Abstract
Open-label, multicenter, randomized study (NCT00289913) evaluated immunogenicity, safety, and tolerability of Vaqta® (hepatitis A vaccine) administered with PedvaxHIB® (Haemophilus b conjugate vaccine [Meningococcal protein conjugate]) & Infanrix® (diphtheria/tetanus/acellular pertussis vaccine) in healthy, 15-month-old children. Five groups were evaluated: Group 1 received Vaqta®/Infanrix® PedvaxHIB® on Day-1 and Vaqta® at Week-24; Group 2 received Infanrix® PedvaxHIB® on Day-1, Vaqta® at Week-4, and Vaqta® at Week-28; Group 3 received Vaqta®/PedvaxHIB® on Day-1 and Vaqta® Week-24; Group 4 received PedvaxHIB® on Day-1, Vaqta® at Week-4, and Vaqta® at Week-28; and Group 5 (safety only) received Vaqta® on Day-1 and Vaqta® at Week-24. Hepatitis A seropositivity rate (SPR: ≥10 mIU/mL), Hib capsular polyribosylribitol phosphate (PRP) antibody response (>1.0 μg/mL), and geometric mean titers (GMT) to pertussis toxin (PT), pertussis filamentous hemagglutinin antibody (FHA), and pertactin were examined. Non-inferiority statistical criteria required a difference >10% in Hepatitis A SPR, PRP >1.0 μg/mL, and a GMT ratio of >0.67 for pertussis antigens. Injection-site and systemic adverse events (AEs) and daily temperatures were collected. Hepatitis A SPRs were 100% for Groups 1–4, regardless of initial serostatus. Anti-PRP titers were comparable (98.1% - 97.0%) for Groups 1–4. GMT and mean fold-rise were comparable for all 3 pertussis antigen components between concomitant and nonconcomitant groups. Criteria for non-inferiority of immune responses for concomitant vs nonconcomitant administration were met for Hepatitis A, Hib, and pertussis antigens. No statistically significant incidence differences of individual AEs were found between concomitant and nonconcomitant groups. No serious vaccine-related AEs or deaths were reported; no subject discontinued due to an AE. Immune responses to Vaqta®, PedvaxHIB®, and Infanrix® given concomitantly were non-inferior to nonconcomitant responses. Vaqta® administered with PedvaxHIB® & Infanrix® had an acceptable safety profile in 15-month-old children.
Introduction
VAQTA® (hepatitis A vaccine, inactivated [Hepatitis A vaccine]; Merck & Co., Inc..) is licensed for immunization against disease caused by hepatitis A virus (HAV) in persons 12 months of age and older.Citation1 Two (2) doses of the vaccine, administered 6 to 18 months apart, are recommended.Citation2 Clinical trials conducted worldwide have demonstrated that Hepatitis A vaccine is efficacious, immunogenic, and well tolerated.
Infanrix® (diphtheria/tetanus/acellular pertussis [DTaP] vaccine; GlaxoSmithKline) is indicated for immunization against diphtheria, tetanus and pertussis in infants and children 6 weeks to 7 y of age.Citation3 DTaP is administered as a 3-dose series at 2, 4, 6, months of age and 2 booster doses administered at 15 months and 4 to 6 y of age.
PedvaxHIB® (Haemophilus influenzae type b [Hib] conjugate vaccine [Meningococcal protein conjugate]; Merck & Co., Inc..) is a conjugate vaccine licensed for immunization against invasive disease caused by Hib in children 2 to 71 months of age.Citation4 Hib is administered as 2 primary doses administered at 2 to 4 months and 1 booster dose at 12 to 15 months of age.
The Advisory Committee on Immunization Practices (ACIP), the American Academy of Family Physicians (AAFP), and the American Academy of Pediatrics (AAP) recommend that children between the ages of 12 and 23 months receive vaccines with antigens including hepatitis B, diphtheria, tetanus, pertussis, Hib, polio, pneumococcal, measles, mumps, rubella, and varicella.Citation5 Hepatitis A vaccine administered to children 12 to 23 months of age would likely be given concomitantly with one or more of the vaccines recommended by these recommending bodies.
A prior study demonstrated comparable immunogenicity and safety of Hepatitis A vaccine administered concomitantly with diphtheria, tetanus, measles, mumps, and rubella vaccine antigens, as compared to separate administration.Citation6 The current study (NCT00289913) was conducted to demonstrate that Hepatitis A vaccine can be administered concomitantly with DTaP and Hib vaccines without impairing the antibody response to the vaccine components or adversely affecting the safety profile.
Results
Participant accounting and demographics
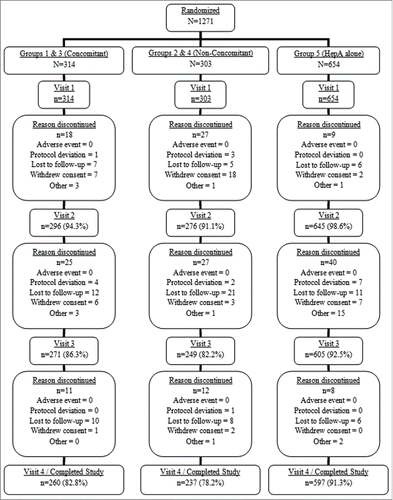
Subjects in all vaccination groups were generally similar across baseline characteristics (). The mean age at enrollment was 15 months for Groups 1 through 4, while a slightly younger mean age (13.2 months) was seen among Group 5 subjects. The racial/ethnic distribution of the overall study group was primarily white (∼65%). Baseline hepatitis A serostatis was generally similar across all enrollment groups (Table S1). A comparable proportion of subjects in the concomitant administration (83%) and nonconcomitant administration (78%) groups completed the study (). The most common reason subjects discontinued was lost to follow-up (86 subjects - 6.8%). No subjects discontinued from the study due to a clinical AE. The percentage of subjects included in the per-protocol immunogenicity analysis for hepatitis A was 60.7% (94/155), 51.7% (78/151), 71.7% (114/159) and 69.1% (105/152) for Groups 1, 2, 3, and 4, respectively.
Table 1. Demographics
Immunogenicity
The observed anti-HAV response rate (percent with titer ≥10 mIU/mL) was 100% for all 4 treatment groups, regardless of baseline hepatitis A serostatus (). For the combined concomitant and nonconcomitant groups, the risk difference (Groups 1 + 3 minus Groups 2 + 4) was 0.0 percentage points with a 95% CI of (−1.8, 2.1) for the per-protocol population (). Non-inferiority of the SPR for hepatitis A antibody at 4 weeks Postdose 2 of Hepatitis A vaccine among subjects who received Hepatitis A vaccine concomitantly or nonconcomitantly with DTaP and Hib vaccines was established based on the pre-specified criteria.
Table 2. Antibody responses
Table 3. Statistical analysis for non-inferiority of antibody responses
The proportion of subjects with anti-polyribosylribitol phosphate (PRP) titers >1.0 μg/mL at Week 4 postvaccination of Hib vaccine was comparable across the 4 treatment groups: 98.1%, 97.0%, 97.3% and 97.2% for Groups 1–4, respectively (). For the combined concomitant and nonconcomitant groups, the risk difference (Groups 1 + 3 minus Groups 2 + 4) was 0.6 percentage points with a 95% CI of (−2.8, 4.1) for the per-protocol population, thus demonstrating non-inferiority ().
The GMTs 4 weeks postvaccination of DTaP vaccine and the mean fold-rise from predose to postdose were comparable for all 3 antigen components (pertussis toxin [PT], filamentous hemagglutinin antibody [FHA], and pertactin) between concomitant and nonconcomitant groups (). For all 3 pertussis components, the lower bound of the 2-sided 95% CI was greater than 0.5 and thus excluded a decrease of 2-fold or more, thereby demonstrating non-inferiority ().
For all antigens, the SPRs and GMTs at 4 weeks Postdose 2 for the full analysis set were comparable to the results observed for the per-protocol population. (Table S2).
Safety
Hepatitis A vaccine was generally well tolerated, regardless of whether it was administered concomitantly with DTaP and Hib vaccines, nonconcomitantly with DTaP and Hib vaccines, or alone. The number and proportion of subjects reporting clinical AEs Days 1 through 14 following any vaccination are summarized in . The nonconcomitant administration and safety cohort groups reported a slightly lower proportion of clinical AEs than the concomitant group, although these differences were not statistically significant.
Table 4. Adverse experience summary
Systemic AEs with the highest incidence rates were pyrexia (Concomitant: 21.5%; Nonconcomitant: 23.4%), irritability (Concomitant: 10.6%; Nonconcomitant: 16.2%), and diarrhea (Concomitant: 9.9%; Nonconcomitant: 9.6%). Similarly, the most frequently reported vaccine-related systemic AEs were pyrexia (Concomitant: 16.2%; Nonconcomitant: 16.2%), irritability (Concomitant: 8.9%; Nonconcomitant: 13.1%), and diarrhea (Concomitant: 2.6%; Nonconcomitant: 2.4%). Serious AEs were reported by 4 subjects in the nonconcomitant vaccination groups and by 2 subjects in the safety cohort group (Table S3). No deaths occurred and no subjects discontinued the study due to an AE. No statistically significant differences were detected between the treatment groups for any of the VRC-prompted injection-site AEs Postdose 1 or Postdose 2 (). No statistical differences between the groups were noted in the incidence of elevated temperatures ().
Table 5. VRC-prompted injection-site adverse experiences (days 1 to 5 following any vaccination)
Table 6. Temperature assessment
Discussion
This study was conducted to demonstrate that Hepatitis A vaccine can be administered concomitantly with Hib and DTaP vaccines to subjects who had completed a primary Hib vaccination series and DTaP vaccination series without impairing the antibody response to any of the vaccine components or adversely affecting the safety profile. A total of 617 subjects were enrolled in Stage I to evaluate the concomitant use of Hepatitis A, DTaP, and Hib vaccines (306 subjects) and Hepatitis A and Hib vaccines (311 subjects). For the assessment of the safety and tolerability of administration of 2 doses of Hepatitis A vaccine alone at least 6 months apart, 654 subjects were enrolled in the safety cohort.
In healthy children 12 to 17 months of age with a negative clinical history of hepatitis A who received Hepatitis A vaccine as a 2-dose regimen at least 6 months apart administered concomitantly or nonconcomitantly with DTaP and Hib vaccines or Hib vaccine, immune responses to Hepatitis A, Hib and DTaP vaccines given concomitantly were non-inferior to each vaccine given nonconcomitantly. Additionally, Hepatitis A vaccine had an acceptable safety profile when given alone or concomitantly with DTaP and Hib vaccines or Hib vaccine, with no vaccine-related serious AEs observed. The incidence of VRC-prompted injection-site AEs at the Dose 1 Hepatitis A vaccine injection-site was higher in the concomitant group than the nonconcomitant group (45.0% vs. 28.5%, respectively), with the majority of all injection-site AEs being reported as mild (<1 % severe in both groups). Of note, there was a low incidence of serious AEs (<1%), and no vaccine-related serious AEs were observed in the study.
The results of this study provide confirmation that Hepatitis A vaccine (VAQTA®, Merck & Co., Inc..) is immunogenic and safe when given in combination with DTaP and Hib vaccines, a result similar to prior concomitant use studies of Hepatitis A vaccine from a different manufacturer (Havrix®, GlaxoSmithKline).Citation7–8 One of the limitations of these studies, including the current one, is an open-label design, which could potentially bias safety reporting. However, a blinded study would require use of placebo injections, which are generally discouraged for younger age groups. Even with the current study design, retention of subjects throughout the study duration was challenging, with higher than anticipated discontinuations. However, none of the discontinuations were due to an adverse event. The immunogenicity results for the more inclusive full analysis set confirmed comparable immunogenicity of concomitantly administered vaccine to the separately administered vaccine group, as shown for the per-protocol population (Table S2).
Conclusion
This study has demonstrated that the immune responses to each vaccine given concomitantly were non-inferior to these vaccines given nonconcomitantly. Concomitant administration of these vaccines had an acceptable safety profile in 12–17 month old children.
Methods
Study design
From April 2006 to June 2010, this open-label, multicenter, randomized, comparative clinical trial (NCT00289913) was conducted across 53 sites in the United States in 2 sequential stages. Stage I (38 sites) evaluated the immunogenicity, safety, and tolerability of the concomitant administration of Hepatitis A vaccine with DTaP and Hib vaccines versus the administration of Hepatitis A vaccine nonconcomitantly with DTaP and Hib vaccines. After enrollment of Stage I was completed, Stage II (Safety Cohort, 25 sites) consisted of subjects who were administered Hepatitis A vaccine with no other vaccines. Within each cohort of Stage I, subjects were stratified prior to randomization based on their prior Hib vaccination history for the primary series (either PedvaxHIB®/COMVAX® or ActHIB®). Subjects were randomly assigned to the investigational/concomitant group or the control/nonconcomitant group within each study site. Subjects who were enrolled into Stage I were not eligible for Stage II.
In Stage I, study subjects must have received at least 2 doses of PedvaxHIB® or Comvax® [Haemophilus b conjugate (Meningococcal Protein conjugate) and Hepatitis B (Recombinant) Vaccine]) or 3 doses of ActHIB® (Haemophilus b Conjugate Vaccine [Tetanus Toxoid Conjugate]; GlaxoSmithKline) prior to 12 months of age. Subjects were randomly assigned to 4 groups (1:1:1:1 ratio) (). The vaccines for subjects in Group 1 were administered at 2 separate study visits: on Day 1, Hepatitis A+DTaP+Hib vaccines were administered concomitantly at separate injection sites; then at Week 24, a second dose of Hepatitis A vaccine was administered. The vaccines for subjects in Group 2 were administered at 3 separate study visits: on Day 1, DTaP+Hib vaccines were administered concomitantly at separate injection sites; then at Week 4, the first dose of Hepatitis A vaccine was administered; and finally at Week 28, a second dose of Hepatitis A vaccine was administered. The vaccines for subjects in Group 3 were administered at 2 separate study visits: on Day 1, Hepatitis A+Hib vaccines were administered concomitantly at separate injection sites; then at Week 24, a second dose of Hepatitis A vaccine was administered. The vaccines for subjects in Group 4 was administered at 3 separate study visits: on Day 1, Hib vaccine was administered; then at Week 4, the first dose of Hepatitis A vaccine was administered; and finally at Week 28, a second dose of Hepatitis A vaccine was administered.
Table 7. Vaccination and blood draw timelines
Stage II subjects (Group 5) received Hepatitis A vaccine on Day 1 and at Week 24 (). The Safety Cohort in Stage II received open-label vaccine.
Study objectives
The study objectives were (1) to demonstrate that Hepatitis A vaccine can be administered concomitantly with either DTaP and Hib vaccines or Hib vaccine without impairing the antibody response to hepatitis A compared with the administration of Hepatitis A vaccine alone; (2) to demonstrate that Hepatitis A vaccine can be administered concomitantly with Hib vaccine without impairing the antibody responses to Haemophilus influenzae type b (Hib) capsular polysaccharide (polyribosylribitol phosphate [PRP]) compared with Hib vaccine administered nonconcomitantly with Hepatitis A vaccine; (3) to demonstrate that Hepatitis A vaccine can be administered concomitantly with DTaP and Hib vaccines without impairing the antibody responses to pertussis toxin (PT), pertussis filamentous hemagglutinin antibody (FHA), and pertactin compared with DTaP and Hib vaccines administered nonconcomitantly with Hepatitis A vaccine; (4) to demonstrate that Hepatitis A vaccine administered concomitantly with either DTaP and Hib vaccines or Hib vaccine is generally well tolerated compared with Hepatitis A vaccine administered alone; and (5) to demonstrate the safety and tolerability of 2 doses of Hepatitis A vaccine in children 12 to 23 months of age in the Safety Cohort.
Study population
For Stage I (Concomitant Use), healthy subjects 15 months of age with a negative clinical history of hepatitis A had no immune impairment or deficiency, neoplastic disease, or depressed immunity including those resulting from corticosteroid use, and no history of allergy or anaphylactoid reaction to any component of the vaccines. For Stage II (Safety Cohort), subjects required similar inclusion criteria to Stage I, however there was no Hib vaccination history requirement and subjects could be 12 to 17 months of age upon receipt of the first study vaccination. The study was conducted in accordance with principles of Good Clinical Practice, approved by the Ethical Review Committee of each participating site, and written informed consent was obtained from each subject prior to study entry.
Vaccine descriptions
Each 0.5 mL dose of Hepatitis A vaccine contains approximately 25 U of hepatitis A virus antigen adsorbed onto approximately 0.225 μg of aluminum, provided as amorphous aluminum hydroxyphosphate sulfate, and 35 μg of sodium borate as a pH stabilizer, in 0.9% sodium chloride. Hepatitis A vaccine was used as supplied; no reconstitution was necessary.
DTaP vaccine is a fully liquid combination of diphtheria and tetanus toxoids and 3 pertussis antigens: inactivated pertussis PT, FHA, and pertactin (69 kiloDalton outer membrane protein) approved for the active immunization against diphtheria, tetanus, and pertussis. Each antigen is individually adsorbed onto aluminum hydroxide. Each 0.5 mL dose is formulated to contain 25 Lf of diphtheria toxoid, 10 Lf of tetanus toxoid, 25 μg of inactivated pertussis PT, 25 μg of FHA, and 8 μg of pertactin. The vaccine was used as supplied.
Hib conjugate vaccine [Meningococcal protein conjugate] is a fully liquid polysaccharide-protein conjugate vaccine approved for active immunization against Hib, the leading cause of meningitis in children. Each 0.5 mL dose of Hib vaccine contains 7.5 μg of Haemophilus b PRP and 125 μg of Neisseria meningitidis OMPC as active ingredients. The vaccine was used as supplied.
Measures
Immunogenicity
During Stage I, ∼5 mL of blood was drawn from all subjects immediately prior to vaccination at the designated time points in each cohort (). Subsequently, blood samples were drawn on Day 1, at Week 4, and 4 weeks Postdose 2 of Hepatitis A vaccine (Week 28 in Groups 1 and 3; Week 32 in Groups 2 and 4). Serum samples from subjects in Group 1 were tested for antibodies to hepatitis A, PRP, diphtheria, tetanus, pertussis PT, FHA, and pertactin at Day 1; antibodies to PRP, diphtheria, tetanus, pertussis PT, FHA, and pertactin at Week 4; and hepatitis A at Week 28. Serum samples from subjects in Group 2 were tested for antibodies to PRP, diphtheria, tetanus, pertussis PT, FHA, and pertactin at Day 1 and Week 4, and hepatitis A at Week 4 and at Week 32. Serum samples from subjects in Group 3 were tested for antibodies to hepatitis A and PRP at Day 1, antibodies to PRP at Week 4, and antibodies to hepatitis A at Week 28. Serum samples from subjects in Group 4 were tested for antibodies to PRP at Day 1 and Week 4, and for antibodies to hepatitis A at Week 4 and Week 32. Antibody responses to each antigen of interest were evaluated by an appropriately sensitive and reliable, validated, quantitative assayCitation6, in qualified laboratory.
Safety
All subjects were followed for safety for 14 d after Visits 1, 2, and 3 (Table S4). This included a 14-day follow-up period after Visit 2 for Groups 1 and 3 when no vaccines were administered so that all comparisons of post-vaccination safety data after Visits 1 and 2 combined included 28 d of follow-up. Temperatures and injection-site adverse events (AEs) were recorded Day 1 through Day 5 on a standardized Vaccination Report Card (VRC) following each study visit. Any injection-site AEs or elevated temperatures that occurred during the remainder of the safety follow-up period (through Day 14) were also recorded on the VRC. Systemic AEs were recorded on the standardized VRC for Day 1 through 14 following each study visit. All vaccine-related serious AEs (SAEs) and deaths that occurred during the study were reported.
Statistical analysis
Immunogenicity
The first primary immunogenicity hypothesis regarding the non-inferiority of the hepatitis A SPR (percent with titer ≥10 mIU/mL) 4 weeks after 2 doses of Hepatitis A vaccine in the concomitant group compared with the hepatitis A SPR 4 weeks after 2 doses of Hepatitis A vaccine administered nonconcomitantly in the control group was based on 2 one-sided non-inferiority tests (each conducted at a 0.025 significance level). The statistical criterion for demonstrating non-inferiority of the SPR to hepatitis A corresponded to the lower bound of the 2-sided 95% CI on the difference in SPRs (concomitant minus nonconcomitant) being >−0.1, i.e. excluding a decrease of 10.0 percentage points or more.
The second primary immunogenicity hypothesis regarding the non-inferiority of the PRP antibody response (proportion of subjects with anti-PRP titers >1.0 μg/mL) 4 weeks after administration of Hepatitis A vaccine concomitantly with either DTaP and Hib vaccines or Hib vaccine compared with the PRP antibody response 4 weeks after the administration of either DTaP and Hib vaccines or Hib vaccine was based on a 1-sided non-inferiority test (conducted at a 0.025 significance level) comparing antibody response rates between groups. The statistical criterion for demonstrating non-inferiority of the antibody response rate corresponded to the lower bound of the 2-sided 95% CI on the antibody response rate difference between the 2 groups (concomitant minus nonconcomitant) being >−0.1, i.e., excluding a decrease of 10.0 percentage points or more.
The third primary immunogenicity hypothesis regarding the non-inferiority of the GMTs to pertussis PT, FHA, and pertactin 4 weeks after the administration of Hepatitis A vaccine concomitantly with DTaP and Hib vaccines (Group 1) compared with the antibody responses 4 weeks after the administration of DTaP and Hib vaccines (Group 2) was based on 3 one-sided non-inferiority tests (each conducted at a 0.025 significance level) comparing the ratio of GMTs for each component. The statistical criterion for demonstrating non-inferiority of the GMTs to pertussis PT, FHA, and pertactin corresponded to the lower bound of the 2-sided 95% CI on the ratio of GMTs (Group 1/Group 2) being >0.5 for each antigen, i.e. excluding a decrease of 2-fold or more.
The power summary for Stage I is provided in Table S5.
Safety
All subjects who were vaccinated and had safety follow-up data were included in the safety analyses and summaries. Groups 1 and 3 combined and Groups 2 and 4 combined were compared across the combined safety follow-up periods after Visits 1 and 2 (for a total of 28 days); Group 5 safety follow-up period included Days 1 to 14 following each dose. Analysis of safety data included risk differences, 95% CIs for the risk difference, and p values for the comparisons of the treatment groups involving daily temperatures and injection-site AEs solicited on the VRC within Days 1 to 5 (which included erythema, swelling, and pain or tenderness). For Stage I, if no SAEs were observed in 310 subjects who received Hepatitis A vaccine concomitantly with either DTaP and Hib vaccines or Hib vaccine, this study provided 97.5% confidence that the true rate was <1.2%.
For Stage II, if no SAEs were observed in 650 subjects who received Hepatitis A vaccine alone, this study provided 97.5% confidence that the true rate was <0.57%.
Disclosure of Potential Conflicts of Interest
FPS was employed by Merck at the time of this research; now employed by Philimmune. Other than employees of Merck & Co., Inc. (as indicated on the title page), all authors have been investigators for the sponsor. Employees may hold stock and/or stock options in the company.
Acknowledgments
The authors would like to thank all the subjects who participated in this study along with the VAQTA® Protocol 068 Study Group: Abuhammour (MI), Aguilar (ID), Andrews (GA), Ashley (AL), Bromberg (NY), Cantor (CA), Caro (OH), Concannon (AZ), Congeni (OH), Cruz (CA), Daly (KY), Davis (AR), Duke (AR), Giordano (FL), Goswami (IL), Grogg (OK), Harvey (AR), Hines (MI), Holmes (AR), Iqbal (OH), Johnston (AL), Kamdar (TX), Lam (UT), Lauer (KS), Leader (MA), Leonardi (SC), Levin (NJ), Marshall (KY), Matthews (AR), McLaughlin (ID), Melamed (CO), Menzel (PA), Mubarak (SC), Mufson (WV), Nassim (KY), Newton (NC), Pamaran (CA), Parker (LA), Purnell (TX), Ramsey (UT), Restrepo (CA), Reynolds (KS), Sanchez-Bal (CA), Schear (OH), Schechtman (FL), Schwartz (VA), Severson (MN), Shapiro (PA), Sheikh (CA), Simon (KY), Soberano (CA), Spiegel (MO), Stacks (MA), Sullivan (CT), Wiseman (VA), Yogev (IL), Yudovich (TX), Zissman (FL).
Authors' Contributions
Ramsey: enrollment of subjects and/or data collection, analysis and interpretation of data, and preparation of manuscript. Stek, Petrecz: analysis and interpretation of data, and preparation of manuscript. Klopfer, Kuter, Schödel, Lee: study concept and design, analysis and interpretation of data, and preparation of manuscript.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Supplemental_material.doc
Download MS Word (124 KB)Funding
This study was funded by Merck & Co., Inc. In conjunction with the external investigators, this study was designed, executed, and analyzed by the sponsor. Although the sponsor formally reviewed a penultimate draft, the opinions expressed are those of the authorship and may not necessarily reflect those of the sponsor. All co-authors approved the final version of the manuscript.
References
- US. Package Circular: VAQTA™ (Hepatitis A Vaccine, Inactivated): February 2003
- Centers for Disease Control and Prevention. Prevention of Hepatitis A through Active or Passive Immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2006;55(RR07):1-23
- US. Package Circular: Infanrix™ (diphtheria/tetanus/acellular pertussis [DTaP] vaccine; GlaxoSmithKline): July 2012
- US. Package Circular: PedvaxHIB™ (H. influenzae type b [Hib] conjugate vaccine [Meningococcal protein conjugate]; Merck & Co., Inc.): December 2010
- Centers for Disease Control and Prevention. Child, Adolescent & “Catch-up” Immunization Schedules. Available at: http://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed: August 22, 2012
- Guerra FA, Gress J, Werzberger A, Reisinger K, Walter E, Lakkis H, Grosso AD, Welebob C, Kuter BJ; Pediatric Study Group for VAQTA. Safety, tolerability and immunogenicity of VAQTA given concomitantly vs. nonconcomitantly with other pediatric vaccines in healthy 12-month-old children. Pediatr Infect Dis J 2006; 25:912-9; PMID:17006287; http://dx.doi.org/10.1097/01.inf.0000238135.01287.b9
- Nolan T, Bernstein H, Blatter MM, Bromberg K, Guerra F, Kennedy W, Pichichero M, Senders SD, Trofa A, Collard A, et al. Immunogenicity and safety of an inactivated hepatitis A vaccine administered concomitantly with diphtheria-tetanus-acellular pertussis and haemophilus influenzae type B vaccines to children less than 2 years of age. Pediatrics 2006; 118:e602-9; PMID:16950952; http://dx.doi.org/10.1542/peds.2005-2755
- Trofa AF, Klein NP, Paul IM, Michaels MG, Goessler M, Chandrasekaran V, Blatter M. Immunogenicity and safety of an inactivated hepatitis A vaccine when coadministered with Diphtheria-tetanus-acellular pertussis and haemophilus influenzae type B vaccines in children 15 months of age. Pediatr Infect Dis J 2011; 30:e164-9; PMID:21494175; http://dx.doi.org/10.1097/INF.0b013e31821b8a7d