Abstract
Successful vaccination policies for protection from invasive pneumococcal diseases (IPD) dependent on determination of the exact serotype distribution in each country. We aimed to identify serotypes of pneumococcal strains causing IPD in children in Turkey and emphasize the change in the serotypes before and after vaccination with 7-valent pneumococcal conjugate vaccine (PCV-7) was included and PCV-13 was newly changed in Turkish National Immunization Program. Streptococcus pneumoniae strains were isolated at 22 different hospitals of Turkey, which provide healthcare services to approximately 65% of the Turkish population. Of the 335 diagnosed cases with S. pneumoniae over the whole period of 2008–2014, the most common vaccine serotypes were 19F (15.8%), 6B (5.9%), 14 (5.9%), and 3 (5.9%). During the first 5 y of age, which is the target population for vaccination, the potential serotype coverage ranged from 57.5 % to 36.8%, from 65.0% to 44.7%, and from 77.4% to 60.5% for PCV-7, PCV-10, and PCV-13 in 2008–2014, respectively. The ratio of non-vaccine serotypes was 27.2% in 2008–2010 whereas was 37.6% in 2011–2014 (p=0.045). S. penumoniae serotypes was less non-susceptible to penicillin as compared to our previous results (33.7 vs 16.5 %, p=0.001). The reduction of those serotype coverage in years may be attributed to increasing vaccinated children in Turkey and the increasing non-vaccine serotype may be explained by serotype replacement. Our ongoing IPD surveillance is a significant source of information for the decision-making processes on pneumococcal vaccination.
Abbreviations
| IPD | = | Invasive pneumococcal diseases |
| PCV-7 | = | Pneumococcal Conjugate Vaccine 7 valent |
| PCV-10 | = | Pneumococcal Conjugate Vaccine 10 valent |
| PCV-13 | = | Pneumococcal Conjugate Vaccine 13 valent |
| WHO | = | World Health Organization |
| NIP | = | National Immunization Program |
| MIC | = | Minimal inhibitory concentration |
| SD | = | Standard deviations |
| IQR | = | Interquartile range |
| PNS | = | Penicillin nonsusceptible |
| CSF | = | Cerebrospinal fluid |
| CDC | = | Centers for Disease Control and Prevention. |
Introduction
Streptococcus pneumoniae (pneumococcus) is a bacterium that can lead to serious infections such as meningitis, sepsis, pneumonia, cellulitis, arthritis, mastoiditis, and peritonitis. Pneumococcal infection is a major cause of morbidity and mortality worldwide. World Health Organization (WHO) estimated that 1.6 million deaths were caused by S. pneumoniae in 2005; of these deaths, 0.7–1 million were children less than 5 y of age.Citation1,2
Pneumococci, which have more than 90 serotypes due to different capsular polysaccharides having different antigenic characteristics, induce serotype-specific immune response.Citation3 It has been reported that serotype distribution of the pneumococcus changes in time due to various factors including clonal enlargement, capsular transformation, mass and routine pneumococcal vaccination, and widespread antibiotic use in population.Citation4,5 Thus, obtaining information about local serotype distribution of pneumococci and observing potential changes in this distribution in time is essential for effective vaccination strategies.
In 2007, 7-valent pneumococcal conjugate vaccine (PCV-7), which contains 7 (4, 6B, 9V, 14, 18C, 19F, 23F) of the most common serotypes encountered in childhood, was recommended by WHO to be included in national immunization programs.Citation6 Routine PCV-7 vaccination had a major impact on the incidence of invasive and noninvasive pneumococcal diseases in children worldwide.Citation7 Higher valency PCVs, 10-valent PCV (PCV-10) (contains serotypes 1, 5, and 7F in addition to the PCV-7 serotypes) and 13-valent PCV (PCV-13) (contains serotypes 3, 6A, and 19A in addition to the PCV-10 serotypes), were introduced in 2009.Citation8 In Turkey, PCV-7 was introduced into the Turkish National Immunization Program (NIP) in 2009 at 2, 4, 6, and 12 months of age and it was replaced with PCV-13 in November 2011 based on the local seroepidemiology of invasive pneumococcal diseases (IPD)Citation9.
In our study conducted between July 2008 and February 2010,Citation10 the most common serotypes were 19F and 6B. In children ≤2 y of age, the potential coverage rate of PCV-7 was 69.5%. The ongoing development and introduction into routine immunization schedules of glycoconjugate pneumococcal vaccines has markedly reduced the incidence of disease caused by vaccine serotypes, initially against the 7 serotypes in the first vaccine, and now with coverage increasing from 10 or 13 serotypes in recent years. As many differences between countries and years in serotype distribution after PCV usage were reported in several studies,Citation4,11-13 surveillance of pneumococcal diseases on the basis of serotypes should be done by all countries or regions.
Hospital based pneumococal surveillance study across several regions of Turkey have been performing since 2008 because local epidemiology knowledge is required to support policymakers' decision on the most appropriate vaccine to be used against serotypes of pneumococal strains which cause invasive pneumocaccal (IPD) disease in children. We present here the results from 2008 to 2014.
Results
Streptococcus pneumoniae strains were isolated and serotyped in 335 samples between 2008 and 2014. The median age of cases was 4 y (interquartile range [IQR], 1.5–9.0) and the boy-to-girl ratio was 1.33:1 in whole period. The median age of cases was 4 y (IQR, 1.0–9.0) in 2008–2010 and the median age of cases was 5 y (IQR, 2.0–9.0) in 2011–2014. Among the cases, the site of infection during 2008–2014 showed that the most prevalent disease was bacteremia/sepsis followed by meningitis and empyema (). Of the 335 diagnosed cases with S. pneumoniae over the whole period of 2008–2014, the most common vaccine serotypes were 19F (15.8%), 6B (5.9%), 14 (5.9%), and 3 (5.9%). The most common vaccine serotypes were 19F (n = 39, 19.3%), 6B (n = 16, 7.9%), 4 (n = 14, 6.9%), and 14 (n = 12, 5.9%) in 2008–2010. Of the 67 diagnosed cases with S. pneumoniae in the period of 2011–2012, the most common vaccine serotypes were 19F (n = 7, 10.4%) and 3 (n = 5, 7.5%). Among 66 diagnosed cases with S. pneumoniae in 2013–2014, the most common vaccine serotypes were 19F (n = 7, 10.6%), 14 (n = 6, 9.0%), and 3 (n = 5, 7.6%). Among the patients diagnosed with S. pneumoniae in 2008–2010, the most common non-PCV-13 serotypes were 7A, 8, 15 and 15C (n = 5, 2.5%) in each. Among the patients diagnosed with S. pneumoniae in 2011–2014, the most common non-PCV-13 serotypes were 15C (n = 4, 3%) and 8 (n = 3, 2.3%) (). The ratio of non-vaccine serotypes was 27.2% (55/202) in 2008–2010 whereas was 37.6% (50/133) in 2011–2014 for all age groups and the difference was significant (p = 0.045). Twenty two of the S. pneumoniae-positive samples could not be serotyped in all age groups ().
Table 1. The site of infection according to the years
Table 2. Serotype distribution of Streptococcus pneumoniae according to age groups per year in Turkey
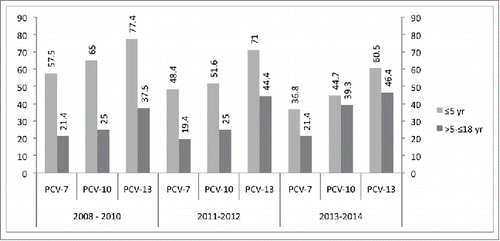
During the first 5 y of age, the potential serotype coverage rates of PCV-7, PCV-10, and PCV-13 were 57.5 %, 65%, and 77.4%, respectively; coverage rates of these vaccines were 21.4 %, 25 %, and 37.5% for >5 -≤18 y age group in 2008–2010. Of the cases during 2011–2012, the potential serotype coverage rates of PCV-7, PCV-10, and PCV-13 were 48.4%, 51.6%, and 71% for the first 5 y of age, respectively; coverage rates of these vaccines were 19.4%, 25%, and 44.4% for >5 -≤18 y age group. The potential serotype coverage rates of PCV-7, PCV-10, and PCV-13 for the first 5 y of age were 36.8%, 44.7%, and 60.5%, respectively; coverage rates of these vaccines were 21.4%, 39.3%, and 46.4% for >5 -≤18 y age group in 2013–2014 ().
Figure 1. Vaccine serotype coverage rates for PCV-7, PCV-10 and PCV-13 before and after inclusion of PCV-7 and PCV-13 in Turkey's NIP according to the years.
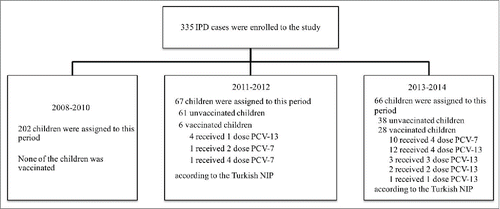
Children <1 year of age was agreed upon in Turkey at the end of 2008 and was included in the National Immunization Schedule in 2009. PCV-7 was used for 2 y in Turkey before being replaced by PCV-13 in November 2011.Citation9 The children in our previous studyCitation10 had not been vaccinated with PCV-7. Six of the 67 patients were vaccinated with either PCV-7 or PCV-13 in 2011–2012 (). Of the 6 vaccinated cases (4 of them diagnosed with bacteremia/sepsis and 2 of them diagnosed with meningitis), the isolated serotypes were 3 (n = 2), 9V (n = 1), and 19A (n = 1) in 4 of the PCV-13 vaccinated cases and were 3 and Q (+) in 2 of the PCV-7 vaccinated cases. Among the 28 patients (18 of them diagnosed with bacteremia/sepsis, 5 of them diagnosed with meningitis, and 5 of them diagnosed with empyema), 10 of them were vaccinated with PCV-7 and 18 of them were vaccinated with PCV-13 in 2013–2014 (). Of the 18 PCV-13 vaccinated cases, the isolated serotypes were 19F (n = 3), 3 (n = 2), 4 (n = 2), G (+) (n = 2), 23F, 9V, 2, 8, 14, 19A, 23A-F-, and D (+) in one patient each. One serotype was not identified. Of the 10 PCV-7 vaccinated cases, the isolated serotypes were 19F (n = 2), 15B (n = 2), 6B, 3, 20, and R (+) in one patient each. Two serotypes were not identified. During the first 5 y of age in 2013–2014, the serotype coverages for PCV-7, PCV-10, and PCV-13 were 30%, 60%, and 80% in unvaccinated children and were 39.3%, 39.3%, and 53.6% in vaccinated children, respectively.
Figure 2. CONSORT flow diagram showing number of IPD cases enrolled in the study, study periods and vaccination status of the IPD cases according to the years.
Of 202 invasive isolates, 68 (33.7%) were penicillin-resistant (minimal inhibition concentration (MIC) >0.06 microgram/ml) Streptococcus pneumoniae (PRSP) during 2008–2010 in our previous study.Citation10 Of 133 invasive isolates between 2011–2014, 22 (16.5 %) were PRSP. Penicillin-resistant Streptococcus pneumoniae ratio was significantly changed from 33.7% to 16.5% in 2008–2014 (p = 0.001). Of these PRSP isolates, 50% (n = 11) were from children ≤5 y of age. The proportions of PRSP isolates potentially covered by the PCV-7, PCV-10, and PCV-13 vaccines were 50%, 50%, and 63.3%, respectively.
Among IPD cases, cases in subjects ≤5 y and >5 - ≤18 y old were 72.3 % and 27.7 % in 2008–2010, respectively. However, in 2011–2012 IPD was predominant in children >5 - ≤18 y old (53.7%). Among IPD cases, cases in subjects ≤5 y and >5 - ≤18 y old were 57.6% and 42.4% in 2013–2014, respectively. When we consider the entire study period (2008–2014), more IPD cases were occurred in children under 5 y of age (64.2 % of all cases).
The regional distribution of S. pneumoniae during 2008–2014 showed that agent was most prevalent in the Central Anatolia (n = 96, 29%) followed by Mediterranean (n = 80, 24%) and Marmara regions (n = 67, 20%). The prevalence of regional distribution of S. pneumoniae isolates in other regions of Turkey were East Anatolia (n = 28, 8%), Aegean (n = 23, 7%), Black Sea (n = 21, 6%), and (n = 20, 6%), in decreasing order.
Discussion
Efficient implementation of regional and national immunization programs is important in terms of recent epidemiologic data on vaccine-preventable diseases because it provides critical piece of information from those areas. Therefore, we have monitored the serotype distribution of S. pneumonia in Turkey from 2008 to 2014. In our previous study, the most common serotypes in order of frequency were 19F, 6B, 4, 14, 19A, and 3 during the time period when PCV-7 was newly included in the country's NIP.Citation10 The most common serotypes in the order of frequency were 19F, 3, 23F, 6B, and 19A among the age groups ≤5 y and 14, 6A, and 19F among the age group >5 - ≤18 y in the period of 2011–2014. In consistently with our findings, according to the studies published from 1990 to 2008 of the epidemiology and serotype distribution of IPD in European children, the most common serotypes causing IPD were 14, 6B, 19F, and 23F, all of which are included in PCV-7.Citation2,12
PCVs has greatly reduced the incidence of disease by the vaccine serotypes both in vaccinated young children and among non-vaccinated groups due to herd immunity, and has led to public health benefits throughout the developed world where it has been used.Citation14,15 The median age of children included in the recent study was 5 y This possibly means the indirect effect of the PCVs that cause the reducing of the vaccine serotypes and serotype replacement. Therefore, no change was seen in the older age group. Among our cases, the site of infection during 2008–2014 showed that the most prevalent disease was bacteremia/sepsis followed by meningitis and empyema.
In several studies, regional and temporal variations in serotype distribution were reported after PCV usage. One of the our previous study carried out with 31 CSF samples collected from 13 medical centers in Turkey between the years 2005 and 2007 (pre-PCV era), the most common pneumococcal serotypes were 5 and 19F, followed by serotypes 1 and 23F.Citation16 The theoretical coverage rates by PCV-7, PCV-10, and PCV-13 were 48.1%, 85.2%, and 92.6%, respectively, for all age groups. In a study from Poland, where mass vaccination against pneumococcal diseases has not been introduced, the most common serotypes were 3, 14, 19A, 4, 9V, 19F, 1, and 23F in the pre-PCV era. The PCV-10 and PCV-13 covered 60.4 and 78.6 % of cases involving children under 5 y of age, respectively.Citation17 Imöhl et al.Citation12 reported that serotype coverage PCV-7, PCV-10, and PCV-13 were 62.3%, 75.5%, and 84.8 % for children, respectively. Although the overall reported burden of IPD varies among countries in Europe, vaccine serotype coverage ranged from 37% to 100% for PCV-7, with mean increases in coverage of 7% and 16% for PCV-10 and PCV-13, respectively.Citation2,18 During the first 5 y of age in the present study, which is the target population for vaccination, the potential serotype coverage ranged from 57.5 % to 36.8%, from 65% to 44.7%, and from 77.4% to 60.5% for PCV-7, PCV-10, and PCV-13 in 2008–2014, respectively. The potential vaccination coverages of children aged 5–18 y for PCV-7, PCV-10, and PCV-13 in whole period were not changed in our cohort. Routine vaccination with PCV-7 for children < 1 y of age was included in the Turkish NIP in 2009, and PCV-7 was replaced by PCV-13 in November 2011. Between 2010–2013, approximately 97 % of the target population was vaccinated with PCV (see http://www.sgk.gov.tr). The reduction of those serotype coverage in years may be explained by the impact of Turkish vaccination rates and increasing the vaccinated children in the study. According to those results, our ongoing IPD surveillance is a significant source of information for the decision-making processes on pneumococcal vaccination.
The Centers for Disease Control and Prevention (CDC) calculated the projected number of IPD cases prevented by PCV-7 among children aged <5 years, by age and direct or indirect effects and reported that the overall incidence of IPD among children aged <5 years declined.Citation19 Ben-Shimol et al.Citation20 reported that a 63% reduction of all-serotype IPD episodes was observed in children under 5 y of age. They attributed this finding to the sequential introduction of PCV-7 and PCV-13 on IPD. Regev-Yochay et al.Citation14 introduced the early impact of PCV-7/PCV-13 sequential introduction to the national pediatric immunization plan whereas an increase in non-vaccine type IPD. However, they reported the need of additional follow-up for the long-term impact of PCV-13. Concomitant with the decrease in incidence of IPD caused by the vaccine serotypes, there has been an increase in the incidence of IPD caused by non-vaccine serotypes. This phenomenon is referred to as serotype replacement.Citation2 Studies have revealed that widescale use of PCV-7 has caused serotype replacement responsible for IPD in populations.Citation2,21-23 The proportion of non-vaccine serotypes was 27.2% in our previous study.Citation10 We determined a significant increase (37.6%) in non-vaccine serotypes and a significant reduction in the number of isolates in the 2011–2014 period when compared to our previous results reflecting the period between 2008–2010 possibly due to the impact of national pediatric vaccination program with PCVs.
The level of PRSP isolates was higher in the present study than the European average (8.9%), with the highest proportion in Southern and Eastern European countries.Citation24 However, the proportion of PRSP isolates was significantly decreased in the present study as compared with our previous results. These results may possibly be attributed that decreasing number of antimicrobial resistant infections due to pneumococcal immunization.Citation24 Therefore, the need for continued surveillance is important to monitor changes in antimicrobial susceptibilities as well as serotypes in order to guide strategies for prevention and treatment as highlighted in literature.Citation28
This study has several limitations. First, we did not find enough cases and our study likely missed many cases with IPD to drive accurate rates of serotypes for S. penumoniae, thus limiting the generalizability of those data. The surveillance included 15 healthcare centers and 55% of the population in the period of 2008–2010 whereas included 22 healthcare centers and 65% of the population in the period of 2011–2014. Our smaller sample size as compared the previous years may possibly be affected by these operational changes. Additionally, all the data presented in this study were gathered through study centers. Our small sample size may possibly be attributed that this hospital based surveillance method and our data showed that we need nationwide study centers in Turkey where we may catch more cases through an active surveillance system. Second, despite the high vaccination rate of Turkey, the children in our study had been vaccinated with either PCV-7 or PCV-13 because the majority of the children were older than the requisite age for routine vaccination, according to the Turkish Ministry of Health Schedule. We really do not know the serotype distribution in post-PCV era. Third, we could not mention the incidences of the pneumococcal diseases in the present study because of the nature of our study. Besides incidences, we organized the study to find the serogroup distribution instead of case numbers. In addition, the difficulty of culture-confirmed diagnosis is an ongoing problem and both previous antibiotic usage and culture techniques limit to have accurate incidence rates. Despite these limitations, this project has provided useful insights into the serotype distribution of S.pneumoniae in Turkey. Because the data on the prevalence and serotype distribution of S.penumoniae isolates causing invasive diseases in post-PCV era are limited as well as pre-PCV era in Turkey.
It has been shown that introduction of conjugate vaccines can dramatically reduce IPD incidence, if vaccines are administered to the target population with high vaccine coverage.Citation14,15,19 The epidemiology and etiology of IPD may change over time and by regions in a way that cannot be predicted. In our study we showed that the most common serotypes were 19F, 3, 23F, 6B, and 19A among the age groups ≤ 5 years, which is the target population for vaccination, with a potential serotype coverage ranged from 57.5 % to 36.8%, from 65% to 44.7%, and from 77.4% to 60.5% for PCV-7, PCV-10, and PCV-13, respectively, over the entire study period (2008–2014). These results highlight the need for ongoing surveillance of pneumococcal disease is essential to monitor the disease dynamics after routine use of PCVs in Turkey.
Materials and Methods
Study design
This multicenter, hospital-based, prospective, epidemiological study was conducted in Turkey between July 2008 and December 2014 among children and adolescents younger than 18 y of age. The study was separated some periods as 2008–2010 (July 2008-February 2010),Citation10 2011–2012 (January 2011-December 2012), and 2013–2014 (January 2013-December 2014). The study was reviewed and approved by the Hacettepe University Institutional Ethics Committee. This ethical approval was sufficient for the other study sites as well. All patients admitted and treated for invasive infections attributable to S. pneumoniae were screened in 22 hospitals located in 7 regions of Turkey [Central Anatolia, Marmara, South East Anatolia, Aegean, East Anatolia, Mediterranean, and Black Sea], which provide healthcare services to approximately 65 % of the Turkish population. Cases were eligible for evaluation if S. pneumoniae was isolated from a normally sterile body site and was identified on the basis of typical colony morphology on blood agar as well as Gram strain, optochin sensitivity and bile solubility tests. After patients' parents/legal guardians had given informed consent, specimens were involved in the study. Duplicate isolates from the same patient were not accepted. The diagnosis of isolate was confirmed at the central study laboratory (Department of Microbiology and Infection Disease; Istanbul Faculty of Medicine).
We obtained isolates of S. penumoniae as a part of the routine clinical diagnostic practice, from blood-culture system (Bactec 9050, Becton Dickinsen, Temse, Belgium), from lung aspirate by inoculation of culture media at the patients' bedside, and from cerebrospinal fluid (CSF) using standard microbiological procedures.Citation26
Laboratory analyses
Susceptibility tests to antimicrobial agents were performed by standard disc diffusion method on Muller Hinton agar supplemented with 5% sheep blood. The susceptibility for penicillin was detected with a 1 –ml oxacillin disc. The minimal inhibitory concentration (MIC) of the antibiotics was determined by E test.Citation27 Disc diffusion tests were performed according to the guidelines of the Clinical and Laboratory Standards Institute (formerly known as the NCCLS guidelines).Citation28 An inoculum density equivalent to 0,5 MacFarland Standard was prepared in Muller Hinton broth.Citation27 Serotyping was performed by the Quellung reaction using serotype- specific antisera according to the manufacturer's instructions (Statens Seruminstitut, Copenhagen, Denmark). Vaccine-type strains included serotypes 4, 6B, 9V, 14, 18C, 19F, 23F, 1, 5, 7F, 3, 6A, and 19A. All other serotypes were considered non-vaccine types.
Statistical analysis
Data were analyzed using the SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA). Descriptive statistics were used to summarize the participants' baseline characteristics, including means, standard deviations (SDs), medians, and interquartile ranges for continuous variables and frequency distributions for categorical variables. To compare the differences in frequencies, we used the χ2 or Fisher exact tests. The normality of quantitative variables was tested by Kolmogorov–Smirnov test. In all analyses, 2-tailed p-values ≤ 0.05 were regarded as statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by Pfizer.
References
- World Health Organization. Pneumococcal disease [cited 2015 Feb 20]. n.d. Available from: http://www.who.int/ith/diseases/pneumococcal/en/
- Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis 2010; 14:e197-209; PMID:19700359; http://dx.doi.org/10.1016/j.ijid.2009.05.010
- Pletz MW, Maus U, Krug N, Welte T, Lode H. Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Agents 2008; 32:199-206; PMID:18378430; http://dx.doi.org/10.1016/j.ijantimicag.2008.01.021
- Feikin DR, Klugman KP. Historical changes in pneumococcal serogroup distribution: implications for the era of pneumococcal conjugate vaccines. Clin Infect Dis 2002; 35:547-55; PMID:12173128; http://dx.doi.org/10.1086/341896
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962-73; PMID:21492929; http://dx.doi.org/10.1016/S0140-6736(10)62225-8
- World Health Organization. Pneumococcal conjugate vaccine for childhood immunization – WHO position paper. Weekly epidemiological report. Weekly Epidemiological Record 2007; 82:93-104; PMID:17380597
- Weil-Olivier C, van der Linden M, de Schutter I, Dagan R, Mantovani L. Prevention of pneumococcal diseases in the post-seven valent vaccine era: a European perspective. BMC Infect Dis 2012; 12:207; PMID:22954038; http://dx.doi.org/10.1186/1471-2334-12-207
- Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Muenz LR, O'Brien KL. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: The pneumococcal global serotype project. PLoS Med 2010; 7:e1000348; PMID:20957191; http://dx.doi.org/10.1371/journal.pmed.1000348
- Ceyhan M, Ozsurekci Y, Gürler N, Ozkan S, Sensoy G, Belet N, Hacimustafaoglu M, Celebi S, Keser M, Dinleyici EC, et al. Serotype distribution of Streptococcus pneumoniae causing parapneumonic empyema in Turkey. Clin Vaccine Immunol 2013; 20:972-6; PMID:23637041; http://dx.doi.org/10.1128/CVI.00765-12
- Ceyhan M, Gurler N, Yaman A, Ozturk C, Oksuz L, Ozkan S, Keser M, Salman N, Alhan E, Esel D, et al. Serotypes of Streptococcus pneumoniae isolates from children with invasive pneumococcal disease in Turkey: baseline evaluation of the introduction of the pneumococcal conjugate vaccine nationwide. Clin Vaccine Immunol 2011; 18:1028-30; PMID:21508171; http://dx.doi.org/10.1128/CVI.00526-10
- Feikin DR, Klugman KP, Facklam RR, Zell ER, Schuchat A, Whitney CG. Increased prevalence of pediatric pneumococcal serotypes in elderly adults. Clin Infect Dis 2005; 41:481-7; PMID:16028155; http://dx.doi.org/10.1086/432015
- Imöhl M, Reinert RR, van der Linden M. Regional differences in serotype distribution, pneumococcal vaccine coverage, and antimicrobial resistance of invasive pneumococcal disease among German federal states. Int J Med Microbiol 2010; 300:237-47; PMID:19604721; http://dx.doi.org/10.1016/j.ijmm.2009.05.005
- Jacobs MR, Good CE, Bajaksouzian S, Windau AR. Emergence of Streptococcus pneumoniae serotypes19A, 6C, and 22F and serogroup 15 in Cleveland, Ohio, in relation to introduction of the proteinconjugated pneumococcal vaccine. Clin Infect Dis 2008; 47:1388-95; PMID:18959493; http://dx.doi.org/10.1086/592972
- Regev-Yochay G, Paran Y, Bishara J, Oren I, Chowers M, Tziba Y, Istomin V, Weinberg M, Miron D, Temper V, et al. Early impact of PCV7/PCV13 sequential introduction to the national pediatric immunization plan, on adult invasive pneumococcal disease: A nationwide surveillance study. Vaccine 2015; 33:1135-42; PMID:25613717; http://dx.doi.org/10.1016/j.vaccine.2015.01.030
- Lepoutre A, Varan E, Georges S, Dorleans F, Janoir C, Gutmann L, Levy-Bruhl D, Microbiologists of Epibac; ORP Networks. Impact of the pneumococcal conjugate vaccines on invasive pneumococcal disease in France, 2001-2012. Vaccine 2015; 33:359-66; PMID:25448105; http://dx.doi.org/10.1016/j.vaccine.2014.11.011
- Ceyhan M, Yildirim I, Sheppard CL, George RC. Pneumococcal serotypes causing pediatric meningitis in Turkey: application of a new technology in the investigation of cases negative by conventional culture. Eur J Clin Microbiol Infect Dis 2010; 29:289-93; PMID:20087750; http://dx.doi.org/10.1007/s10096-009-0853-y
- Skoczynska A, Kuch A, Sadowy E, Wasko I, Markowska M, Ronkiewicz P, Matynia B, Bojarska A, Wasiak K, Golebiewska A, et al. Recent trends in epidemiology of invasive pneumococcal disease in Poland. Eur J Clin Microbiol Infect Dis 2015; 34:779-87; PMID:25475124; http://dx.doi.org/10.1007/s10096-014-2283-8
- Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, Thomas AR, Harrison LH, Bennet NM, Farley MM, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med 2006; 354:1455-63; PMID:16598044; http://dx.doi.org/10.1056/NEJMoa051642
- Centers for Disease Control and Prevention. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction-eight states, 1998-2005. JAMA 2008; 299:1253-5; http://dx.doi.org/10.1001/jama.299.11.1253
- Ben-Shimol S, Greenberg D, Given-Lavi N, Schlesinger Y, Somekh E, Aurier S, Miron D, Dagan R. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children. Vaccine 2014; 32:3452-9; PMID:24690148; http://dx.doi.org/10.1016/j.vaccine.2014.03.065
- Dagan R. Serotype replacement in perspective. Vaccine 2009; 27(Suppl 3):C22-4; PMID:19545935; http://dx.doi.org/10.1016/j.vaccine.2009.06.004
- Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherion T, Levine OS, Whitney CG, O'Brien KL, Moore MR; Serotype Replacement Study Group. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med 2013; 10:e1001517; PMID:24086113; http://dx.doi.org/10.1371/journal.pmed.1001517
- Ben-Shimol S, Greenberg D, Given-Lavi N, Elias N, Glikman D, Rubinstein U, Dagan R. Israeli bacteremia and meningitis active surveillance group. Rapid reduction in invasive pneumococcal disease after introduction of PCV7 into the national immunization plan in Israel. Vaccine 2012; 30:6600-7; PMID:22939907; http://dx.doi.org/10.1016/j.vaccine.2012.08.012
- Navarro Torne A, Dras JG, Quinten C, Hruba F, Busana MC, Lopalca LP, Gauci AJ, Pastore-Celentano L. ECDC country experts for pneumococcal disease. European ehanced surveillance of invasive pneumococcal disease in 2010: data from 26 European countries in the post-heptavalent conjugate vaccine era. Vaccine 2014; 32:3644-50; PMID:24795228; http://dx.doi.org/10.1016/j.vaccine.2014.04.066
- Phanhsamart W, Srifeung S, Chatsuwan T, Nunthapisud P, Treerauthaweeraphong V, Rungnobhakhun P, Sricharoenchai S, Chokephaibulkit K. Changing trends in serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae causing invasive diseases in central Thailand, 2009-2012. Hum Vaccin Immunother 2014; 10:1866-73; PMID:25424794; http://dx.doi.org/10.4161/hv.28675
- Ceyhan M, Gürler N, Ozsurekci Y, Keser M, Aycan AE, Gurbuz V, Salman N, Camcioglu Y, Dinleyici EC, Ozkan S, et al. Meningitis caused by Neisseria meningitidis, haemophilus influenzae type B and streptococcus pneumoniae during 2005-2012 in Turkey: A multicenter prospective surveillance study. Hum Vaccin Immunother 2014; 10:2706-12; PMID:25483487; http://dx.doi.org/10.4161/hv.29678
- Yalcin I, Gurler N, Alhan E, Yaman A, Turgut M, Celik U, Akcakaya N, Camcioglu Y, Diren S, Yildirim B. Serotype distribution and antibiotic susceptibility of invasive Streptococcus pneumoniae disease isolates from children in Turkey, 2001-2004. Eur J Pediatr 2006; 165:654-7; PMID:16602003; http://dx.doi.org/10.1007/s00431-006-0128-x
- Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard-Eleventh Edition; 9-10 [cited 2016 Jan 31]. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. Available from: http://www.antimicrobianos.com.ar/ATB/wp-content/uploads/2012/11/01-CLSI-M02-A11-2012.pdf
