Abstract
The objective of this study was to calculate Vaccine Effectiveness (VE) in healthcare workers (HCW) and to compare VE between patients and HCW. A case-control investigation based on the prospective study was conducted between 2004 and 2009 in a teaching hospital. All HCW with influenza-like illness (ILI) from participating units (n = 24) were included, and vaccination status was characterized by interview. A total of 150 HCW presented ILI; 130 (87%) were female, 27 (18%) were positive for influenza, and 42 (28%) were vaccinated. Adjusted VE was 89% (95% CI 39 to 98). Among patients, adjusted VE was 42% (95% CI −39 to 76). The difference of VE (VEhcw - VEpat) was 46.15% (95% CI 2.41 to 144). The VE ratio (VEhcw / VEpat) was 2.09 (95% CI −1.60 to 134.17). Influenza VE differed between HCW and patients when the flu season was taken into account. This finding confirms the major impact of host determinants on influenza VE.
Abbreviations
| VE | = | Vaccine Effectiveness |
| ILI | = | Influenza-Like Illness |
| HCW | = | Healthcare Workers |
| CI | = | Confidence Interval |
| OR | = | Odds Ratio. |
Introduction
Influenza vaccine effectiveness (VE) is associated with determinants related to the vaccine, to circulating virus strains and to the host. VE is optimal when circulating and vaccine strains are similar and when the host provides efficient immunological responses. A meta-analysis determined that VE ranged between 50% to 80% in older children or adultsCitation1 and was 23% against influenza-like illness (ILI) in the elderly.Citation2 A recent randomized trial in adults 65 years or older showed that a high-dose, trivalent-inactivated influenza vaccine induced higher antibody responses and better protection against influenza than a standard-dose vaccine.Citation3 These results indicate that vaccine dose should be adapted for older patients. Age, underlying diseases, immune function, malnutrition, pregnancy, and tobacco consumption are host determinants which alter VE. Citation1-4
Studies among young adults in good health reported VE ranging from 70% to 90%.Citation5 Healthcare workers (HCW), an important target population for influenza vaccination, are usually considered as healthy adults as well. Nevertheless, studies of VE are scarce in this group. Influenza vaccination is highly recommended in this population because they are exposed to flu from patients but are also sources of infection for patients and colleagues.Citation6 A randomized controlled trial in HCW reported 88% VE against serologically-defined influenza A and 89% against influenza B7 over 3 years.
It appears that VE differs according to host characteristics but, to our knowledge, few data are available on concurrent VE estimation between different populations coming from a similar environment and adjusted to influenza season. Such differences are more pronounced when specificity of the outcome is as high as laboratory-confirmed influenza.Citation1 A case-control study of ILI individuals negative for influenza virus, as the controls, provided accurate VE estimates, and it remained a valid design.Citation8 In addition, multiyear trials are encouraged to estimate vaccine efficacy because of high incidence variability between years.Citation1
Surveillance of nosocomial ILI and confirmed influenza was implemented in Edouard Herriot Hospital, (Lyon, France) since 2004.Citation6 The vaccine status of included patients and HCW between 2004 and 2009 was recorded. We previously reported VE in confirmed influenza restricted to patients by influenza season.Citation9 We seized the occurrence of confirmed influenza cases among HCW to conduct additional analyses, including estimation of influenza VE in this population, and compared HCW and patient VE, taking influenza season into account.
Results
HCW
A total of 150 HCW were included during the study period. The characteristics of these subjects, reported in , did not change with time. Eighty-six percent were women, and median age was 38 years (27–58), with no difference by gender. Forty-two (28%) HCW were vaccinated against influenza during the study period with a range of vaccine coverage of 8% for the 2006–2007 season to 42% for the 2004–2005 season.
Table 1. Baseline characteristics of HCW with ILI and patients with or without confirmed influenza, at Edouard Herriot Hospital in Lyon, France, from 2004 to 2009
During the study, 27 HCW had confirmed influenza – 23 women (85.5%) and 4 men (14.8%) – and were considered as cases. Twenty-two cases (81.4%) were infected with influenza A and 5 with influenza B virus; 2 HCW (7.4%) with influenza were vaccinated (2004–2005 flu season). reports the characteristics of cases and controls among HCW. Patient characteristics, published previously,Citation9 are reported as well to facilitate comparison between populations ().
Pooled vaccine coverage was 28% for the 5 seasons: it was 42%, 38%, 8%, 29% and 30% for the 2004–2005, 2005–2006, 2006–2007, 2007–2008 and 2008–2009 seasons, respectively (). Vaccine coverage was 55% in the geriatric unit, compared to 19% in other wards (p < 0.001) (). It was 50% for medical doctors, and 24% for other HCW (p = 0.015) (). During the 2006–07 season, the vaccine coverage was 0% in the medical wards against 11%, 35%, 24% and 22% in 2004-05, 2005-06, 2007-08 and 2008-09 respectively. Moreover, 8% of HCW came from geriatric ward against 37%, 24%, 25% and 27% in 2004–05, 2005–06, 2007–08 and 2008–09 respectively.
Table 2. Characteristics of 150 HCW enrolled in ILI and confirmed influenza surveillance at Edouard Herriot Hospital, Lyon, 2004–2009
Table 3. Influenza vaccine coverage as percentage of HCW by flu season, Edouard Herriot Hospital, Lyon, France, 2004–2009
VE in HCW and comparison with patients
VE among HCW was 72.2% (95% CI −103% to −96%) for the 2004–2005 flu season. It was not calculated for other seasons because no case occurred in vaccinated HCW. Adjusted VE was 88.5% (95% CI 39% to 98%), independently of patient age, gender and flu season (). VE was 91% (95% CI 33% to 99%) in HCW infected by influenza A, and −101% (95% CI −3,300% to −87.3%) in those infected by influenza B.
Figure 1. Comparison of vaccine effectiveness (VE) among HCW and patients in 5 influenza seasons, Edouard Herriot Hospital, Lyon, France, 2004–2009. *Adjusted for age, gender and influenza season.
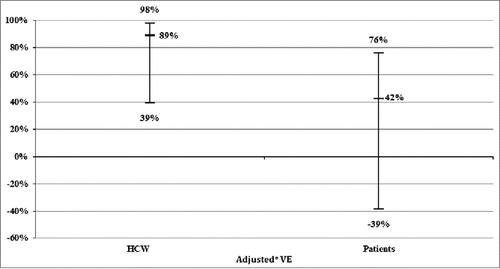
Adjusted VE was 42.3% (ORadjusted = 0.577) in vaccinated patients (p < 0.001), as reported previously.Citation9 The difference of VE (i.e. VEhcw - VEpat) was 46.15% with 95% CI ranging between 2.41 to 144. The VE ratio (i.e., VEhcw / VEpat) was estimated to be 2.09 with 95% CI ranging between −1.60 and +134.17.
Discussion
The objectives of this study were to report flu VE in HCW with a case-test, negative-control, prospective study design and to compare VE among hospitalized patients in similar flu seasons, meaning that both populations were exposed to similar vaccine and virological strains in the community across time.
We found among HCW, a low influenza vaccination rate (8%) during 2006-07. Moreover, only 8% of HCW came from geriatric ward against 24% to 37% for the other seasons. This may explain by the fact that the vaccination rate in geriatric ward was higher compared to other wards. Previous studies have reported that HCW in geriatric ward were more willing to be vaccinated because of their awareness of the vulnerability of elderly patients and willingness to protect them against hospital-acquired influenza.Citation6,10
We observed an adjusted VE of 88.5% among HCW compared to 42% among patients in a previous work.Citation9 The difference was statistically significant in the study population. The results suggest that, under the hypothesis that HCW were considered as a healthy population, being a patient was a strong determinant of decreased of flu VE whatever the flu season, after adjustment for age and gender. The ratio of VE (i.e., VEhcw / VEpat) was estimated to be 2.09 with 95% CI ranging between −1.60 and +134.17. This interval was too wide, which is why our estimates should be interpreted with caution. However, it was clearly asymmetrical, indicating a trend toward a 2-fold increase of VE in HCW versus patients. This large interval was expected. Indeed, in the denominator 1 - ORpat × 100, the 95% CI of adjusted ORpat was broader than that of adjusted ORhcw and contained 1 (95% CI 0.24 to 1.39). The latter point explains why the lower boundary of the 95% CI of the VE ratio was negative.
These results emphasized that host determinants are essential in influenza VE. They could explain various effects of vaccines in different populations. Robust VE estimates are published annually.Citation11,12 However, to our knowledge, such comparisons between the populations investigated across time during the same influenza season have never been reported.
The study design did not provide an opportunity to explore, in detail, patient determinants associated with decreased VE. However, the results indicate that the health status of populations scheduled for vaccination, including age,Citation3 is an important determinant of vaccine impact. Different vaccination policies against the same agent (i.e., influenza virus) might be suggested regarding target populations. Moreover, vaccine composition or factors improving immune responses might be research topics for improved influenza protection. Patients for whom risk factors of vaccine failure cannot be modified (i.e. age) could gain from such new interventions.
This study had some limitations, the confounders for adjustment were similar for HCW and patients, but specific information regarding underlying diseases in the hospitalized population was not available for HCW. While the survey was prospective and standardized, the objective was mostly to describe incidence rates of nosocomial ILI and fluCitation6 and not VE evaluation. However, the results are based on a prospective design less exposed to bias and missing data; the research assistants involved in this study were well trained for years, which might be a positive aspect to control bias regarding data collection. Sample size was not totally appropriate for testing such a hypothesis. Another limitation related the 257 subjects (123 HCW and 134 patients) with ILI not confirmed for influenza. Among them, 34 (13%) have been infected by other virus. Furthermore, it is likely that bacterial infections may have caused ILI. Therefore, we cannot exclude a selection bias nor appraise if our estimates were under- or over-estimated with the inclusion of these subjects. Finally, information regarding type, name and vaccine manufacturer was not collected.
Viral strains were analyzed in the National Reference Center for Influenza for the South of France. Another positive point was the concurrent/simultaneous collection of data on HCW and patients, which gave us an opportunity to make relevant comparisons according to place and time.
In addition, we suggest that these results raised the issue of what vaccine for what population? Do we need a flu vaccine for healthy populations and a different one for individuals with underlying disease? Indeed, for the same agent, VE differed according to the population targeted by the vaccine. Route of administration, composition with or without adjuvant, various epitope and other host characteristics, such as nutritional statusCitation13,14 or specific underlying diseases,Citation15 are interesting topics for discussion but beyond the scope of this article.
In conclusion, with similar exposure to flu virus, VE differed strongly between patients and HCW, suggesting that both vaccine specialists and general practitioners should take population characteristics into account to anticipate flu VE. This type of analyze of VE might be interesting to implement for exploring similar issues for other vaccines.
Materials and Methods
Study design and population
A case-control study, nested in a prospective influenza surveillance program detailed elsewhere,Citation6 was conducted at University of Lyon's Edouard Herriot Hospital in France during the 2004-2005 to 2008–2009 flu seasons. All hospital wards were invited to participate before each season. In 2004–2005, 2005–2006, 2006–2007, 2007–2008 and 2008–2009, 13 (224 beds), 29 (493 beds), 32 (537 beds) and 7 (114 beds) wards respectively from the surgery, medicine (including geriatrics), obstetrics-gynecology, and intensive care specialties agreed to join in. The study period was defined according to alert thresholds of the French national ILICitation16 and influenzaCitation17 surveillance networks. The study was conducted from October 15 to April 15 in 2004–05, 2005–06 and 2006–07, and from January 1 to April 15 in 2007–08 to 2010–11.
Included were adult patients and HCW aged 18 years or older, with ILI at admission or during hospitalization, who agreed to be enrolled. Patients less than 18 years old were excluded. All patients and HCW received written information about the study, and were asked to participate with signed, informed consent. A nasal swab was taken to confirm influenza in every individual included in the study (see below). A detailed description of included patients can be found elsewhere.Citation9 The following will focus on enrolled HCW only.
HCW with ILI were defined as presenting with rectal or axillary temperature ≥37.8°C, in the absence of antipyretics, together with cough or sore throat. Cases were HCW who met these inclusion criteria and were positive for influenza, whereas controls were HCW subjected to the same inclusion criteria but with negative results for influenza virus detection (see below). HCW for whom vaccination status was not documented and/or nasal swabs and diagnosis were not undertaken were excluded.
Data were collected prospectively for each season, and each participating ward was followed actively by research nurses of the infection control team in conjunction with a collaborating HCW in each ward.Citation6 Research nurses contacted every participating ward twice a week to ascertain if ILI occurred, and the collaborating HCW in each ward was also asked to report every probable case to the infection control unit.
The following information was collected for each HCW with ILI: work schedule, underlying diseases, start and stop date of clinical ILI features, treatments against ILI, influenza vaccination status and date of vaccination. Information was obtained by interview and completed with medical records from the occupational health department.
Virological diagnosis
A nasal swab was collected for laboratory virological diagnosis of influenza from each included HCW. The swabs were immediately sent to an in-hospital lab which is the national reference center of influenza for the South France area. The nasal swabs were taken by Virocult kit. Antigens for influenza A and B viruses were identified by immunocapture enzyme-linked immunosorbent assay (ICE). In addition, virus cultures were produced by inoculating the samples on fibroblastic MRC-5, lipid metabolism of monkey kidney cells (LLC-MK2), Madin-Darby canine kidney (MDCK), epithelial HEp-2 and Vero cells. Antigens were detected on MDCK cells by ICE after 5 days of culture. Influenza A and B viruses were also detected by reverse transcription-polymerase chain reaction. A nasal swab was considered positive if at least 1 of these 3 tests was positive for influenza. It was deemed negative if all 3 tests were negative. HCW with negative results were designated as controls in this analysis.
Statistical analysis
Categorical variables were reported as percentages and compared by Fisher's exact test. Continuous variables were analyzed as medians (min-max) and compared by the Mann-Whitney test. VE was estimated as 1 – odds ratio (OR) x 100, with OR being calculated by logistic regression. A protective effect was assumed in case of positive VE with 95% confidence interval (95% CI) that excluded 0. To evaluate VE independently of confounding factors, an adjusted VE and its 95% CI were also calculated. This model was applied by step-wise backward removal with inclusion of age, gender, influenza season and all variables with P < 0.1 values. The adjusted model was built similarly to the adjusted model used for VE among patients.Citation9 Adjustments were made for age, gender and influenza season.
VE ratio and the difference in VE was calculated with its 95% CI by the bootstrap method with Bias corrected and accelerated (BCa) method Citation18,19 and with stratification on season and the status of participants (HCW or patients). The interaction with age and vaccination was tested. The final model excluded the age-vaccination effect interaction. These analyses were conducted by SPSS 17.0 for Windows and Stata software version 13.1 (Stata Corp., College Station, TX, USA).
Disclosure of Potential Conflicts of Interest
Pr. B. Lina has received travel grants from Roche, research grants from Roche and Sanofi Pasteur, and consulting fees from Roche, GlaxoSmithKlein, Biocryst, Merck, and Sanofi Pasteur. Pr. P. Vanhems has received research grants from Sanofi MSD and consulting fees from Biomerieux.
Authors' Contributions
Dr Vanhems had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: PV, BL. Acquisition of data: CR, BL, OR. Statistical analysis: PV, YB, SR, RE, NV, SA. Interpretation of data: PV, YB, SA, TB, OR. Drafting of the manuscript: PV, YB, SA. Critical revision of the manuscript for important intellectual content and approval of the final draft: PV, YS, SR, TB, CR, BL, OR, NV, RE, SA. Obtained funding: PV, NV, BL. Study supervision: PV, BL.
Acknowledgments
We thank the following services and personnel for their close cooperation: Burns Unit – Prof. Fabienne BRAYE; Emergency and Resuscitation – Prof. Dominique ROBERT; Federation of Digestive Specialties – Prof. Jean Alain CHAYVIALLE, Prof. Olivier BOILLOT, Prof. Jean BOULEZ; General and Digestive Surgery – Prof. Etienne TISSOT, Prof. Xavier BARTH, Dr. Olivier MONNEUSE; Geriatrics – Dr. Henriette PAULET-LAFUMA; Haematology – Prof. Mauricette MICHALLET; Internal Medicine – Prof. Jacques NINET; Nephrology – Prof. Maurice LAVILLE, Prof. Jean Pierre FAUVEL, Prof. Denis FOUQUE; Obstetrics-Gynecology – Prof. Pascal GAUCHERAND; Ophthalmology – Prof. Philippe DENIS, Prof. Carole BURILLON; orthopedic Surgery – Prof. Jean Paul CARRET, Prof. Jacques BEJUI-HUGUES; Otolaryngology – Prof. Eric TRUY, Prof. François DISANT; Rheumatology – Prof. Pierre Dominique DELMAS, Prof. Roland CHAPURLAT, Dr. Aurélie FONTANA; Transplantation Medicine – Prof. Jean Louis TOURAINE; Urology – Prof. Xavier MARTIN. Thanks are also due to Ovid DA SILVA, for editing this manuscript.
Funding
The main hospital-based prospective surveillance study of ILI was supported by the “Program Hospitalier de Recherche Clinique (PHRC) Régional” from the French Ministry of Health and Sanofi Pasteur.
References
- Fiore AE, Bridges CB, Katz JM, Cox N. Inactivated influenza vaccines. In: Plotkin S, Orenstein W, Offit P (eds.), Vaccines (6th ed.). Philadelphia, PA: Elsevier; 2012, 257-293.
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 2005; 366:1165-74; PMID:16198765
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, Martin E, Gurunathan S, Nathan R, Greenberg DP, Tornieporth NG, Decker MD, Talbot HK. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635-45
- Cruijff M, Thijs C, Govaert T, Aretz K, Dinant GJ, Knottnerus A. The effect of smoking on influenza, influenza vaccination efficacy and on the antibody response to influenza vaccination. Vaccine 1999; 17:426-32; PMID:10073719
- Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, Rangarajan B, Newton DW, Boulton ML, Monto AS. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med 2006; 355:2513-22; PMID:17167134
- Landelle C, Vanhems P, Saadatian-Elahi M, Voirin N. Influenza vaccination coverage among patients and healthcare workers in a university hospital during the 2006–2007 influenza season. Vaccine 2012; 31:23-6
- Wilde JA, McMillan JA, Serwint J, Butta J, O'Riordan MA, Steinhoff MC. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA 1999; 281:908-13; PMID:10078487
- Orenstein EW, De Serres G, Haber MJ, Shay DK, Bridges CB, Gargiullo P, Orenstein WA. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol 2007; 36:623-31; PMID:17403908
- Amour S, Voirin N, Regis C, Bouscambert-Duchamp M, Comte B, Coppere B, Pires-Cronenberger S, Lina B, Vanhems P. Influenza vaccine effectiveness among adult patients in a University of Lyon hospital (2004–2009). Vaccine 2012; 30:821-4
- Thomas RE, Jefferson TO, Demicheli V, Rivetti D. Influenza vaccination for health-care workers who work with elderly people in institutions: a systematic review. Lancet Infect Dis 2006; 6:273-9; PMID:16631547
- McLean HQ, Thompson MG, Sundaram ME, Kieke BA, Gaglani M, Murthy K, Piedra PA, Zimmerman RK, Nowalk MP, Raviotta JM, et al. Influenza vaccine effectiveness in the United States during 2012-2013: variable protection by age and virus type. J Infect Dis 2015; 211:1529-40; PMID:25406334
- Valenciano M, Kissling E, Reuss A, Jimenez-Jorge S, Horvath JK, Donnell JM, Pitigoi D, Machado A, Pozo F, Team IMMCCS. The European I-MOVE Multicentre 2013–2014 Case-Control Study. Homogeneous moderate influenza vaccine effectiveness against A(H1N1)pdm09 and heterogenous results by country against A(H3N2). Vaccine 2015; 33:2813-22; PMID:25936723
- Hara M, Tanaka K, Hirota Y. Immune response to influenza vaccine in healthy adults and the elderly: association with nutritional status. Vaccine 2005; 23:1457-63; PMID:15670881
- Sagawa M, Kojimahara N, Otsuka N, Kimura M, Yamaguchi N. Immune response to influenza vaccine in the elderly: association with nutritional and physical status. Geriatr Gerontol Int 2011; 11:63-8
- Emborg HD, Krause TG, Hviid A, Simonsen J, Molbak K. Effectiveness of vaccine against pandemic influenza A/H1N1 among people with underlying chronic diseases: cohort study, Denmark, 2009–10. BMJ 2012; 344:d7901.
- Flahault A. Global monitoring of influenza: potential contribution of national networks from a French perspective. Exp Rev Anti Infect Ther 2006; 4:387-93; PMID:16771616
- Hannoun C, Dab W, Cohen JM. A new influenza surveillance system in France: the Ile-de-France “GROG”. 1. Principles and methodology. Eur J Epidemiol 1989; 5:285-93; PMID:2792306; http://dx.doi.org/10.1007/BF00144828
- Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med 2000; 19:1141-64; PMID:10797513; http://dx.doi.org/10.1002/(SICI)1097–0258(20000515)19:9%3c1141::AID-SIM479%3e3.0.CO;2-F
- Efron B. Better bootstrap confidence intervals. J Am Stat Association 1987; 82:171-85; http://dx.doi.org/10.1080/01621459.1987.10478410
