledge, only one study has evaluated the immunogenicity and safety of the intradermal vaccine in HIV-infected individuals.Citation17 The study compared a low-antigen-content intradermal vaccine (9 μg HA per strain) with a conventional intramuscular vaccine (9 μg HA per strain), and targeted HIV-infected adults. The reported seroprotection rate was approximately 80% and the seroconversion rate was nearly 50–60%. MFI ranged widely from 2.5 to 10.0. The study concluded that the half-antigen-dose intradermal vaccine was safe and showed similar immunogenicity with the standard-dose intramuscular vaccine. Our results are similar. The IDflu9μg and the conventional intramuscular vaccine elicited similar immune reactions and both groups satisfied the CHMP criteria. Similar finding was also demonstrated in another study.Citation18 Although the study compared the immunogenicity of booster dose of inactivated polio vaccine, a 60% dose reduction in the standard dose could elicit comparable boosting reaction through intradermal vaccination. Therefore, fractional dose of intradermal vaccine could be effective even in the HIV-infected individuals.
Seroprotection rates in HIV-infected adults treated with the conventional intramuscular vaccine against seasonal influenza vaccine have been reported to be 70–90%, while seroconversion rates of 40–60% have been reported.Citation12-14, 19 Although the immune response was suboptimal compared with healthy subjects, the majority of values met the CHMP criteria. Surprisingly, overall seroprotection rates were very low (approximately 15–60%) during the pre-ART era.Citation6,20, 21 This improvement in immunogenicity is probably due to the effects of active use of ART. In this study, 74.4% of participants were taking ART and had a mean CD4+ T-cell count of nearly 500 cells/mm3. HIV viral load was also well-suppressed at 60.7%. High CD4+ T-cell counts and low HIV viral loads may have contributed to fundamental improvement in immune reactions to vaccination.
Age, low CD4+ T-cell counts and high HIV viral loads are considered to be important factors in immune reactions. In this study, the IDflu9μg group was younger than the other groups, and ART treatment was less common. To adjust the factors and find factors which were related to immunogenicity, we conducted univariate analysis. Our analysis of factors including age, HIV viral load, CD4+ T-cell counts, ART status and vaccine type found no association with seroprotection. These results were consistent with the findings of a recent study.Citation22 This may also have been due to the correction of previously well-known risk factors by widespread use of ART. However, recent studies, including this study, may have been underpowered to identify all factors due to the small number individuals in the studied populations.
There are limited data about the impact of antigen dose increases on immunogenicity in HIV-infected individuals. Two previous studies, which compared a double dose (30 μg/strain) with a standard dose (15 μg/strain) vaccination, reported that immune response was not improved.Citation15,23 Although our study used an intradermal vaccine, an antigen dose (15 μg/strain) increase also did not improve immune response compared with a low-antigen-dose (9 μg/strain) intradermal vaccine. The low-antigen-dose intradermal vaccine was sufficient to elicit satisfactory immunogenicity. Therefore, it is thought that a dose increase is unnecessary in HIV-infected adults in the era of ART.
This study has some limitations. First, this study compares different vaccine brands. Intramuscular vaccine is one of the subunit vaccine while intradermal vaccines are classified as a split vaccine. Previous meta-analysis found no relevant differences in immunogenicity in the same condition, but subunit vaccine showed a lower frequency of systemic and local reactions. The discrete component might contribute to different immunogenicity and safety profiles regardless of the route of administration. Second, this study was not a double-blinded study, which may have influenced the safety data. However, it is well known that intradermal vaccines result in a higher number of at least local adverse events than intramuscular vaccines.Citation18 Third, immunogenicity against the B strain was lower than reported in other studies. The B strain is known to produce poor reactivity in the HI test, especially when using egg-propagated vaccine virus.Citation24 Use of a neutralization assay might yield more precise measurements. Fourth, the sample size may have been too small to assess statistical differences. This study corrected the different baseline characteristics and GMT levels through statistical methods such as a multivariate analysis and an ANCOVA model. If the sample size was bigger, each group would have similar baseline values, which resulted in different outcomes. When performing this study, there were no previous studies with similar study design. Therefore, we were limited in our ability to calculate a statistically powerful sample size. Fifth, a previous vaccination and an influenza infection history were not available. These factors can influence immunogenicity.
Although our study has several limitations, the results suggest that the use of an intradermal vaccine does not improve immunogenicity in HIV-infected adults compared with a conventional intramuscular vaccine, even when an increased antigen dose is used. Although more mild local reactions were observed in the intradermal vaccine group, the 3 vaccines were tolerable to all participants.
Materials and Methods
Ethics statement
This study was approved by the institutional review board (IRB) of Korea University Guro Hospital, Ewha University Mokdong Hospital and Kangnam Sacred Heart Hospital, all of which are located in Seoul City of the Republic of Korea. This study was performed in accordance with the Helsinki Declaration and Good Clinical Practices.
Study subjects
This multicenter, randomized, controlled, open-label study was conducted at 3 university hospitals during the 2011/2012 pre-influenza season. HIV-infected individuals aged 18–60 y who were not immunized with the 2011/2012 influenza vaccine were recruited. Informed consent was obtained from all participants following IRB recommendations. Exclusion criteria included: a known allergy to eggs, presentation of any febrile illness ≥37.5°C on the day of vaccination, any history of hypersensitive reaction to a previous influenza vaccination, any other vaccinations within the past month, use of immunosuppressive agents, having received blood products or immunoglobulins during the previous 3 months, and any other conditions that might interfere with the study results.
Vaccines
Participants were centrally randomized to one of 3 vaccines with no stratification. Study vaccines were a standard dose trivalent subunit inactivated intramuscular vaccine (Agrippal® S1, Novartis Vaccines and Diagnostics S. R. L., Italy), a reduced-content intradermal split vaccine (IDflu9μg®, Sanofi Pasteur SA, France) and a standard-content intradermal split vaccine (IDflu15μg®, Sanofi Pasteur SA, France). The three vaccines contained an A/California/7/2009 (H1N1)-like virus, an A/Perth/16/2009 (H3N2)-like virus, and a B/Brisbane/60/2008-like virus, as recommended by the WHO.
Antibody assay
Blood samples were taken from all participants prior to vaccination and 4 weeks after vaccination. Hemagglutination-inhibiting (HI) antibodies against each of the 3 antigen components were measured by standard microtiter assay.Citation25 In brief, serum was treated with a receptor-destroying enzyme (Sigma, St. Louis, MO, USA). Serum dilutions ranging from 1:5 to 1:5,120 were then prepared. HI titer was read after a 0.5% suspension of washed chicken erythrocytes was added.
The antibody response was interpreted according to the criteria of the Committee for Medical Products for Human Use (CHMP). Geometric mean titer (GMT), seroprotection rate (the proportion of participants with a HI titer ≥1:40), seroconversion rate (the proportion of participants with a ≥fold4- increase in titer from baseline or a post-vaccination HI titer ≥1:40 if the baseline titer was <1:40), and mean fold increase (MFI) (GMT ratio of post-vaccination HI titer to pre-vaccination HI titer) were calculated. The vaccine approval criteria in adults younger than 60 were applied: seroprotection rate >70%, seroconversion rate >40%, and MFI >2.5.
IFN-γ ELISpot assay
Gamma interferon enzyme-linked immunosorbent spot assays were performed on a randomly selected subset of 6 participants from each group using cryopreserved peripheral blood mononuclear cells (PBMCs).Citation25 In brief, duplicate cultures of 300,000 PBMCs per well were stimulated with the influenza vaccines. For negative and positive controls, PBMCs were incubated with phosphate-buffered saline and phytohemagglutinin, respectively. Then, IFN-γ spot-forming units (SFUs) were determined using an ELISpot analyzer (Cellular Technology Ltd., Shaker Heights, OH). A response was considered positive when antigen-specific IFN-γ SFUs were greater than 10 SFUs per 300,000 PBMCs. PBMCs were repeatedly stimulated using 3 concentrations of HA (2.0 g, 1.0 g and 0.5 g).
Safety assessment
At the time of vaccination, participants were provided with a thermometer, a ruler and a diary and were asked to monitor for any local or systemic reactions for 7 d The diary was based on the Food and Drug Association (FDA) Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. The contents of the diary included details about body temperature, pain, tenderness, redness and induration diameter at the injection site, and the severity of systemic symptoms such as headache, malaise, chills, muscle aches, arthralgia, and any other adverse events. Redness and induration diameter were considered mild if the diameter was 1–4 mm, moderate if the diameter was 5–9 mm and severe if the diameter ≥10 mm.
Statistical analysis
The sample size (n = 90 ) was calculated based on a statistical power of 0.8, a significance level of 0.05, and a drop-out rate of 10%. One-way analysis of variance (ANOVA) with post-hoc Bonferroni analysis and Dunnett T3 analysis was used to compare the continuous variables among the 3 groups. The chi-square and Fisher's exact tests were used for bivariate analyses. An ANCOVA model adjusted according to pre-vaccination levels was used to compare post-vaccination GMTs.
Univariate analysis was additionally performed to evaluate factors related to seroprotection. Variables included gender, age, body mass index (BMI), smoking history, comorbidity, AIDS-defining events, duration of HIV infection, CD4+ T-cell counts, HIV viral load, ART status (subjects on ART vs. without ART), and influenza vaccine group. All p-values were 2-sided and accepted when the values were <0.05. All analyses were performed using the SPSS 18.0 software (SPSS Korea, Seoul, Korea).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethics Statement
This retrospective chart review was conducted in the department infectious disease of Kangnam Sacred Heart Hospital and Ewah Womans University Mokdong Hospital in Korea and approved by the medical ethics committee at each hospital. (Clinical Trial Registration: NCT02398097).
References
- Neuzil KM, Coffey CS, Mitchel EF, Jr., Griffin MR. Cardiopulmonary hospitalizations during influenza season in adults and adolescents with advanced HIV infection. J Acquir Immune Defic Syndr 2003; 34:304-7; PMID:14600576; http://dx.doi.org/10.1097/00126334-200311010-00008
- Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch Intern Med 2001; 161:441-6; PMID:11176770; http://dx.doi.org/10.1001/archinte.161.3.441
- Pinto LA, Blazevic V, Anderson SA, Venzon DJ, Trubey CM, Rowe T, Katz JM, Liewehr D, Dolan MJ, Shearer GM. Influenza virus-stimulated generation of anti-human immunodeficiency virus (HIV) activity after influenza vaccination in HIV-infected individuals and healthy control subjects. J Infect Dis 2001; 183:1000-8; PMID:11237823; http://dx.doi.org/10.1086/319277
- Santini-Oliveira M, Camacho LA, Souza TM, Luz PM, Vasconcellos MT, Giacoia-Gripp CB, Morgado MG, Nunes EP, Lemos AS, Ferreira AC, et al. H1N1pdm09 adjuvanted vaccination in HIV-infected adults: a randomized trial of two single versus two double doses. PloS one 2012; 7:e39310; PMID:22761759; http://dx.doi.org/10.1371/journal.pone.0039310.
- Crum-Cianflone NF, Eberly LE, Duplessis C, Maguire J, Ganesan A, Faix D, Defang G, Bai Y, Iverson E, Lalani T, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in an immunocompromised population: a prospective study comparing HIV-infected adults with HIV-uninfected adults. Clin Infect Dis 2011; 52:138-46; PMID:21148532; http://dx.doi.org/10.1093/cid/ciq019.
- Miotti PG, Nelson KE, Dallabetta GA, Farzadegan H, Margolick J, Clements ML. The influence of HIV infection on antibody responses to a two-dose regimen of influenza vaccine. JAMA 1989; 262:779-83; http://dx.doi.org/10.1001/jama.1989.03430060075029.
- Nelson KE, Clements ML, Miotti P, Cohn S, Polk BF. The influence of human immunodeficiency virus (HIV) infection on antibody responses to influenza vaccines. Ann Intern Med 1988; 109:383-8; PMID:2970238; http://dx.doi.org/10.7326/0003-4819-109-5-383.
- Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, Kottilil S, Gezmu M, Follmann D, Vodeiko GM, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis 2005; 191:1442-50; PMID:15809902; http://dx.doi.org/10.1086/429298.
- Klein MB, Lu Y, DelBalso L, Cote S, Boivin G. Influenzavirus infection is a primary cause of febrile respiratory illness in HIV-infected adults, despite vaccination. Clin Infect Dis 2007; 45:234-40; PMID:17578785; http://dx.doi.org/10.1086/518986.
- Young F, Marra F. A systematic review of intradermal influenza vaccines. Vaccine 2011; 29:8788-801; PMID:21968444; http://dx.doi.org/10.1016/j.vaccine.2011.09.077.
- Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med 2004; 351:2295-301; PMID:15525714; http://dx.doi.org/10.1056/NEJMoa043540.
- Durando P, Fenoglio D, Boschini A, Ansaldi F, Icardi G, Sticchi L, Renzoni A, Fabbri P, Ferrera A, Parodi A, et al. Safety and immunogenicity of two influenza virus subunit vaccines, with or without MF59 adjuvant, administered to human immunodeficiency virus type 1-seropositive and -seronegative adults. Clin Vaccine Immunol 2008; 15:253-9; PMID:18003811; http://dx.doi.org/10.1128/CVI.00316-07.
- Gabutti G, Guido M, Durando P, De Donno A, Quattrocchi M, Bacilieri S, Ansaldi F, Cataldini S, Chiriac∫ PG, De Simone M, et al. Safety and immunogenicity of conventional subunit and MF59-adjuvanted influenza vaccines in human immunodeficiency virus-1-seropositive patients. J Int Med Res 2005; 33:406-16; PMID:16104444; http://dx.doi.org/10.1177/147323000503300406.
- McKittrick N, Frank I, Jacobson JM, White CJ, Kim D, Kappes R, DiGiorgio C, Kenney T, Boyer J, Tebas P. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med 2013; 158:19-26; PMID:23277897; http://dx.doi.org/10.7326/0003-4819-158-1-201301010-00005.
- Cooper C, Thorne A, Klein M, Conway B, Boivin G, Haase D, Shafran S, Zubyk W, Singer J, Halperin S, et al. Immunogenicity is not improved by increased antigen dose or booster dosing of seasonal influenza vaccine in a randomized trial of HIV infected adults. PloS One 2011; 6:e17758; PMID:21512577; http://dx.doi.org/10.1371/journal.pone.0017758.
- Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 2010; 33:464-78; PMID:21029958; http://dx.doi.org/10.1016/j.immuni.2010.10.007.
- Ansaldi F, Valle L, de Florentiis D, Parodi V, Murdaca G, Bruzzone B, Durando P, Setti M, Icardi G. Phase 4 randomized trial of intradermal low-antigen-content inactivated influenza vaccine versus standard-dose intramuscular vaccine in HIV-1-infected adults. Hum Vaccin Immunother 2012; 8:1048-52; PMID:22832261; http://dx.doi.org/10.4161/hv.20347.
- Troy SB, Kouiavskaia D, Siik J, Kochba E, Beydoun H, Mirochnitchenko O, Levin Y, Khardori N, Chumakov K, Maldonado Y. Comparison of the immunogenicity of various booster doses of inactivated polio vaccine delivered intradermally versus intramuscularly to HIV-infected adults. J Infect Dis 2015; 211:1969-76; PMID:25567841; http://dx.doi.org/10.1093/infdis/jiu841.
- Madhi SA, Maskew M, Koen A, Kuwanda L, Besselaar TG, Naidoo D, Cohen C, Valette M, Cutland CL, Sanne I. Trivalent inactivated influenza vaccine in African adults infected with human immunodeficient virus: double blind, randomized clinical trial of efficacy, immunogenicity, and safety. Clin Infect Dis 2011; 52:128-37; PMID:21148531; http://dx.doi.org/10.1093/cid/ciq004.
- Schneider MM, Sprenger MJ, Hoepelman IM, van der Graaf Y, Borleffs JC. Antibody response to tetravalent influenza subunit vaccine in patients infected with human immunodeficiency virus type 1. Int J Antimicrob Agents 1996; 6:195-200; PMID:18611709; http://dx.doi.org/10.1016/0924-8579(95)00038-0.
- Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. Aids 1994; 8:469-76; PMID:7912086; http://dx.doi.org/10.1097/00002030-199404000-00008.
- Cooper CL. Pandemic H1N12009 influenza and HIV: a review of natural history, management and vaccine immunogenicity. Curr Opin Infect Dis 2012; 25:26-35; PMID:22183114; http://dx.doi.org/10.1097/QCO.0b013e32834ef56c.
- Kroon FP, van Dissel JT, de Jong JC, Zwinderman K, van Furth R. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine 2000; 18:3040-9; PMID:10825608; http://dx.doi.org/10.1016/S0264-410X(00)00079-7.
- Kishida N, Fujisaki S, Yokoyama M, Sato H, Saito R, Ikematsu H, Xu H, Takashita E, Tashiro M, Takao S, et al. Evaluation of influenza virus A/H3N2 and B vaccines on the basis of cross-reactivity of postvaccination human serum antibodies against influenza viruses A/H3N2 and B isolated in MDCK cells and embryonated hen eggs. Clin Vaccine Immunol 2012; 19:897-908; PMID:22492743; http://dx.doi.org/10.1128/CVI.05726-11.
- Cheong HJ, Song JY, Park JW, Yeon JE, Byun KS, Lee CH, Cho HI, Kim TG, Kim WJ. Humoral and cellular immune responses to influenza vaccine in patients with advanced cirrhosis. Vaccine 2006; 24:2417-22; PMID:16406176; http://dx.doi.org/10.1016/j.vaccine.2005.11.064.