Abstract
Dendritic cell (DC)-based vaccines designed to target cancer stem cells (CSC) can induce significant antitumor responses via conferring host anti-CSC immunity. Our recent studies have demonstrated that CSC-DC vaccine could inhibit metastasis of primary tumors and induce humoral immune responses against cancer stem cells. This approach highlights the promise of cancer stem cell vaccine in cancer immunotherapy.
To date, the available cancer-preventive vaccines function by preventing viral infections rather than cancer per se. For example, there are 2 vaccines that prevent viral infections whose long-term consequence of chronic infection include cancer. Hepatitis B vaccine prevents infection by hepatitis B virus (HBV), where chronic HBV infection results in a high risk of progression to cirrhosis and hepatocellular carcinoma (HCC).Citation1,2 In this regard, it has been shown that Hepatitis B vaccination can lower the long-term rate of development of HCC in populations with a high level of vaccination.Citation3,4 Similarly, human papilloma virus (HPV) vaccine prevents infection, where chronic HPV infection is a risk factor for development of cervical cancer.Citation5,6 HPV vaccine is now being used in 11–14 year-old girls. It is reported that about 90% of invasive cervical cancer, high-grade cervical neoplasia, genitals warts and anal cancer cases could be prevented by a HPV vaccine.Citation7
The field of ‘cancer vaccines’ that are not anti-infection vaccines is directed to immunotherapy of diagnosed tumors. For therapy, dendritic cells (DC) pulsed with tumor antigen have shown efficiency and DC-based vaccine represents a promising immunotherapy for cancers.Citation8,9 For example, sipuleucel-T immunotherapy for castration-resistant prostate cancer revealed encouraging result. Sipuleucel-T is a cancer vaccine developed from autologous dendritic cells loaded with engineered fusion protein of prostatic acid phosphatase (PAP) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Recent Phase III studies show that sipuleucel-T therapy can prolong median survival in patients with castration-resistant prostate cancer (CRPC). Use of sipuleucel-T vaccine has been approved by the United States Food and Drug Administration (Clinical Trials.gov number, NCT00065442).Citation10
Many human malignancies are associated with quantitative and qualitative deficiencies in the immune system. Immunotherapy thus holds the promise for cancer treatment, and has demonstrated the complementary role to traditional cancer treatments, e. g. surgery, chemotherapy and radiotherapy. Immunotherapy has revealed several advantages over the traditional cancer treatment, such as less toxicity.Citation11 The current immunological strategies for cancer therapy include vaccines, monoclonal antibodies, and cellular therapies. However, despite of the encouraging preclinical studies in cancer immunotherapy, the clinical responses are limited to a confined patient population.
There is accumulating evidence that tumors contain a distinct subpopulation of stem-like cells, or cancer stem cells (CSCs).Citation12-14 Recent studies have described several biomarkers for cancer stem cells e.g. CD44+/ CD24− for breast cancer,Citation15 CD133+ for brain tumorCitation16 and colon cancer,Citation17 and CD44+ for gastric cancer.Citation18 The isolation and identification of cancer stem cells or CSC-like cells (CSC-LCs) in ovarian cancer have also been reported.Citation19 Importantly, Du et al found that use of anti-CD44 monoclonal antibodies may be a potential strategy for the treatment of human ovarian cancer after conventional therapy via inhibiting tumor growth and promoting apoptosis in sphere-forming cells (SFCs) with stemness.Citation20 More recently, high aldehyde dehydrogenase (ALDH) activity, measured via ALDEFLUOR assay, has been successfully used as a marker to enrich cancer stem cell populations from multiple tumor types.Citation21-27 Ithimakin et al. reported that higher expression of HER2 in the ALDEFLUOR+/ ALDHhigh cancer stem cells further enhances tumor initiation.Citation28 Cancer stem cells are resistant to traditional chemotherapy and radiotherapy,Citation29,30 and have been found to be responsible for the initiation, relapse as well as metastasis of tumor.Citation31,32 Immunological targeting of cancer stem cells may therefore represent a novel approach in cancer immunotherapy.
We have reported the protective antitumor immunity induced by ALDEFLUOR+/ ALDHhigh cancer stem cell-dendritic cell (CSC-DC) vaccine using histologically distinct murine tumor models syngeneic to different immunocompetent hosts. Enriched ALDEFLUOR+/ ALDHhigh cancer stem cells were immunogenic and more effective as an antigen source than unsorted bulk tumor cells or ALDEFLUOR−/ ALDHlow cells in inducing protective antitumor immunity.Citation27 Mechanistic investigations established that CSC-primed antibodies and T cells were capable of selectively targeting cancer stem cells and therefore conferring antitumor immunity.Citation27 The inability to target cancer stem cells with the current immunological approaches may be a significant factor contributing to treatment failure. These results provide a rationale for a new type of cancer immunotherapy based on the development of CSC-DC vaccines that can induce host immunity specifically targeting cancer stem cells.
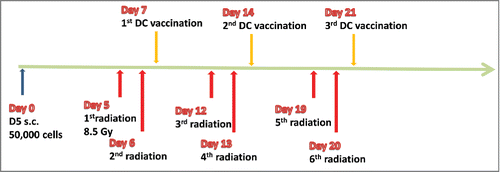
For a vaccine to be clinically relevant, it needs to be examined in the therapeutic models. In a recent study, we developed a strategy to target the cancer stem cell populations in melanoma and squamous cell carcinoma using cancer stem cell lysate-pulsed dendritic cells in established tumors.Citation33 The CSC-DC vaccine was administered after localized radiation therapy of the established tumor. Using mouse models we demonstrated that dendritic cells pulsed with cancer stem cells enriched by virtue of their expression of the CSC marker ALDH (CSC-DC) significantly inhibited tumor growth, reduced development of pulmonary metastases of established primary tumor and prolonged survival. In these experiments, B6 or C3H mice were inoculated s.c. with 0.05 × 106 D5 melanoma cells or 0.5 × 106 SCC7 squamous cell carcinoma cells respectively on day 0. The mice were treated with localized radiation therapy (RT) on day 5 and day 6 followed by the 1st DC vaccine on day 7. The combined RT + vaccine treatment was repeated on day 12, 13, 14 and 19, 20, 21 respectively. Thus, the RT was delivered 6 times, which were on days 5, 6, 12, 13, 19 and 20 with a total dose of 51 Gy (8.5 Gy × 6), while vaccines were administrated 3 times, 1 week apart, which were on days 7, 14 and 21 ().
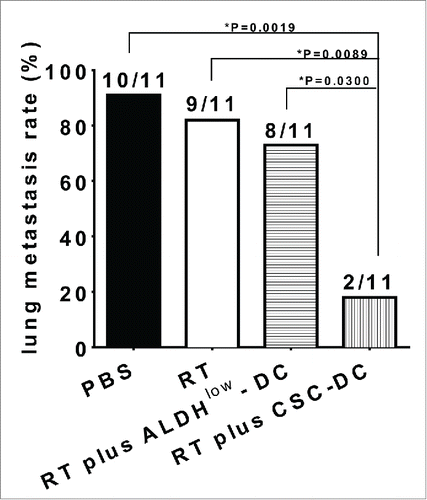
Mice treated with local tumor radiation therapy (RT) plus ALDHhigh CSC-DC vaccine showed a 20.5-day survival advantage over the RT plus ALDHlow-DC vaccinated mice (P = 0.0036), and a 17-day survival advantage over the RT plus heterogeneous, unsorted tumor cell lysate-pulsed dendritic cell (H-DC) vaccinated mice (P = 0.0121). We found that RT followed by ALDHhigh CSC-DC vaccination significantly inhibited the lung metastasis in the established tumor models (). Specifically, in each experiment group (n = 11), there were 10, 9 and 8 mice that resulted in distant lung metastasis after treatment with PBS (control), RT only, or RT plus ALDHlow-DC vaccine respectively. In contrast, RT plus ALDHhigh CSC-DC vaccine treatment only had 2 mice with lung metastasis. As a result, RT plus ALDHhigh CSC-DC vaccination significantly prolonged the overall survival of the animals, showing the significant therapeutic efficacy of ALDHhigh CSC-DC vaccine in combination with radiation therapy in the management of established tumors. CSC-DC vaccine significantly reduced ALDHhigh CSCs in the primary tumors. Direct targeting of CSCs was demonstrated by specific binding of IgG produced by ALDHhigh CSC-DC vaccine-primed B cells to ALDHhigh CSCs, resulting in lysis of these target CSCs in the presence of complement, thus showing the humoral immune responses against cancer stem cells. These data suggest that the CSC-DC vaccine approach may be useful after conventional treatment of cancers, such as radiotherapy, where local and systemic relapse are high.
Figure 2. Local tumor radiation therapy (RT) followed by CSC-DC vaccination significantly inhibited the lung metastasis. Bar graph shows the percentage of lung metastasis.
In order to improve vaccine efficiency, Mitchell et al used tetanus toxoid as an adjuvant. They randomized patients with glioblastoma for pre-conditioning with either mature DCs or TD (tetanus/diphtheria) unilaterally before bilateral vaccination with DCs pulsed with cytomegalovirus phosphoprotein 65 (pp65) RNA. Twelve patients were randomized into this clinical trial. Compared to DC alone-treated patients, TD-pre-conditioned patients showed significantly more draining lymph nodes (DLNs) and increased median progression-free and overall survivals.Citation34 Bacille Calmette-Guérin (BCG) is well known as another adjuvant used in cancer immunotherapy, particularly for bladder tumor. Rossi et al reported that the number of peripheral blood plasmacytoid DCs (pDCs) was partially affected by BCG administration.Citation35 Investigation and application of novel adjuvant may enhance the therapeutic efficacy of CSC-DC vaccine.
Programmed death ligand 1 (PD-L1, also known as B7 homolog 1, B7-H1 or CD274) makes tumor-reactive T cells tolerate to tumor cells by binding to programmed death-1 (PD-1 or CD279).Citation36,37 It is a major barrier to antitumor immunity.Citation38 Durgan et al found that PD-L1−/− mice given dendritic cells pulsed with antigen and α-GalCer showed decreased tumor size and this was associated with increased trafficking of antigen-presenting cells and CD8+ T cells to the tumor. These data demonstrated that interrupting PDL1/PD-1 interaction amplifies an anti-tumor response when coupled with DC vaccination.Citation39 In a clinical trial, Gettinger et al reported that administration of anti–PD-1 antibody Nivolumab produced durable responses and enhanced survival rates in patients with heavily pretreated non-small cell lung cancer (NSCLC).Citation40 In addition, Eggermont AM et al conducted a double-blind phase III trial in patients with stage III cutaneous melanoma. Patients received intravenous infusions of 10 mg/kg ipilimumab, a first-in-class immunological checkpoint blockade agent and monoclonal antibody against cytotoxic T-lymphocyte antigen 4 (CTLA-4) or placebo as a control every 3 weeks for 4 doses, then every 3 months for up to 3 y Median recurrence-free survival was 26.1m in the ipilimumab group versus 17.1m in the placebo group; 3-year recurrence-free survival was 46.5% in the ipilimumab group vs. 34.8% in the placebo group.Citation41 While cancer vaccine and other forms of immunotherapy has been found playing an important role in the treatment of lung cancer,Citation42 renal carcinoma,Citation43 and colorectal cancer,Citation44 immunological targeting of cancer stem cells utilizing cancer stem cell-based vaccine may represent a more potent strategy to prevent tumor metastasis and relapse. Particularly, immunologically targeting cancer stem cells while simultaneously blocking PD-1/PD-L1 and/or CTLA-4-mendiated immune suppression may significantly enhance the outcome of current immunotherapies of cancer.
Disclosure of Potential Conflicts of Interest
There were no conflicts of interest.
Funding
This work was supported by the Elsa U. Pardee Foundation, and partially supported by the Gillson Longenbaugh Foundation.
References
- Vivekanandan PM, Torbenson B. Ramakrishna, Hepatitis B virus-associated hepatocellular carcinoma from India: role of viral genotype and mutations in CTNNB1 (beta-catenin) and TP53 genes. J Gastrointest Cancer 2011; 42(1):20-5; PMID:20963515; http://dx.doi.org/10.1007/s12029-010-9222-4
- Han YF, Zhao J, Ma LY, Yin JH, Chang WJ, Zhang HW, Cao GW. Factors predicting occurrence and prognosis of hepatitis-B-virus-related hepatocellular carcinoma. World J Gastroenterol 2011; 17(38):4258-70; PMID:22090781; http://dx.doi.org/10.3748/wjg.v17.i38.4258
- Kim MN, Han KH, Ahn SH. Prevention of hepatocellular carcinoma: beyond hepatitis B vaccination. Semin Oncol 2015; 42(2):316-28; PMID:25843736; http://dx.doi.org/10.1053/j.seminoncol.2014.12.018
- Chang MH. Prevention of hepatitis B virus infection and liver cancer. Recent Results Cancer Res 2014; 193:75-95; PMID:24008294; http://dx.doi.org/10.1007/978-3-642-38965-8_5
- Botha MH, Richter KL. Cervical cancer prevention in South Africa: HPV vaccination and screening both essential to achieve and maintain a reduction in incidence. S Afr Med J 2015; 105(1):33-4; PMID:26046160; http://dx.doi.org/10.7196/samj.9233
- Demarteau N, Breuer T, Standaert B. Selecting a mix of prevention strategies against cervical cancer for maximum efficiency with an optimization program. Pharmacoeconomics 2012; 30(4):337-53; PMID:22409292; http://dx.doi.org/10.2165/11591560-000000000-00000
- Riethmuller D, Jacquard AC, Lacau St Guily J, Aubin F, Carcopino X, Pradat P, Dahlab A, Prétet JL. Potential impact of a nonavalent HPV vaccine on the occurrence of HPV-related diseases in France. BMC Public Health 2015; 15(1):453; PMID:25934423; http://dx.doi.org/10.1186/s12889-015-1779-1
- Huang C, Ramakrishnan R, Trkulja M, Ren X, Gabrilovich DI. Therapeutic effect of intratumoral administration of DCs with conditional expression of combination of different cytokines. Cancer Immunol Immunother 2012; 61(4):573-9; PMID:22223258; http://dx.doi.org/10.1007/s00262-011-1198-9
- Finkelstein SE, Iclozan C, Bui MM, Cotter MJ, Ramakrishnan R, Ahmed J, Noyes DR, Cheong D, Gonzalez RJ, Heysek RV, et al. Combination of external beam radiotherapy (EBRT) with intratumoral injection of dendritic cells as neo-adjuvant treatment of high-risk soft tissue sarcoma patients. Int J Radiat Oncol Biol Phys 2012; 82(2):924-32; PMID:21398051; http://dx.doi.org/10.1016/j.ijrobp.2010.12.068
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363(5):411-22; PMID:20818862; http://dx.doi.org/10.1056/NEJMoa1001294
- Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol 2015; 33(18):2092-9; PMID:25918278
- Shimamura M, Nagayama Y, Matsuse M, Yamashita S, Mitsutake N. Analysis of multiple markers for cancer stem-like cells in human thyroid carcinoma cell lines. Endocr J 2014; 61(5):481-90; PMID:24531915; http://dx.doi.org/10.1507/endocrj.EJ13-0526
- Chiba T, Iwama A, Yokosuka O. Cancer stem cells in hepatocellular carcinoma: Therapeutic implications based on stem cell biology. Hepatol Res 2015; PMID: 26123821; http://dx.doi.org/10.1111/hepr.12548
- Pozzi V, Sartini D, Rocchetti R, Santarelli A, Rubini C, Morganti S, Giuliante R, Calabrese S, Di Ruscio G, Orlando F, et al. Identification and characterization of cancer stem cells from head and neck squamous cell carcinoma cell lines. Cell Physiol Biochem 2015; 36(2):784-98; PMID:26021266; http://dx.doi.org/10.1159/000430138
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003; 100(7):3983-8; PMID:12629218; http://dx.doi.org/10.1073/pnas.0530291100
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63(18):5821-8; PMID:14522905
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007; 445(7123):106-10; PMID:17122772; http://dx.doi.org/10.1038/nature05372
- Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009; 27(5):1006-20; PMID:19415765; http://dx.doi.org/10.1002/stem.30
- Wang H, Paczulla A, Lengerke C. Evaluation of stem cell properties in human ovarian carcinoma cells using multi and single cell-based spheres assays. J Vis Exp 2015; (95):e52259; PMID:25590994
- Du YR, Chen Y, Gao Y, Niu XL, Li YJ, Deng WM. Effects and mechanisms of anti-CD44 monoclonal antibody A3D8 on proliferation and apoptosis of sphere-forming cells with stemness from human ovarian cancer. Int J Gynecol Cancer 2013; 23(8):1367-75; PMID:24257550; http://dx.doi.org/10.1097/IGC.0b013e3182a1d023
- Visus C, Wang Y, Lozano-Leon A, Ferris RL, Silver S, Szczepanski MJ, Brand RE, Ferrone CR, Whiteside TL, Ferrone S, et al. Targeting ALDH(bright) human carcinoma-initiating cells with ALDH1A1-specific CD8(+) T cells. Clin Cancer Res 2011; 17(19):6174-84; PMID:21856769; http://dx.doi.org/10.1158/1078-0432.CCR-11-1111
- Almanaa TN, Geusz ME, Jamasbi RJ., A new method for identifying stem-like cells in esophageal cancer cell lines. J Cancer 2013; 4(7):536-48; PMID:23983818; http://dx.doi.org/10.7150/jca.6477
- Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck 2010; 32(9):1195-201; PMID:20073073; http://dx.doi.org/10.1002/hed.21315
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007; 1(5):555-67; PMID:18371393; http://dx.doi.org/10.1016/j.stem.2007.08.014
- Januchowski R, Wojtowicz K, Zabel M. The role of aldehyde dehydrogenase (ALDH) in cancer drug resistance. Biomed Pharmacother 2013; 67(7):669-80; PMID:23721823; http://dx.doi.org/10.1016/j.biopha.2013.04.005
- Le Magnen C, Bubendorf L, Rentsch CA, Mengus C, Gsponer J, Zellweger T, Rieken M, Thalmann GN, Cecchini MG, Germann M, et al. Characterization and clinical relevance of ALDHbright populations in prostate cancer. Clin Cancer Res 2013; 19(19):5361-71; PMID:23969936; http://dx.doi.org/10.1158/1078-0432.CCR-12-2857
- Ning N, Pan Q, Zheng F, Teitz-Tennenbaum S, Egenti M, Yet J, Li M, Ginestier C, Wicha MS, Moyer JS, et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res 2012; 72(7):1853-64; PMID:22473314; http://dx.doi.org/10.1158/0008-5472.CAN-11-1400
- Ithimakin S, Day KC, Malik F, Zen Q, Dawsey SJ, Bersano-Begey TF, Quraishi AA, Ignatoski KW, Daignault S, Davis A, et al. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res 2013; 73(5):1635-46; PMID:23442322; http://dx.doi.org/10.1158/0008-5472.CAN-12-3349
- Dingwall S, Lee JB, Guezguez B, Fiebig A, McNicol J, Boreham D, Collins TJ, Bhatia M. Neoplastic human embryonic stem cells as a model of radiation resistance of human cancer stem cells. Oncotarget 2015; 6(26): 22258–69; PMID: 26082437; http://dx.doi.org/10.18632/oncotarget.4165
- Yang Y, Fan Y, Qi Y, Liu D, Wu K, Wen F, Zhao S. Side population cells separated from A549 lung cancer cell line possess cancer stem cell-like properties and inhibition of autophagy potentiates the cytotoxic effect of cisplatin. Oncol Rep 2015; 34(2):929-35; PMID:26081992
- Chen YJ, Zhang X, Wu ZS, Wang JJ, Lau AY, Zhu T, Lobie PE. Autocrine human growth hormone stimulates the tumor initiating capacity and metastasis of estrogen receptor-negative mammary carcinoma cells. Cancer Lett 2015; 365(2):182-9; PMID:26070963; http://dx.doi.org/10.1016/j.canlet.2015.05.031
- Tang KD, Holzapfel BM, Liu J, Lee TK, Ma S, Jovanovic L, An J, Russell PJ, Clements JA, Hutmacher DW, et al. Tie-2 regulates the stemness and metastatic properties of prostate cancer cells. Oncotarget 2015; PMID: 25978029; http://dx.doi.org/10.18632/oncotarget.3950
- Lu L, Tao H, Chang AE, Hu Y, Shu G, Chen Q, Egenti M, Owen J, Moyer JS, Prince ME, et al. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. Oncoimmunology 2015; 4(3):e990767; PMID:25949905; http://dx.doi.org/10.4161/2162402X.2014.990767
- Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, Congdon KL, Reap EA, Archer GE, Desjardins A, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015; 519(7543):366-9; PMID:25762141; http://dx.doi.org/10.1038/nature14320
- Rossi R, Lichtner M, Iori F, Ermocida A, Mascia C, Mengoni F, Sauzullo I, Dini D, Mastroianni CM, Vullo V. Dendritic cells in blood and urine samples from bladder cancer patients undergoing BCG immunotherapy. Arch Ital Urol Androl 2013; 85(4):157-63; PMID:24399114; http://dx.doi.org/10.4081/aiua.2013.4.157
- Berghoff AS, Ricken G, Widhalm G, Rajky O, Hainfellner JA, Birner P, Raderer M, Preusser M. PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL). Clin Neuropathol 2014; 33(1):42-9; PMID:24359606; http://dx.doi.org/10.5414/NP300698
- Tripathi S, Guleria I. Role of PD1/PDL1 pathway, and TH17 and treg cells in maternal tolerance to the fetus. Biomed J 2015; 38(1):25-31; PMID:25355392; http://dx.doi.org/10.4103/2319-4170.143511
- Ostrand-Rosenberg S, Horn LA, Haile ST. The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity. J Immunol 2014; 193(8):3835-41; PMID:25281753; http://dx.doi.org/10.4049/jimmunol.1401572
- Durgan K, Ali M, Warner P, Latchman YE. Targeting NKT cells and PD-L1 pathway results in augmented anti-tumor responses in a melanoma model. Cancer Immunol Immunother 2011; 60(4):547-58; PMID:21240487; http://dx.doi.org/10.1007/s00262-010-0963-5
- Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015; 33(18):2004-12; PMID:25897158
- Eggermont AM, Chiarion-Sileni V, Grob JJ. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015; 16(6):e262; PMID:26065611
- Vansteenkiste J, Zielinski M, Linder A, Dahabreh J, Gonzalez EE, Malinowski W, Lopez-Brea M, Vanakesa T, Jassem J, Kalofonos H, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol 2013; 31(19):2396-403; PMID:23715567; http://dx.doi.org/10.1200/JCO.2012.43.7103
- Wang D, Zhang B, Gao H, Ding G, Wu Q, Zhang J, Liao L, Chen H. Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer. BMC Cancer 2014; 14:251; PMID:24720900; http://dx.doi.org/10.1186/1471-2407-14-251
- Amin M, Lockhart AC. The potential role of immunotherapy to treat colorectal cancer. Expert Opin Investig Drugs 2015; 24(3):329-44; PMID:25519074; http://dx.doi.org/10.1517/13543784.2015.985376