Abstract
A single vaccination of Yellow Fever vaccines is believed to confer life-long protection. In this study, results of vaccinees who received a single dose of 17DD-YF immunization followed over 10 y challenge this premise. YF-neutralizing antibodies, subsets of memory T and B cells as well as cytokine-producing lymphocytes were evaluated in groups of adults before (NVday0) and after (PVday30-45, PVyear1-4, PVyear5-9, PVyear10-11, PVyear12-13) 17DD-YF primary vaccination. YF-neutralizing antibodies decrease significantly from PVyear1-4 to PVyear12-13 as compared to PVday30-45, and the seropositivity rates (PRNT≥2.9Log10mIU/mL) become critical (lower than 90%) beyond PVyear5-9. YF-specific memory phenotypes (effector T-cells and classical B-cells) significantly increase at PVday30-45 as compared to naïve baseline. Moreover, these phenotypes tend to decrease at PVyear10-11 as compared to PVday30-45. Decreasing levels of TNF-α+ and IFN-γ+ produced by CD4+ and CD8+ T-cells along with increasing levels of IL-10+CD4+T-cells were characteristic of anti-YF response over time. Systems biology profiling represented by hierarchic networks revealed that while the naïve baseline is characterized by independent micro-nets, primary vaccinees displayed an imbricate network with essential role of central and effector CD8+ memory T-cell responses. Any putative limitations of this cross-sectional study will certainly be answered by the ongoing longitudinal population-based investigation. Overall, our data support the current Brazilian national immunization policy guidelines that recommend one booster dose 10 y after primary 17DD-YF vaccination.
Introduction
Yellow fever, the original viral hemorrhagic fever, was one of the most feared lethal diseases before the development of an effective vaccine.1 The live attenuated Yellow Fever (17DD-YF and 17D-YF) vaccines are examples of a highly successful prophylactic intervention for controlling disease expansion.
For the 17D-YF vaccine, a single vaccination was proposed as protective and viral neutralizing antibodies could be detected up to 30 y post-vaccination in doses pre-established by World Health Organization.Citation2 A single immunization with YF vaccines induced a strong immune response driven by several cytokines and chemokines such as IFN-γ, TNF-α, IL-2 and MIP-1β.Citation2,3 Viral-neutralizing antibody titers have been used to define immunogenicity and protection; however the establishment of a memory CD8+ T-cell response was shown to be paramount for the duration of immunity.Citation3
In fact, recent studies have shown controversy in regards to the duration of immunity of a single-dose 17DD-YF immunization.Citation3-5 Several studies usually define and characterize the duration of immunity taking into account development of the viral-neutralizing antibodies measured by the Plaque Reduction Neutralization Test (PRNT), disregarding the importance of the cellular responses in the maintenance of specific immunity.Citation3-9 A broad and polyfunctional human memory CD8+ T-cell response was measured in primary vaccinees supporting the finding that a single shot of 17D-YF vaccine is protective against Yellow Fever.Citation2-9 It is well described, however, that continuous antigen exposure is necessary for the generation of a strong memory response, which gives rise to a controversial discussion on whether one single immunization with 17DD-YF vaccine is sufficient for the life-long protection.Citation4,5,10 It is clear that a better understanding of the several immunological correlates of protection among cellular responses upon YF vaccination is needed in order to provide insight into the mechanisms that lead to successful and long-lasting immunization, which are yet to be fully described. In addition, it is not clear when re-exposure to antigen by re-vaccination would be critical for the development of efficient memory subsets in the context of the 17DD-YF vaccination. Previous recent study has reported decreased seropositivity in 17DD-YF vaccinated subjects after PVyear5-9, which may indicate failure in primary immunization, or waning of protective immunity to levels below the protection threshold after 5 to 10 y after vaccination.Citation4
Considering the above, the present study aimed at evaluating several subsets of memory T cells and their timeline in 17DD-YF primary vaccinated adults. The current results demonstrate a descendent trend of several memory subsets over time among 17DD-YF vaccinees, which highly advises for a booster dose 10 y after primary 17DD-YF vaccination.
Results
Humoral immunogenicity kinetics upon 17DD-YF primary vaccination: Decreased PRNT titers after 10 y of immunization
shows the kinetics of neutralizing antibody in 17DD-YF vaccinated adults over 10 y. Six time-points referred as NVday0; PVday30-45; PVyear1-4; PVyear5-9; PVyear10-11 and PVyear12-13 were evaluated. The median PRNT titers decrease progressively along time from the highest at PVday30-45 (4.1Log10mIU/mL) to the lowest at PVyear10-11 and PVyear12-13 (3.3Log10mIU/mL) ().
Figure 1. Flowchart illustrating the study population and experimental design. The study was based on two branches: The humoral (A) and cellular (B) immunology analysis. The eligible population comprises 171 adults. Blood collections (without anticoagulant for humoral analysis and in heparinized tubes for cellular immunology assessment) were performed prior vaccination (NVday0 (n=39) and at different timepoints after primary vaccination: PVday30-45 (n=39); PVyear1-4 (n=36); PVyear5-9 (n=12); PVyear10-11 (n=45) and PVyear12-13 (n=39).
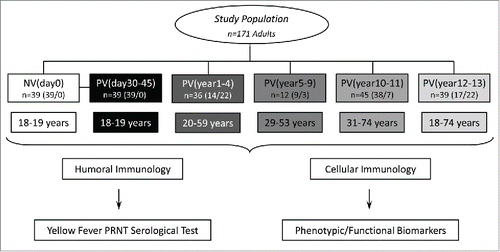
Figure 2. Immunogenicity following 17DD-YF primary vaccination. (A) Anti-YF neutralizing antibody titers were measured by PRNT carried out before NVday0 (n=39) and at different time-points after primary vaccination: PVday30-45 (n = 39); PVyear1-4 (n = 36); PVyear5-9 (n = 12); PVyear10-11 (n = 45) and PVyear12-13 (n = 39). PRNT antibody titers are expressed in log10 mIU/mL. (B) Seropositivity rates were determined by the PRNT value of 2.9 log10 mIU/mL as the cut-off point to segregate seropositive from seronegative samples and data analysis performed by multivariate logistic regression analysis modeled as a function of the time (in months) elapsed since vaccination as categories. (C) Correlation analysis of Anti-YF neutralizing antibody titers after primary vaccination at different time-points using linear regression fit curve. Spearman's correlation r index and p values are displayed in the lower corner of the graph. Significant differences at p < 0.05 as compared to NVday0 time-point are displayed as “a” and differences as compared to PVday30-45 are displayed as “b”.
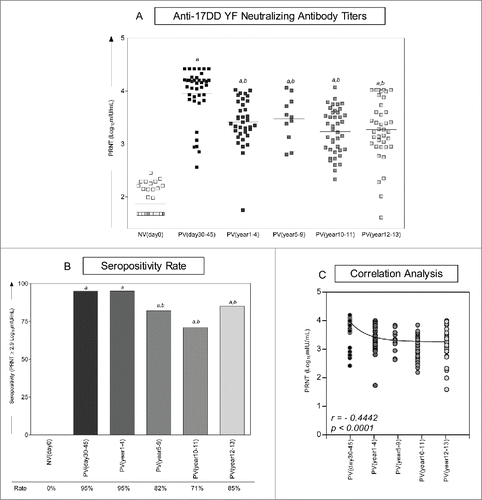
Despite the significant decrease on the median PRNT titers observed at PVyear1-4 (3.5Log10mIU/mL) as compared to the reference time-point (PVday30-45 = 4.1Log10mIU/mL), the seropositivity rate (PRNT ≥ 2.9Log10mIU/mL) significantly decreased from 95% observed at PVday30-45 to 82% and 71% at PVyear5-9 and PVyear10-11, respectively. Surprisingly, the seropositivity rate increases from 71% at PVyear10-11 to 85% at PVyear12-13 with an evident cluster of vaccinees presenting high PRNT titers (≥4.0Log10mIU/mL), which resembles the ones observed at PVday30-45 (). A negative correlation found between PRNT titers and time-points after 17DD-YF primary vaccination reinforce the progressive reduction of PRNT titers along time of vaccination ().
Fragility of effector memory T-cells and classical memory B-cells after 10 y of vaccination
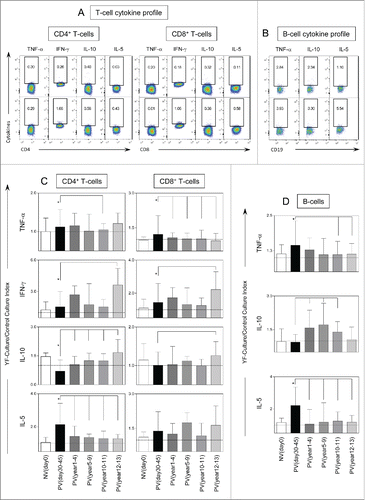
shows the cellular immunology components in 17DD-YF vaccinated adults up to 13 y of immunization. Six groups were referred by their time after primary vaccination and classified as NVday0; PVday30-45; PVyear1-4; PVyear5-9; PVyear10-11 and PVyear12-13. Distinct gating strategies were applied to determine a range of T and B-cell memory phenotypic/functional subsets (, respectively). The phenotypic features including, T-cell subsets (naïve, early effector, central memory and effector memory) and B-cell subsets (naïve, classical memory and nonclassical memory) were expressed as YF-culture/control culture indexes as described in methods. The phenotypic features were evaluated initially in paired-wise fashion. For this approach, samples from PVday30-45 were paired to their respective baseline (NVday0) and then compared accordingly with significant differences highlighted by *. Subsequently, a comparative analysis was performed by comparing each group with the reference group (PVday30-45).
Figure 3. Timeline of memory phenotypic features following 17DD-YF primary vaccination. (A) Flow cytometric dot plots representing the memory T-cell phenotypes and (B) memory B-cell phenotypes. (C) memory T-cell phenotypes such as naïve, early effector, central and effector memory T-cells as well as (D) B-cell phenotypes such as naïve, nonclassical and classical memory are represented by YF-Culture/Control culture index plotted as bar graphs for healthy adults prior vaccination NVday0 (n=39) and at different time-points after primary vaccination: PVday30-45 (n = 39); PVyear1-4 (n = 36); PVyear5-9 (n = 12); PVyear10-11 (n = 45) and PVyear12-13 (n = 39). Significant differences at p < 0.05 as compared to NVday0 time-point are displayed as “*” and differences as compared to PVday30-45 time-point are displayed as connecting lines.
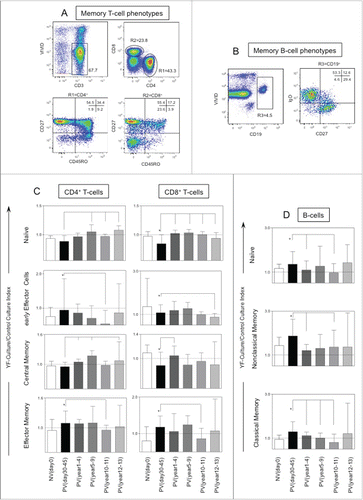
The YF-specific memory phenotypes - effector memory CD4+ and CD8+T-cells along with classical memory B-cells - significantly increased at PVday30-45 as compared to NVday0 baseline (). The early effector memory CD4+ and CD8+ T-cells as well as the classical memory B-cells (, respectively) are decreased in PVyear10-11 indicating the fragility of effective T and B cell recall after 10 y of immunization.
The balance between pro-inflammatory versus regulatory response shifts along time
displays the results of intracytoplasmic cytokine analysis of CD4+, CD8+ and B cells after in vitro YF-specific stimulation. Significant increases in intracytoplasmic TNF-α and IFN-γ in CD4+ and CD8+ T-cells besides higher levels of IL-10+CD4+T-cells were observed along time (). Increased IL-5 production was also observed in CD4+ T-cells and B-cells as early as PVday30-45 (, respectively). A decrease of YF-specific T and B-cell responses with reduced TNF-α+ and IL-5+ CD4+ T-cells and B-cells and increased IL-10+ CD4+ T-cells and B-cells were important changes observed in PVyear10-11. Moreover, decreased levels of TNF-α -producing CD8+ T-cells and increased of IFN-γ+ CD8+T-cells were observed in PVyear12-13 ().
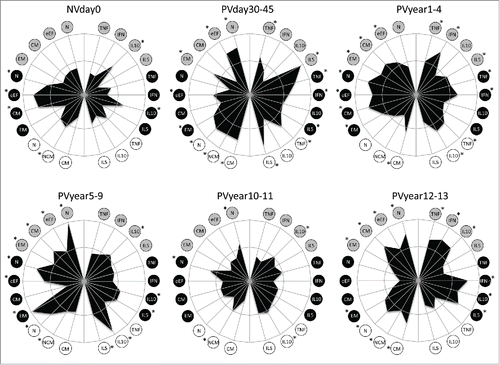
shows the representation of the immunological subsets tested at each time point, plotted in radar graphs. Memory features were plotted on the left half, while the cytokine-producing B and T-cells were plotted at the right half of each graph. The inner circle represents the 50th percentile, which was taken as threshold to define higher (*) and lower production. Before primary vaccination, the radar graph displays a small area, almost contained within the 50th percentile line, except by naïve, early effector and central memory phenotypes accompanied by IL-10+-secreting T-cells. There is a clear expansion of the area composed of both memory and cytokine-producing B and T-cells after vaccination and a discrete area expansion from PVday30-45 to PVyear5-9 (). Pro-inflammatory cytokine-producing T and B-cells exceed the threshold along with effector memory T-cells and non-classical and classical B-cells in PVyear5-9. Singularly, IL-10+ B-cells are over-passing substantially the 50th percentile line, however the same is not observed for other IL-10-producing subsets. At PVyear10-11, the radar area reduces considerably, assuming similar profile observed in NVday0. Sudden expansion was observed later in PVyear12-13, with increase in the pro-inflammatory/regulatory cytokine-producing T-cells along with naïve and central memory T-cells and naïve and classical B-cells ().
Figure 5. Phenotypic and functional memory analysis following 17DD-YF primary vaccination along time. Radar graphs represent the frequency of high producers of memory and functional phenotypic subsets relevant to assessing the immune response before and different times following primary vaccination. Memory phenotypic features were plotted on the left half, while the functional cytokine-producing T and B-cells were plotted at the right half of each radar graph. The inner circle represents the 50th percentile for each parameter, which was taken as threshold to define relevant frequency of subjects with higher levels of a given biomarker (*). Circles in gray, black and white refer to CD4+, CD8+ T-cells and B-cells, respectively. N – naïve, eEF – early effector, CM – Central Memory and EM – Effector Memory.
Systems biology analysis – From micro- to macro-systems
To model the complex interactions between the biomarkers evaluated in the study, networks were assembled by assessing the association between the cytokines profile from distinct cell subset and the memory phenotypes at each time-point (). The biomarker network observed in NVday0 was characterized by micro-nets formed with very few biomarkers. Following, PVday30-45 displayed abundantly rearranged biomarker network with several strong correlations among nodes. Interestingly, some negative correlations start to emerge in PVyear1-4, especially among memory phenotypes. Negative correlations between memory phenotypes and cytokine-producing T and B cells were noticed in a restricted selection of biomarkers participates in PVyear5-9. Several negative correlations emerge and are predominant in PVyear10-11 upsurge of subnets. An abundant and strongly connected macro-net is formed in PVyear12-13, which is composed of several connections between memory phenotypes and pro-inflammatory/regulatory cytokine-secreting cells ().
Figure 6. Systems biology analysis of 17DD-YF primary vaccination over time. Networks were assembled by assessing the association memory and functional phenotypic subsets relevant to assessing the immune response after 17DD-YF primary vaccination at each time-point. Only significant positive and negative correlations (p < 0.05) are displayed. Continuous lines represent direct or positive correlations, whereas dotted lines represent inverse or negative correlations defined by spearman's correlation r index, as described in material and methods. The correlation index (r) defined the strength of association as moderate when 0.36 > r < 0.67 (thin lines) or strong if r > 0.68 (thick lines). Circles in gray, black and white refer to CD4+, CD8+ T-cells and B-cells, respectively. N – naïve, eEF – early effector, CM – Central Memory and EM – Effector Memory.
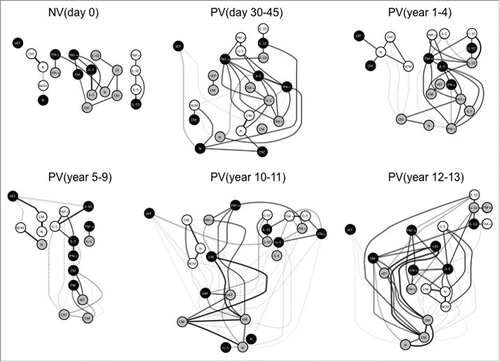
Early effector CD8+ T cells represent a satellite peripheral cell compartment with a negative and weak correlation with classical memory B cells. The satellite early effector CD8+ T cell node displays different associations throughout time with specific and stronger associations with naïve B cells (PVyear1-4), with central memory B cells (PVyear5-9) and with IL-10+CD4+T cells (PVyear10-11) ().
A preserved interaction among IL-10+B/IL-10+CD4+T/IL-10+CD8+T nodes (triple IL-10 node) is seen from PVday30-45 until PVyear12-13, except in PVyear5-9. TNF-α-producing cells seem to be key players within the net, connecting important associations between memory phenotypes and the triple IL-10 node. TNF-α-producing CD8+ T-cells are separated from the major net in PVyear5-9, and this node is not connected to IL-10. These features are characteristic of a weakening net observed in PVyear5-9. IL-5+/ IFN-γ+/ TNF-α+CD8+ T-cells are consistently connected after vaccination in all time-points except in PVyear5-9 and PVyear10-11. A highly connected triangle formed by IL-5+/ IFN-γ+/ TNF-α+CD8+ T-cells are observed in PVyear12-13 ().
In regards to the memory phenotypes and their association with other subsets, stronger correlations between central memory and effector memory CD4+ and CD8+ T-cells are mainly observed in PVyear10-11 and PVyear12-13 ().
Discussion
Large scale YF vaccination has been very effective throughout the world. However, where vaccination campaigns have ceased and vaccination coverage has not been sustained, the disease has recurred, leading to major outbreaks in countries where the disease was considered as eradicated. In face of the endemic transmission of yellow fever virus, WHO recommends that all endemic countries ought to introduce YF vaccine into their routine vaccination programs, considering the efficacy and safety of YF vaccination.Citation11 Reliable but few data have been generated in regards to the duration of immunity associated with 17D-YF vaccination.Citation7-9 WHO advises that single immunization of 17D-YF vaccine is sufficient for large-scale vaccination.Citation11 However, the same findings are yet to be demonstrated for 17DD-YF vaccination, generating controversy on whether a booster dose is required for duration of memory to YF vaccines. It is possible that use of distinct substrains of YF vaccines (17D-204, 17D-213 and 17DD) is not the major factor that contributes to the differences in the duration of immunity following YF vaccination, considering the very few genetic differences among these substrains. However, it is important to mention that we have previously demonstrate that despite an overall similar mixed pro-inflammatory/regulatory cytokine pattern induced by the substarins17D-213 and 17DD induced, the 17DD substrain trigger a slight higher pro-inflammatory profile in the innate immunity compartment.Citation12 This fact may count to putative differences in the duration of the immune response. Moreover, it is important to mention that the systematic review on the need of booster of yellow fever vaccination every 10 years,Citation5 give important details about the studies used as evidence on duration of immunity in healthy individuals. The seven studies addressing the duration of immunity over 10 y were performed in residents in non-endemic countries or non-endemic areas in Brazil. A recent investigation of duration of YF vaccineCitation10 has demonstrated that there is a significant difference in the duration of immune response in populations resident in endemic or non-endemic areas. In fact, this study have demonstrated that an activated immune microenvironment prior to vaccination impedes efficacy of the 17D-YF vaccine in an residents of endemic areas (African cohort) as compared to non-endemic area (Swiss cohort) and suggest that vaccine regimens may need to be boosted in African populations to achieve efficient immunity. Moreover, this study showed that the reduced persistence of memory T and B cell responses in pre-immunized volunteers from Africa could be boosted by a second vaccination. These findings in association with the data presented in our investigation support the proposal of Brazilian authorities to still recommend a booster dose every 10 y in endemic and transition areas. In order to contribute to the understanding of the duration of memory responses after primary vaccination with 17DD-YF vaccine, in this study, neutralizing antibodies along with memory T-cell responses were evaluated in primary vaccinees from 45 d to 13 y after vaccination.
The results indicate that the effector memory CD4+ and CD8+ T-cells, the classical memory B-cells as well as the median PRNT titers decreased at PVyear10-11, indicating a fragility of memory responses after 10 y of 17DD-YF primary vaccination. Recent findings support the results encountered in this studyCitation4 and reveal that an activated immune microenvironmentCitation12,13 prior to vaccination may impede efficacy of the 17D-YF vaccine and suggest that YF vaccination in pre-immunized volunteers should need a booster dose in order to achieve efficient immunity.Citation10 The results in the African cohort are in agreement with the results found here for the Brazilian cohort, indicating that YF-specific T and B cell memory subsets may be altered in the presence of a distinct immune milieu as evidenced by low neutralizing antibody titers after 6 y of vaccination and undetectable levels after longer periods of time.Citation4,10
During infection as well as immunization, a specific repertoire of memory T-cell pool, which is specific for a particular antigen remains relatively constant and it is maintained through homeostatic proliferation driven by pro-inflammatory cytokines, modulated by regulatory cytokines as well as sensor factors produced by antigen-presenting cells upon encountering antigen.Citation14 This genuine memory T-cell collection conveys protection throughout life against that particular pathogen or at least halts severe stages of a second infection. It was recently shown that long-lived epithelial immunity by tissue-resident memory T-cells are fully functional even in the absence of persisting local antigen presentation in the tissue.Citation15
There is strong evidence, however, that repetitive exposure to antigen does improve the generation and are sine qua non for inducing artificial protective immunity and long-lived memory T-cell responses.Citation16-21 Even though highly hypothesized and scrutinized, it still remains unclear why vaccines sometimes fail in inducing this robust and highly specific repertoire of memory cell responses upon a singular exposure to the vaccine antigen. Therefore, re-vaccination with a booster dose for an additional antigen exposure or modification of the antigen delivery system using a homologous/heterologous prime-and-boost regimen may be required for eliciting conspicuous and protective memory T-cell responses for specific cohorts of individuals.Citation19-26 The fragility on memory recall of 17DD-YF -specific central memory and effector memory of CD4+ T and CD8+ T-cell responses, as well as neutralizing antibodies, may indicate that a booster dose is required after primary vaccination with 17DD-YF vaccine for eliciting YF-specific long-lived memory T-cell responses in humans.Citation4
In this matter, generation of a long-lasting protection relies on inducing specific and robust memory and regulatory CD4+ T and CD8+ T-cell responses. The relative proportions of central memory and effector memory in blood differ in the CD4+ and CD8+ T-cell compartments; central memory is predominant in CD4+ whereas effector memory in CD8+.Citation15,22 CD4+ T-cells are essential for the immunological events that lead to Ig class-switch and continuation of antibody assembly and secretion. On the other hand, CD8+ regulatory T-cells are critical for the activation and proliferation of immune cells as well as maintenance of antibody secretion by B cells.Citation24-27 The establishment of optimal memory CD4+ T-cell response is extremely important and depends on cytokine stimuli for maintaining high neutralizing antibody titers.
Previous studies have demonstrated negative correlation of IL-4+CD4+ T-cells, as well as the positive correlation of TNF-α and negative correlation of IL-10+ in CD8+ T-cells with the levels of neutralizing antibodies.Citation10 The present findings demonstrate that a regulatory network of IL-10-producing cell subsets (IL-10+B/IL-10+CD4+T/IL-10+CD8+T) is present in PVday30-45. Even though IL-10 circulating levels rise at even earlier stages, after only a few days post vaccination, this increase in IL-10 levels is observed at minimum doses of 17DD-YF vaccine and in a dose-independent manner, which may indicate that the increase in IL-10 is possibly a bystander outcome of the innate sensing immunity instead of direct effect of vaccination.Citation26 IL-10 kinetics correlates inversely with IFN-γ and TNF-α peak production, as well as the viremia peak post-vaccination, suggesting an important role of this cytokine in modulating the production of the first YF-specific effector function T-cell clones.Citation26
Interestingly, the triple IL-10+B/IL-10+CD4+T/IL-10+CD8+T association is not observed exclusively in PVyear5-9 after primary vaccination, in which very few and weak connections are observed. The results observed in this time-point along with withdrawal of the regulatory system may prelude the beginning of failure in recall responses to YF, in which a regulatory network is no longer necessary for maintaining homeostasis and balanced T-cell proliferation. Novel connections and intertwining of memory T-cell responses and cytokine-producing cells are observed at PVyear10-11 and PVyear12-13 accompanied by the regulatory IL-10 micro network, resembling the first years of primary vaccination.
However, stronger connections between CD4+ and CD8+ central memory and effector memory are now present. In agreement with these findings, a subtle increase in the seropositivity rates was observed at PVyear12-13, which may suggest that undisclosed re-vaccination could have occurred among participants of the study after 10 y of vaccination, which were not reported in the vaccine registration card. Another hypothesis to explain increased seropositivity at PVyear12-13 is the immunosenescence of regulatory responses in elderly vaccinees. Immunosenescence phenomenon is accompanied by weakening of regulatory events, leading to the remodeling of the immune system and its effector and memory functions. This new remodeled immune response may be composed of robust CD8+ T-cell responses as well as specific antibody production.Citation27
It is important to point out that the present investigation may present putative limitations that should be covered in further studies focusing on issues affecting the duration of protective immunity, such as: influence of age at primary vaccination, the impact of distinct number of viral particles/doses, intrinsic of the vaccine batches used at primary vaccination and the number of volunteers on each study groups. Several of these issues will be addressed by a 10 y follow-up longitudinal investigation already setup. This ongoing investigation includes a broader number of volunteers, covering a wide age range at primary vaccination and overcomes the matter of viral particles/doses by using a single vaccine batch. Then, the matter regarding the number of viral particles/dose will be properly covered, supporting a more confident data interpretation of the duration of immune response following primary vaccination. Moreover, the inclusion of a broad age-stratified study population will cover the matter regarding the influence of decade of age at primary vaccination on the duration of protective immunity. Any putative limitations of the cross-sectional experimental design of the present investigation (age and number of viral particles/dose at primary vaccination), will be properly addressed in the ongoing longitudinal population-based study.
In conclusion, altogether our findings confirm previous serologic data, which suggest that a booster dose of 17DD-YF vaccine should be given to Brazilian subjects in order to prevent a decline of seropositivity after primary vaccination. In these cases, one booster dose seems to be necessary to ensure longer protection, possibly sooner than currently recommended. Therefore, the present study supports the current Brazilian national immunization policy guidelines that recommend one booster dose 10 y after primary 17DD-YF vaccination in adults.
Patients and Methods
Study population
This study had formal approval from the Ethics Committee for studies with human subjects (CPqRR/FIOCRUZ #24/2010) and was conducted by the Collaborative Group for Studies of Yellow Fever Vaccine. The protocol included the analysis of “humoral immunology” (neutralizing antibodies) and “cellular immunology” (phenotypic and functional memory-related biomarkers) in all subjects.
The study population consisted of 171 healthy adults (129 males and 49 females, age ranging from 18 to 74 y old) from army military units (RJ, Brazil) and health units from Alfenas (MG, Brazil).Citation4 These geographic areas have been selected due to their low exposure to natural infection (areas with no epidemiological risks for the occurrence of sylvatic transmission of yellow fever, and with no cases of epizootic reports in non-human primates). It was important to minimize the chances of natural infection or second viral exposure that could lead to a natural boosting, which may interfere in the assessment of memory immunity elicited by the YF-vaccine.
The experimental design was structured first as a paired longitudinal arm to identify early correlates of protection at 30 to 45 d after primary vaccination (PVday30-45, ▪ = 39) as compared to baseline observed in Non-Vaccinated conscripts (NVday0, □ = 39). The cross-sectional arm was structured in 4 groups, categorized according to the time after 17DD-YF primary vaccination (PVyear1-4, ▪ = 36; PVyear5-9, ▪ = 12; PVyear10-11, ▪ = 45; PVyear12-13, ▪ = 39) (Fig. 1).
Volunteers with seropositive results at baseline (PRNT>2.9Log10 mIU/mL) were not included in this study. Primary vaccination was confirmed by consulting immunization cards or employee's records. All participants have signed a written consent form. Two blood samples were collected from each volunteer and included 5 mL of whole blood without anticoagulant for humoral immunology assay and 20 mL of heparinized whole blood for cellular immunology analyses.
Plaque Reduction Neutralization Test
Neutralizing antibody titer against Yellow Fever virus was defined by the Plaque Reduction Neutralization Test (PRNT) performed at Virologic Technology Laboratory of Bio-Manguinhos (LATEV, FIOCRUZ). This test was conducted as previously described.Citation23,26 After blood collection, sera was obtained by centrifugation (400xg, 10min, 18°C) of samples obtained from each participant. All sera samples were previously inactivated and submitted to serial two-fold dilutions, starting at 1:5, in 96-well tissue culture plates. A positive serum control for YF neutralizing antibody (internal standard), properly calibrated by the First International Reference Preparation (NIBSC code: YF), was included in each run of the PRNT. For neutralization step, each and every well received a yellow fever viral suspension (strain 17D 213/77, lot UEXVFB01, Dec 2011) at concentration about 30 plaque forming units per well. After neutralization time, a Vero cell suspension was added to the test and the plates were incubated to occur the adsorption of the virus not neutralized and formation of the cell monolayer. After 6 d with semi-solid medium it was possible to count formed plaques. PRNT titer was defined as the reciprocal of the last serum dilution which reduced the plaque numbers in 50% relative to the virus control (included in each run test). Linear regression was used to determine neutralizing antibody titers, by interpolation of the dilutions corresponding to the plaque numbers immediately above and below the 50% endpoint value of the test. Titers expressed in mIU/mL were calculated relative to the antibody content in the International Reference Serum (143 IU/mL), which was used to determine the nominal value of the internal standard (1,115 mIU/mL). Therefore, it was possible to transform neutralizing antibody titer represented by dilution into mIU/mL. In this study, results are represented in Log10 mIU/mL. In order to segregate seropositive from seronegative samples, a cut-off point of 2.9Log10 mIU/mL was applied as described previously.Citation23
In vitro long-term lymphocytes culture
Peripheral blood mononuclear cell (PBMC) were isolated from 14mL of heparinized blood by density gradient centrifugation on Histopaque-1077 (Sigma Aldrich, #H8889) as previously described.Citation13 Cell culture experiments were performed using 48-well microplates for 4h at 37°C in a 5% CO2 humidified atmosphere. For 17DD vaccine antigen stimulation, 1.0 × 106 PBMC were added per well, in a final volume of 800 μl of complete RPMI-1640 (Gibco, # 23400-021) containing 5% heat-inactivated normal human AB serum, antibiotic/antimycotic solution (100U/mL penicillin, 100 mg/mL streptomycin, 0.25 mg/mL amphotericin-B; Sigma, # P3539), and 2 mM L-glutamine (Winlab, # G-6392). The final concentrations of stimuli established as optimal under the cell culture conditions employed were 250 LD50 per well of live attenuated 17DD-YF vaccine (lot# 103VFC015Z – Bio-Manguinhos – FIOCRUZ). Cells were incubated for 144 hours in the presence of 17DD vaccine antigen or medium (control culture). After stimulation, the cell suspension was stained for memory markers and intracytoplasmic cytokines by flow cytometry.
Memory lymphocyte phenotypic analysis
Following in vitro stimulation, cell suspension was washed in wash buffer (phosphate buffered saline [PBS], pH 7.2, supplemented with 0.5% bovine serum albumin and 0.1% sodium azide, all from Sigma Chemical Company) and stained with Live/Dead Dye (Life Technologies, #L34955) for 15 minutes at room temperature, in the dark. After that, the cell suspension was incubated with a cocktail of several monoclonal antibodies for surface and memory molecules. The cocktail was comprised of: 1) Memory T-cells: FITC anti-CD4 (RPA-T4), PerCP-Cy5.5 anti-CD8 (SK1), PE anti-CD27 (M-T271) and PE-Cy7 anti-CD45RO (UCHL1) and 2) Memory B-cells: FITC anti-IgD (IA6-2), PE anti-CD27 (M-T271), PerCP anti-CD19 (HIB19) and APC-Cy7 anti-CD3 (SK7). All antibodies were purchased from BD PharMingen. The cells were incubated for 30 minutes at room temperature, in the dark. After the incubation, the cell suspension was washed with FACS buffer, resuspended in FACS fixing solution (10 g/L paraformaldehyde, 10.2 g/L sodium cacodylate and 6.63 g/L sodium chloride, pH 7.2) and stored at 4°C in the dark until acquisition on BD LSR Fortessa (BD Biosciences).
Intracytoplasmic cytokine analysis
The analysis of intracytoplasmic cytokines in lymphocytes subsets was performed as described previously.Citation28,29 After YF-specific stimulation, 10g/mL of Brefeldin A-BFA (Sigma, # P7651) was added and samples were re-incubated for an additional 4h at 37°C in a 5% CO2 humidified atmosphere. Following incubation, the cultures were treated with 2mM ethylenediaminetetraacetic acid – EDTA, (Sigma, # ED2SS) and maintained at room temperature for 15 min. Following EDTA treatment, the wells were washed 3 times with 500 μL of FACS buffer by centrifugation at 600xg for 10min at room temperature. After additional centrifugation, the supernatant was discarded and the cell pellet was suspended in 500 μL of FACS buffer. Briefly, cell suspension was stained by Live/Dead Dye (Life Technologies, #L34955) for 15 minutes at room temperature, in the dark and then stained with anti-human monoclonal antibodies: Qdot605 anti-CD3 (UCHT1/Invitrogen, #Q10054), APCe-Fluor780 anti-CD4 (GK1.5/eBioscience, #47-0049-42), PerCP anti-CD8 (SK1/BD Biosciences, #347314) and AlexaFluor700 anti-CD19 (HIB19/eBioscience, #56-0199-42). Following incubation at room temperature for 30 min in the dark, stained samples were washed, centrifuged at 600xg for 10min at room temperature, and the supernatant discarded. The cell pellet was resuspended in 2mL of FACS permeabilizing solution (FACS buffer supplemented with 0.5% of saponin) and incubated for 10 min at room temperature in the dark. Following incubation, the samples were washed as described previously and then stained with Alexa Fluor 488 anti-IFN-γ (clone B27, #557718), PE IL-5 (clone JES1-39D10, #559332), APC IL-10 (clone JES3-19F1, #554707) and PE-Cy7 TNF-α (clone MAb11) (BD-PharMingen, #557647). After incubation for 30 min at room temperature in the dark, the cell suspension was washed with FACS permeabilizing solution first and then with FACS buffer, fixed with FACS fixing solution and stored at 4°C in the dark until acquisition on a BD LSR Fortessa (BD biosciences).
Flow cytometric acquisition and analysis
Flow cytometry acquisition was performed in a BD LSR Fortessa flow cytometer (Becton Dickinson), using the FACS DIVA software (BD biosciences). After acquiring 100,000 events/tube gated for lymphocytes, distinct gating strategies were used to analyze the different memory T and B-cells () as well as cytokine-expressing lymphocyte subsets () using FlowJo version 9.3.2 (TreeStar). All samples were first gated on singlets, then on live CD3+ cells, before excluding any potential aggregates, as well as disturbances due to variations in the flow rate (time gate), followed by gating on lymphocytes. Subsequently, CD4+ and CD8+ T-cells were identified for memory analysis. T-cell subsets were defined by expression of CD45RO and CD27. Boolean gates were created from CD45RO vs. CD27 gates and the subpopulations of memory T cells defined as: naïve T cells- CD4+or CD8+ CD27+ CD45RO−; early effector - CD4+or CD8+ CD27− CD45RO−; central memory - CD4+or CD8+ CD27+ CD45RO+; effector memory - CD4+or CD8+ CD27− CD45RO+; B-cell subsets (CD19+) were defined by expression of CD27 and IgD. Boolean gates were created from CD27 vs. IgD gates and the subpopulations of memory B cells defined as: naïve B cells - CD19+ CD27− IgD+; classical memory - CD19+ CD27+ IgD−; nonclassical memory - CD19+ CD27+ IgD+; cytokine-producing cells were defined by expression of CD4, CD8 or CD19 along with TNF-α, IFN-γ, IL-10 and IL-5. Boolean gates were created from CD4; CD8 or CD19 vs. cytokine gates (TNF-α, IFN-γ, IL-10 and IL-5) to define the frequency of cytokine+ cells.
Statistical analysis
In the multivariate analysis, the humoral immune response (indicated by Log10 of titers in the multiple regression model and seropositivity in the logistic regression model) was modeled as a function of the time (in months) elapsed since vaccination as categories, including: NVday0; PVday30-45; PVyear1-4; PVyear5-9; PVyear10-11 and PVyear12-13. The statistical analysis of humoral immune response was performed using the software SPSS® (SPSS Inc., Chicago, IL).
In the analysis of cellular immune response, the memory lymphocyte phenotyping and intracytoplasmic cytokine of peripheral blood leukocytes initially yielded the percentage of memory phenotype and cytokine-positive cells. The memory phenotype and cytokine indexes were then calculated as the ratio between the percentages observed in stimulated culture over the percentage detected in the control culture. Statistical analyses between indexes of NVday0 and PVday30-45 were performed by Mann-Whitney test (unpaired non-parametric) and represented by “*.” Comparisons between indexes of PVday30-45 and other groups (PVyear1-4; PVyear5-9; PVyear10-11 and PVyear12-13) were performed using Kruskal–Wallis followed by a Dunn´s post-test. The differences were demonstrated by connecting lines. Statistical significance was considered if p < 0.05, which was estimated by GraphPad Prism, version 5.0 (San Diego, CA).
Systems biology analysis
In order to further characterize the systemic phenotypic and molecular profiles of the immunological response induced by 17DD-YF vaccine over time, radar charts were plotted using the frequency of high producers following primary vaccination. To calculate the frequency of high producers, a global median of the YF-Culture/Control Culture ratio was estimated and used as a threshold to define high and low producers. The frequency of subjects with biomarkers levels higher than the global median cut-off was plotted in the radar graphs and relevant frequency (represented by *) were considered when the percentage of subjects with higher levels of a given biomarker exceeded the 50th percentile.
To assemble networks, spearman rank correlation test was performed to assess the positive and negative association between the memory phenotypes and intracytoplasmic cytokine production for all groups. These associations were mapped as networks, in which each node represents a different biomarker. The nodes were connected according to their statistical correlation given by Spearman's test. The positive and negative correlations were significant when the p < 0.05. The correlation index (r) were applied to define the association strength as negative (r < 0), moderate (0.36 > r < 0.67) and strong (r > 0.68). Networks were displayed in a hierarchical layout plotted using the Cytoscape software (Version 3.1.0).
Disclosure
Eight authors (VRvD, MS, MSF, AMYY, AH, RMM, RHGF, MLSM) are employees at the 17DD-YF vaccine manufacturer (Bio-Manguinhos, Fundação Oswaldo Cruz), and 6 authors work in other units of Fundação Oswaldo Cruz (LRVA, CTF, LABC, IRC, ATC and OAMF). Bias from competing interest was prevented by: (1) collaboration of one general clinical Physician (HRP) with experience in infectious disease from Brazilian Army; (2) one general clinical Nurse (JACL) with experience in vaccine epidemiological vigilance from State Health Department; (3) 2 Immunologists (LCCM and MR) from Brazilian Research Academy and United States Department of Health; and (5) 4 independent professionals working as Undergraduate student (RAS), PhD student (CCP) or Post-Doc Researchers (ACCA, JGCR and FMFC) in the field of infectious diseases. The FIOCRUZ extramural coworkers contributed with critical overview of the study design, volunteers' immunization and medical care, blood sample collection, blind sample handling and processing, data collection, statistical analysis and data interpretation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank the program for technological development in tools for health-PDTIS-FIOCRUZ for the use of its facilities.
Funding
The authors thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Bio-Manguinhos/FIOCRUZ; PROEP/CPqRR/FIOCRUZ; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Programa Nacional de Imunizações (PNI) – Ministério da Saúde Brazil, FIOTEC, PDTIS, CAPES and Secretaria de Vigilância em Saúde (SVS) for financial support. ACCA and JGCdR received financial support from CNPq MCTI/CNPQ/Universal 14/2014 (ACCA - Grant # 444417/2014-1 and JGCdR - Grant # 458134/2014-7). OAMF and ATC thank the CNPq for the fellowships (PQ).
References
- Monath TP. Yellow fever: an update. Lancet Infect Dis 2001; 1:11-20; PMID:11871403; http://dx.doi.org/10.1016/S1473-3099(01)00016-0
- Poland JD, Calisher CH, Monath TP, Downs WG, Murphy K. Persistence of neutralizing antibody 30-35 years after immunization with 17D yellow fever vaccine. Bull World Health Organ 1981; 59: 895-900; PMID:6978196
- Akondy RS, Monson ND, Miller JD, Edupuganti S, Teuwen D, Wu H, Quyyumi F, Garg S, Altman JD, Del Rio C, et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol 2009; 183:7919-30; PMID:19933869; http://dx.doi.org/10.4049/jimmunol.0803903
- Collaborative group for studies on yellow fever vaccines. Duration of post-vaccination immunity against yellow fever in adults. Vaccine 2014; 32:4977-84; PMID:25090646; http://dx.doi.org/10.1016/j.vaccine.2014.07.021
- Gotuzzo E, Yactayo S, Córdova E. Efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am J Trop Med Hyg 2013; 89:434-44; PMID:24006295; http://dx.doi.org/10.4269/ajtmh.13-0264
- Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR, Castro E, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med 2008; 205:3119-31; PMID:19047440; http://dx.doi.org/10.1084/jem.20082292
- Bassi MR, Kongsgaard M, Steffensen MA, Fenger C, Rasmussen M, Skjødt K, Finsen B, Stryhn A, Buus S, Christensen JP, Thomsen AR. CD8+ T cells complement antibodies in protecting against yellow fever virus. J Immunol 2015; 194: 1141-53; PMID:25539816; http://dx.doi.org/10.4049/jimmunol.1402605
- Querec TD, Rama SA, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol 2008; 10:116-25; PMID:19029902; http://dx.doi.org/10.1038/ni.1688
- Monath TP, Nichols R, Archambault WT, Moore L, Marchesani R, Tian J, Shope RE, Thomas N, Schrader R, Furby D, et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg 2002; 66: 533-41; PMID:12201587
- Muyanja E, Ssemaganda A, Ngauv P, Cubas R, Perrin H, Srinivasan D, Canderan G, Lawson B, Kopycinski J, Graham AS, et al. Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. J Clin Invest 2014; 124:3147-58
- (WHO) 2008. WHO: Expert Committee on Biological Standardization: Requirements for yellow fever vaccine. In Bolletin Requirements for Biological Substances. Geneve: WHO.
- Campi-Azevedo AC, de Araújo-Porto LP, Luiza-Silva M, Batista MA, Martins MA, Sathler-Avelar R, da Silveira-Lemos D, Camacho LA, de Menezes M R, de Lourdes de S. et al. 17DD and 17D-213/77 yellow fever substrains trigger a balanced cytokine profile in primary vaccinated children. PLoS One 2012; 7(12):e49828; PMID:23251351; http://dx.doi.org/10.1371/journal.pone.0049828
- Martins MA, Silva ML, Marciano AP, Peruhype-Magalhães V, Eloi-Santos SM, Ribeiro JG, Correa-Oliveira R, Homma A, Kroon EG, Teixeira-Carvalho A, et al. Activation/modulation of adaptive immunity emerges simultaneously after 17DD yellow fever first-time vaccination: is this the key to prevent severe adverse reactions following immunization? Clin Exp Immunol 2007; 148:90-100; PMID:17309541; http://dx.doi.org/10.1111/j.1365-2249.2006.03317.x
- Ravindran R, Khan N, Nakaya HI, Li S, Loebbermann J, Maddur MS, Park Y, Jones DP, Chappert P, Davoust J, et al. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science 2014; 343:313-7; PMID:24310610; http://dx.doi.org/10.1126/science.1246829
- Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA 2012; 109:7037-42; PMID:22509047; http://dx.doi.org/10.1073/pnas.1202288109
- Tan WG, Jin HT, West EE, Penaloza-MacMaster P, Wieland A, Zilliox MJ, McElrath MJ, Barouch DH, Ahmed R. Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J Virol 2013; 87:1359-72; PMID:23175355; http://dx.doi.org/10.1128/JVI.02055-12
- Virnik K, Hockenbury M, Ni Y, Beren J, Pavlakis GN, Felber BK, Berkower I. Live attenuated rubella vectors expressing SIV and HIV vaccine antigens replicate and elicit durable immune responses in rhesus macaques. Retrovirology 2013; 10-99; PMID:23369348
- Hatch GJ, Graham VA, Bewley KR, Tree JA, Dennis M, Taylor I, Funnell SG, Bate SR, Steeds K, Tipton T, Bean T, Hudson L, Atkinson DJ, McLuckie G, Charlwood M, Roberts AD, Vipond J. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J Virol 2013; 87:7805-15; PMID:23658452; http://dx.doi.org/10.1128/JVI.03481-12
- Ledgerwood JE, Zephir K, Hu Z, Wei CJ, Chang L, Enama ME, Hendel CS, Sitar S, Bailer RT, Koup RA, Mascola JR, Nabel GJ, Graham BS. Prime-boost interval matters: a randomized phase 1 study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect. J Infect Dis 2013; 208:418-22; PMID:23633407; http://dx.doi.org/10.1093/infdis/jit180
- Khurana S, Wu J, Dimitrova M, King LR, Manischewitz J, Graham BS, Ledgerwood JE, Golding H. DNA priming prior to inactivated influenza A(H5N1) vaccination expands the antibody epitope repertoire and increases affinity maturation in a boost-interval-dependent manner in adults. J Infect Dis 2013; 208:413-7; PMID:23633404; http://dx.doi.org/10.1093/infdis/jit178
- Odunsi K, Matsuzaki J, Karbach J, Neumann A, Mhawech-Fauceglia P, Miller A, Beck A, Morrison CD, Ritter G, Godoy H, et al. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci USA 2012; 109:5797-802; PMID:22454499; http://dx.doi.org/10.1073/pnas.1117208109
- Campbell JJ, Murphy KE, Kunkel EJ, Brightling CE, Soler D, Shen Z, Boisvert J, Greenberg HB, Vierra MA, Goodman SB, et al. CCR7 expression and memory T cell diversity in humans. J. Immunol 2001; 166:877-84; PMID:11145663; http://dx.doi.org/10.4049/jimmunol.166.2.877
- Simões M, Camacho LAB, Yamamura AMY, Miranda EH, Cajaraville ACRA, Freire MS. Evaluation of accuracy and reliability of the plaque reduction neutralization test (micro-PRNT) in detection of yellow fever virus antibodies. Biologicals 2012; 40:399-404; PMID:23034357; http://dx.doi.org/10.1016/j.biologicals.2012.09.005
- Reinhardt B, Jaspert R, Niedrig M, Kostner C, L'age-Stehr J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol 1998; 56: 159-67; PMID:9746073; http://dx.doi.org/10.1002/(SICI)1096-9071(199810)56:2%3c159::AID-JMV10%3e3.0.CO;2-B
- Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 2008; 28:710-22; PMID:18468462; http://dx.doi.org/10.1016/j.immuni.2008.02.020
- Campi-Azevedo AC, Estevam P de A, Coelho-dos-Reis JG, Peruhype-Magalhães V, Villela-Rezende G, Quaresma PF, Maia M de L S, Farias RHG, Camacho LAB, Freire M da S, et al. Subdoses of 17DD yellow fever vaccine elicit equivalent virological/immunological kinetics timeline. BMC Infect Dis 2014; 15:14-391
- Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol 2013; 13:875-87; PMID:24157572; http://dx.doi.org/10.1038/nri3547
- Peruhype-Magalhães V, Martins-Filho OA, Prata A, Silva L de A, Rabello A, Teixeira-Carvalho A, Figueiredo RM, Guimarães-Carvalho SF, Ferrari TC, Van Weyenbergh J, et al. Mixed inflammatory/regulatory cytokine profile marked by simultaneous raise of interferon-gamma and interleukin-10 and low frequency of tumour necrosis factor-alpha(+) monocytes are hallmarks of active human visceral Leishmaniasis due to Leishmania chagasi infection. Clin Exp Immunol 2006; 146: 124-32; PMID:16968407; http://dx.doi.org/10.1111/j.1365-2249.2006.03171.x
- Silva ML, Martins MA, Espírito-Santo LR, Campi-Azevedo AC, Silveira-Lemos D, Ribeiro JG, Homma A, Kroon EG, Teixeira-Carvalho A, Elói-Santos SM, et al. Characterization of main cytokine sources from the innate and adaptive immune responses following primary 17DD yellow fever vaccination in adults. Vaccine 2011; 29:583-92; PMID:20732465; http://dx.doi.org/10.1016/j.vaccine.2010.08.046