ABSTRACT
Objective: To assess the efficacy and safety of infliximab for the treatment of psoriasis in a meta-analysis framework.
Methods: Data were extracted by searching the EMBASE (1974–2014), PubMed(1966–2014) and the Cochrane library2013.4th databases. Only randomized and placebo-controlled studies were selected in this study.
Results: Statistically significant differences in efficacy were found for the infliximab (3 or 5 mg/kg) group compared with the control group which received placebo in the treatment of psoriasis vulgaris [OR 13.55, 95%CI (11.14,16.48)]or[OR85.45, 95%CI (39.13,186.58)]. There were also significant differences in efficacy between the infliximab (5 mg/kg) group and the placebo control group during treatment of psoriasis arthritis (PsA) [OR8.36, 95%CI (5.63, 12.40)]. A controlled trial used infliximab (5 mg/kg) in the treatment of palmoplantar psoriasis. This study showed that the effective rate of the infliximab group was 33.3% (4/12) when compared to the placebo control group, which was 8.3% (1/12).
Conclusion: Infliximab is significantly associated with symptom relief, skin lesion improvement, and an increase in the quality of life of psoriasis patients. The most common drug-induced adverse events were pain, hepatic dysfunction, and infusion reaction.
Psoriasis is a common chronic skin disease. This disease is characterized by excessive and rapid growth of the epidermal layer of the skin.Citation1 T lymphocytes are thought to play a vital role in the pathogenesis of psoriasis. Previous reports have shown that the psoriatic T cells are activated memory skin-homing T cells which induce the characteristic disease features via cytokine production.Citation2 The most recent studies on the mechanisms involved in the establishment of psoriasis have shown that phenotypic and functional changes of T lymphocytes can cause proliferation and abnormal differentiation of epidermal keratinocytes to induce psoriasis.Citation3,4 TNF – α can induce T cells to express VEGF mRNA (Vascular Endothelial Growth Factor).Citation5 VEGF is a secreted protein produced by cells that stimulates vasculogenesis and angiogenesis.Citation6 It is highly associated with psoriatic plaque formation, telangiectasia, and capillary twist of the dermal papilla layer.Citation7 TNF-α is produced early on during the induction of inflammation and is involved in the regulation of immune cells. It was found that primarily CD4+ T cells, along with some CD8+ T cells, secreted TNF-α and TNF-2α when T cells were isolated from psoriatic lesions and analyzed by flow cytometry.Citation8,9 Infliximab is a chimeric human-murine anti-TNF-α monoclonal antibody, which has been approved for the treatment of rheumatoid arthritis (RA), ankylosing spondylitis (AS), Crohn's disease, and intractable retinal uveitis in Behcet's disease. Infliximab inhibits or prevents the effective binding of TNF-α with its receptors. Theoretically, it could be approved for the treatment of various types of psoriasis. Our study will analyze the applications of randomized and controlled clinical trials of infliximab in the treatment of psoriasis by meta-analysis in order to evaluate the efficacy and safety of infliximab for the treatment of psoriasis.
Materials and Methods
Inclusion and exclusion criteria
Inclusion criteria
Type of research
Randomized and quasi-randomized controlled trials;
Participants
Psoriasis Patients
Interventions
The experimental group for infliximab, the control group for placebo or methotrexate
Outcomes
The efficacy of infliximab therapy was determined according to the patients' Psoriasis Area and Severity Index (PASI) score before and after treatment. The observed Total Efficiency, TE= (cure + markedly effective) /total number of cases × 100%. The adverse events were also observed during treatment.
Exclusion criteria
Have serious heart, liver, kidney and blood system diseases Infliximab allergies
Methods
Search strategy
A systematic search of PubMed, Medline, EMBASE and Web of science from 1966 to 2014 using the search terms 'infliximab' and 'psoriasis' were conducted.
An electronic search was conducted in the EMBASE (1974–2014), PubMed(1966–2014) and the Cochrane library 2013.4th databases. The search was limited to the English language. Key words: infliximab, psoriasis, randomized, controlled.
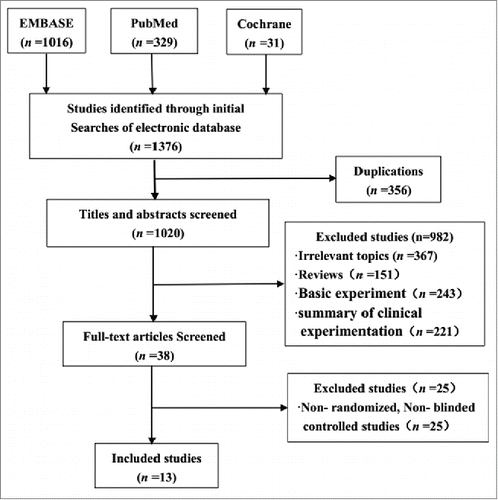
For the process and method of searching publications, see .
Data extraction
Two reviewers independently screened titles and abstracts for potential eligibility. Full text was screened to determine final eligibility. Disagreements were resolved by consultation. Two reviewers re-read the article, analyzed paragraph ambiguity, corrected errors, and reached a consensus, if they made different initial conclusions. Quality analysis was carried out using the method described in JüniCitation10 et al. Four quality evaluation criteria were used for the assessment of randomized controlled trials: 1. Did the trial use the correct randomized method? 2. Is concealment of allocation assessed and is the method correct? 3. Was the blind method used in the trial? 4. Does the trial have withdrawals or dropouts? Does the trial have the intention to treat analysis if follow ups or drop outs occur? If all 4 evaluation criteria are met then there is a low risk of bias. However if one or more of the quality evaluation criteria is only partly met then there is a moderate risk of bias. If one or more of the quality evaluation criteria are totally not met then there is a high risk of bias. In addition, we reviewed the literature by the quality criteria of the Jadad scale (see ). 1–2 points were used to indicate low-quality research, while 3–5 points were assigned to high-quality research.
Table 1. Methodology quality assessment-jadad scale
Allocation concealments of Cochrane systematic review are classified according to the following standards: Grade A: adequate, that considered allocation concealment to be appropriate in the research if centralized randomization, numbered, coded vehicles, or opaque, sealed, and sequentially numbered envelopes were reported; Grade B:unclear, that considered allocation concealment to be unclear in the research if the author did not reported allocation concealments or the reported concealments did not belong in the Grade A category; Grade C: inadequate, that considered concealments were not sufficient or the allocations were not concealed, such as cross-assignment or allocation of record number.
Statistical analysis
The Cochrane collaboration RevMan 5.2 version software was used for meta-analysis. All the outcome measures were categorical data in our systematic review. We therefore took the Odds ratio (OR) and 95%CI for statistical analysis of efficacy. Subgroup analysis was based on heterogeneous factors that may arise. We used the fixed effect mode to summarize each subgroup of similar research for meta-analysis when the research of sub groups have enough similarity (p ≥ 0.1); we used the random effects model to calculate the OR value of total results when there was heterogeneity between subgroups of included research (between subgroups p < 0.05); we used a sensitivity analysis method when heterogeneity was caused by low quality research. Efficacy analysis of the research used the same meta-analysis when there was only one research study in the subgroup.
Results
The general data of included research
The initial search identified 1376 articles. There were 38 articles with controlled clinical trials after exclusion based on repetition, review, basic experiment, and the summary of clinical experimentation. It was found that 13 controlled trial articles met the inclusion standard after a thorough read of the entire articles. Included in the 13 trial articles were 7 articles on psoriasis vulgaris treated by infliximab, 5 articles regarding the treatment of psoriasis arthritis (PsA) by infliximab, and 1 article covering the treatment of palmoplantar psoriasis by infliximab. However, a number of articles were unsuitable for meta-analysi, because the categorical data classified standard is not unified.
The baseline data of the included research
Quality analysis of 13 included research articles (see ).
Table 2. Baseline data of included researches
Results of meta-analysis
The efficacy of infliximab and placebo in the controlled treatment of psoriasis vulgaris
The efficacy of infliximab (5mg/kg) and placebo in the controlled treatment of psoriasis vulgaris
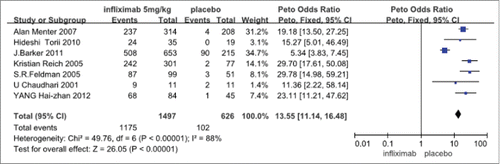
The 7 research studies had both clinical and statistical homogeneity (χ2 = 49.76, p < 0.00001).The meta-analysis results showed that statistically significant differences in efficacy were found for the infliximab (5 mg/kg) group compared with control group which received placebo in treatment of psoriasis vulgaris[OR 13.55, 95%CI (11.14,16.48)]11-17 (see ).
The efficacy of infliximab (3mg/kg) and placebo in controlled treatment of psoriasis vulgaris
Two research papers had clinical homogeneity and statistical homogeneity(χ2 = 1.81, p = 0.18).The results of meta-analysis showed that statistically significant differences in efficacy were found between the infliximab (3 mg/kg) group compared with control group that received placebo in treatment of psoriasis vulgaris. [OR85.45, 95% CI (39.13, 186.58)]Citation11,15 (see ).
Figure 3. The meta-analysis result of infliximab (3 mg/kg) group in treatment of psoriasis vulgaris in efficacy.

For statistical results, see .
Table 3. The meta-analysis result (grouped by the dose of infliximab)
The efficacy of infliximab (5mg/kg) and placebo in the controlled treatment of psoriasis arthritis (PsA)
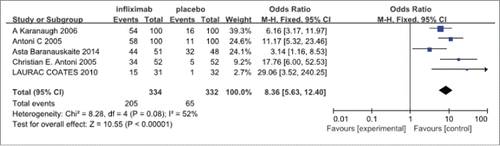
The 5 research studies had clinical homogeneity and statistical homogeneity (χ2=8.28, p=0.08).The results of meta-analysis showed that statistically significant differences in efficacy were found for the infliximab (5 mg/kg) group compared with the control group, which received placebo in treatment of psoriasis arthritis(PsA) [OR8.36, 95% CI (5.63, 12.40)]Citation18-22 (see ).
The efficacy of infliximab (5mg/kg) and placebo in controlled treatment of palmoplantar psoriasis
One controlled trial used infliximab (5 mg/kg) for the treatment of palmoplantar psoriasis. Twenty-four patients were randomized (1:1) to receive either infliximab 5 mg/kg or placebo. At week 14, 33.3% (4 of 12) of patients randomized to receive infliximab reached m-PPPASI 75 compared to 8.3% (1 of 12) on placebo.Citation21
Adverse events
The occurrence of adverse events during infliximab treatment was reported in 13 articles. These adverse events included: pain, headache, nausea, diarrhea, vomiting, infusion reactions, abnormal liver function tests, development of antinuclear antibodies, nasopharyngitis, malaise, leucopenia, pruritus, musculoskeletal pain, allergic reaction, lymphoproliferative disorders, and the development of malignancies. Pain, abnormal liver function tests, and infusion reactions are more common adverse effects associated with infliximab treatment.
For statistical results, see .
Table 4. The incidence rates of adverse events during infliximab treatment
Discussion
Study quality
This meta-analysis investigated the efficacy as well as the adverse effects of infliximab in the treatment of patients with psoriasis. The use of evidence-based medicine to study the published articles provides the best clinical evidence for use of infliximab in the treatment of psoriasis. A number of articles can be retrieved by querying domestic and foreign websites. Unfortunately, few articles meet the standards of meta-analysis and can be combined due to the outcome measures used in the statistical methods employed in this study. Meta-analysis of the infliximab clinical trials found that 13 controlled trial articles met the inclusion standard. The analysis results included 7 articles on the treatment of psoriasis vulgaris by infliximab, 5 articles regarding psoriasis arthritis (PsA) treated by infliximab, and one article on the treatment of palmoplantar psoriasis by infliximab. Eleven out of 13 articles were randomized controlled trials and these were considered as high quality reports. Only 2 low quality articles were non-blinded randomized trials.
Efficacy
Our systematic review confirms that infliximab is effective in the treatment of psoriasis vulgaris, psoriasis arthritis, and palmoplantar psoriasis. The results of our literature meta-analysis show that statistically significant differences in efficacy were found for the infliximab (5 mg/kg) group compared with the control group which received placebo during treatment of psoriasis vulgaris [OR 13.55, 95% CI (11.14, 16.48). Statistically significant differences in efficacy were found for the infliximab (3 mg/kg) group in the treatment of psoriasis vulgaris as compared with the control group which received placebo [OR 85.45, 95% CI (39.13, 186.58)]. There were also significant differences in the efficacy between infliximab (5 mg/kg) treated group and the placebo control group when administered to patients with psoriasis arthritis (PsA) [OR 8.36, 95% CI (5.63, 12.40)]. One research report examined the use of 5mg/kg infliximab in the treatment of palmoplantar psoriasis in a controlled clinical trial. Twenty-four patients were randomized (1:1) to receive infliximab 5 mg/kg or placebo. At week 14, 33.3% (4 of 12) of patients randomized to infliximab reached m-PPPASI 75 as compared to only 8.3% (1 of 12) on placebo.
In adults, infliximab can be administered at a single dose of 3 mg/kg, 5kg/kg or 10mg/kg, with repeated infusions at 2 and 6 weeks. Continued repeated treatment can be applied at 14 and 22 weeks depending on the disease. According to the observations made in our searched articles, the effects of the drug on patient's condition can last up to 1 y.
Safety
The main cause of withdrawal or dropouts in the infliximab studies was due to the occurrence of adverse events. The most common drug-induced adverse events associated with infliximab were pain, hepatic dysfunction and infusion reaction. These symptoms were subsequently followed by secondary infections such as hepatitis and fungal disease.
Implications for future research
First, the studies contained a relatively small sample size. Therefore the results need to be further validated and confirmed. However, the high quality of studies on other populations such as Asian populations improves the credibility of our results. Second, the baseline of the severity of the disease and the prevalence of patients with other comorbidities were diverse, which could also limit the accuracy of this meta-analysis.
Infliximab is a new therapeutic agent for the treatment of severe psoriasis. The expensive price and adverse events associated with infliximab are limiting the widespread application of this drug in the clinic. In the future, we hope that a thorough large scale clinical trial of infliximab on patients with psoriasis will provide valuable data on the long-term efficacy and recurrence.
In conclusion, infliximab treatment is well tolerated and leads to significant associated with symptom relief in psoriasis patients. More clinical trials and economic studies are needed to establish infliximab as a front line treatment for psoriasis.
Disclosure of Potential Conflicts of Interest
The authors do not have any conflicts of interest on this paper.
Author Contributions
L.T.Z and J.W. designed the project, supervised experiments. J.W, and Q.X.Z collected and analyzed the data. J.W. and L.T.Z wrote the manuscript. Other authors performed data analysis and revised the manuscript.
Acknowledgments
We thank Dr. Ying Li (Peking University Health Science Center (PUHSC)) for critical reading of this manuscript and preparing the manuscript.
Funding
Funding was supported by Tianjin Natural Science Foundation, grant no. 12JCYBJC17200.
References
- Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA, Korman NJ, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis:Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008; 58 (5):826-50; PMID:18423260; http://dx.doi.org/10.1016/j.jaad.2008.02.039.
- Prinz JC. Psoriasis vulgaris–a sterile antibacterial skin reaction mediated by cross-reactive T cells? An immunological view of the pathophysiology of psoriasis. Clin Exp Dermatol 2001; 26(4):326-32; PMID:11422184; http://dx.doi.org/10.1046/j.1365-2230.2001.00831.x.
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 2009; 182(9):5836-45; PMID:19380832; http://dx.doi.org/10.4049/jimmunol.0802999.
- Nakajima A, Matsuki T, Komine M, Asahina A, Horai R, Nakae S, Ishigame H, Kakuta S, Saijo S, Iwakura Y. TNF, but not IL-6 and IL-17, is crucial for the development of T cell-independent psoriasis-like dermatitis in Il1rn-/- mice. J Immunol 2010; 185(3):1887-93; PMID:20610641; http://dx.doi.org/10.4049/jimmunol.1001227.
- Sato K, Takaishi M, Tokuoka S, Sano S. Involvement of TNF-α converting enzyme in the development of psoriasis-like lesions in a mouse model. PLoS One 2014; 9(11):e112408; PMID:25384035; http://dx.doi.org/10.1371/journal.pone.0112408.
- Yang L, You S, Kumar V, Zhang C, Cao Y. In vitro the behaviors of metastasis with suppression of VEGF in human bone metastatic LNCaP-derivative C4-2B prostate cancer cell line. J Exp Clin Cancer Res 2012 May 1; 31:40; http://dx.doi.org/10.1186/1756-9966-31-40.
- Flisiak I, Zaniewski P, Rogalska M, Myśliwiec H, Jaroszewicz J, Chodynicka B. Effect of psoriasis activity on VEGF and its soluble receptors concentrations in serum and plaque scales. Cytokine 2010; 52(3):225-9; PMID:20980160; http://dx.doi.org/10.1016/j.cyto.2010.09.012.
- Austin LM, 0zawa M, Kikuchi T, Walters IB, Krueger JG.The majority of epidermal in psoriatic patients. J Invest Dermatol 1999; 113 (5):752-759; PMID:10571730; http://dx.doi.org/10.1046/j.1523-1747.1999.00749.x.
- Duan H, Kohda T. Interleukin-8-positive neutrophils in psoriasis. J Dermatol Sci 2001; 26 (2):119-24; http://dx.doi.org/10.1016/S0923-1811(00)00167-5.
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trial. Br Med J 2001; 323(7 303):42-46; PMID:11440947; http://dx.doi.org/10.1136/bmj.323.7303.42.
- Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, Li S, Dooley LT, Arnold C, Gottlieb AB. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2007; 56(1):31.e1-15; PMID:17097378; http://dx.doi.org/10.1016/j.jaad.2006.07.017.
- Torii H, Nakagawa H. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci 2010; 59(1):40-9; PMID:20547039; http://dx.doi.org/10.1016/j.jdermsci.2010.04.014.
- Barker J, Hoffmann M, Wozel G, Ortonne JP, Zheng H, van Hoogstraten H, Reich K. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol. 2011; 165(5):1109-17; PMID:21910713; http://dx.doi.org/10.1111/j.1365-2133.2011.10615.x.
- Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, Li S, Dooley LT, Griffiths CE; EXPRESS study investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005, Oct 15-21; 366(9494):1367-74; http://dx.doi.org/10.1016/S0140-6736(05)67566-6.
- Feldman SR, Gordon KB, Bala M, Evans R, Li S, Dooley LT, Guzzo C, Patel K, Menter A, Gottlieb AB. Infliximab treatment results in significant improvement in the quality of life of patients with severe psoriasis: a double-blind placebo-controlled trial. Br J Dermatol 2005, May; 152(5):954-60; http://dx.doi.org/10.1111/j.1365-2133.2005.06510.x.
- Chaudhari U, Romano P, Mulcahy LD. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomized trial. Lancet 2001, Jun 9; 357(9271):1842-7; http://dx.doi.org/10.1016/S0140-6736(00)04954-0.
- Yang HZ, Wang K, Jin HZ, Gao TW, Xiao SX, Xu JH, Wang BX, Zhang FR, Li CY, Liu XM, et al. Infliximab monotherapy for Chinese patients with moderate to severe plaque psoriasis: a randomized, double-blind, placebo-controlled multicenter trial. Chin Med J (Engl) 2012; 125(11):1845-51; PMID:22884040.
- Kavanaugh A, Krueger GG, Beutler A, Guzzo C, Zhou B, Dooley LT, Mease PJ, Gladman DD, de Vlam K, Geusens PP, et al. Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis 2007; 66(4):498-505; PMID:17114188; http://dx.doi.org/10.1136/ard.2006.058339.
- Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, Zhou B, Dooley LT, Kavanaugh A; IMPACT 2 Trial Investigators. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005; 64(8):1150-7; PMID:15677701; http://dx.doi.org/10.1136/ard.2004.032268.
- Baranauskaite A, Raffayová H, Kungurov NV, Kubanova A, Venalis A, Helmle L, Srinivasan S, Nasonov E, Vastesaeger N; RESPOND investigators. Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the RESPOND research. Ann Rheum Dis 2012; 71(4):541-8; PMID:21994233; http://dx.doi.org/10.1136/ard.2011.152223.
- Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, Furst DE, Molitor J, Keystone E, Gladman D, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005; 52(4):1227-36; PMID:15818699; http://dx.doi.org/10.1002/art.20967.
- Coates LC, Helliwell PS. Validation of minimal disease activity criteria for psoriatic arthritis using interventional trial data. Arthritis Care Res (Hoboken) 2010; 62(7):965-9; PMID:20589696; http://dx.doi.org/10.1002/acr.20155.
- Bissonnette R, Poulin Y, Guenther L, Lynde CW, Bolduc C, Nigen S. Treatment of palmoplantar psoriasis with infliximab: a randomized, double-blind placebo-controlled research. J Eur Acad Dermatol Venereol 2011; 25(12):1402-8; http://dx.doi.org/10.1111/j.1468-3083.2011.03984.x.