Abstract
Vaccine biomarkers are critical to many aspects of vaccine development and licensure, including bridging findings in pre-clinical studies to clinical studies, predicting potential adverse events, and predicting vaccine efficacy. Despite advances in our understanding of various biological pathways, and advances in systems analyses of the immune response, there remains much to learn about qualitative and quantitative aspects of the human host response to vaccination. To stimulate discussion and identify opportunities for collaborative ways to advance the field of vaccine biomarkers, A Next Generation Vaccine Biomarker workshop was held in Ottawa. The two day workshop, sponsored by the National Research Council Canada, Canadian Institutes of Health Research, Public Health Agency of Canada, Pfizer, and Medicago, brought together stakeholders from Canadian and international industry, government and academia. The workshop was grouped in themes, covering vaccine biomarker challenges in the pre-clinical and clinical spaces, veterinary vaccines, regulatory challenges, and development of biomarkers for adjuvants and cancer vaccines. The use of case studies allowed participants to identify the needs and gaps requiring innovation. The workshop concluded with a discussion on opportunities for vaccine biomarker discovery, the Canadian context, and approaches for moving forward. This article provides a synopsis of these discussions and identifies steps forward for advancing vaccine biomarker research in Canada.
Introduction
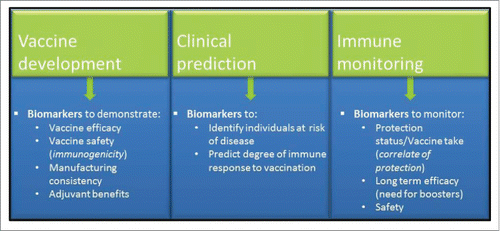
Vaccines remain a highly cost effective way to improve public health, reducing morbidity and mortality. According to the World Health Organization (WHO), between 2 and 3 million deaths are prevented annually as a result of immunizations.Citation1 A vaccine biomarker is a measurable biological indicator used to evaluate physiological, toxicological, pharmacological, or clinical significance of vaccination. They are essential components for many stages of the vaccine development pipeline, from disease biomarkers, to post marketing monitoring ().
The Next Generation Vaccine Biomarker workshop built upon the Canadian Adjuvant Initiative Workshop,Citation2 bringing together key experts and opinion leaders to stimulate further discussions in the vaccine biomarker space (, participant list).
Table 1. Participant list for the Next Generation Vaccine Biomarker Workshop. Contributors represented government, industry, regulatory bodies, academia, public-private partnerships, and funding agencies
The workshop was sponsored by the National Research Council Canada (NRC), Public Health Agency of Canada (PHAC), Canadian Institutes for Health Research (CIHR), Pfizer, and Medicago. The 2 day workshop began with a welcome and introductory commentary from the master of ceremonies, Dr. Luis Barreto. This was followed by opening remarks from 3 key drivers of the workshop, Dr. Jim Richards (Director, Vaccine Program, NRC), Dr. Marc Ouellette (Scientific Director, CIHR Institute of Infection and Immunity), and Dr. John Spika (Director General, Center for Immunization and Respiratory Infectious Diseases PHAC).
In his address, Dr. Richards shared the NRC mandate to provide strategic research support to meet industry needs and biomedical innovation, particularly in vaccine development. A key highlight of NRC's contribution to the vaccines field was the development of the Group C Meningitis glycoconjugate vaccine by Dr. Harold Jennings. NRC's involvement with the Federal Vaccine Innovation and Development plan stems from this foundation in vaccine development.
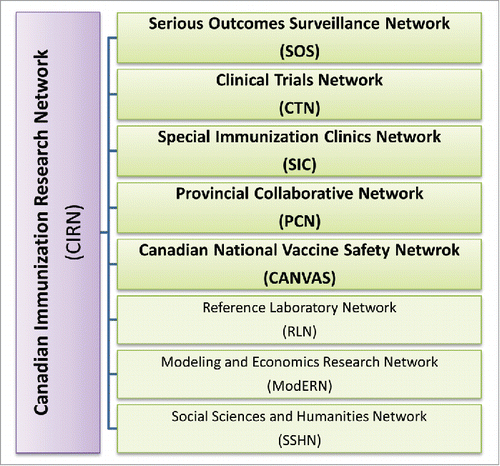
Dr. Ouellette described the Federal Vaccine Innovation and Development plan as a multi-departmental initiative which began in 2012, facilitating vaccine research efforts in Canada at a national level. Both the Canadian Adjuvant Initiative Workshop and the present Next Generation Vaccine Biomarkers Workshop have arisen from the collaborative efforts of those involved with the action plan. Dr. Ouellette also described the contributions of CIHR and PHAC to fund the Canadian Immunization Research Network (CIRN, ), to support research involving vaccines, immunization, and infectious disease.
Figure 2. CIRN Infrastructure. CIRN is comprised of 5 core networks, as well as 3 support networks, that work collaboratively to advance vaccine research in Canada.
Dr. Spika went on to acknowledge the long history of vaccine development in Canada. Of particular note are the activities for more than 50 years of the National Committee of Immunization, reporting to the Assistant Deputy Minister, Infectious Disease Prevention and Control, currently lead by Dr. Judith Bossé. He highlighted the alignment of the Federal Vaccine Innovation and Development plan with the National Immunization Strategy, which has been in place since 2003, and has a key component focused on vaccine innovation.
Next Generation of Vaccine Biomarkers
Session 1, entitled ‘Next Generation of Vaccine Biomarkers,’ was chaired by Dr. Luis Barreto and Dr. John Spika. It opened with a Keynote Address and included perspectives from industry, public/private collaborative organizations, and regulatory bodies.
Keynote Address
Keynote Speaker, Dr. Judie Alimonti of the National Microbiology Laboratory (NML), Public Health Agency of Canada, spoke about ‘Perspectives of Biomarkers on the Development and Use of Ebola Monoclonal Antibodies and Vaccines―A Paradigm Shift.’ Ebola (EBOV) is a negative sense single stranded RNA virus, responsible for more than 10000 infections in West Africa by October 2014, with a mortality rate close to 50%. Onset of symptoms is typically 3-21 days post-exposure with symptoms including fever, muscle aches, diarrhea, vomiting, and hemorrhage. The cause of death is multi-organ failure and shock in up to 90% of cases. At the time of the workshop, the West African outbreak of Ebola was at its height, and the largest in terms of number of cases and geographical range. Dr. Alimonti described 2 approaches being pursued by the NML: a live attenuated vaccine and monoclonal antibody therapy. The vaccine candidate is a live attenuated Indiana strain of the Vesicular Stomatitis Virus (VSV) expressing the EBOV glycoprotein, which helps mediate immunity. A non-human primate (NHP) study demonstrated efficacy in vaccinated Cynomologus monkeys challenged with Zaire ebola virus (ZEBOV).Citation3 The vaccine candidate was also used twice as a post-exposure therapy during the 2014 West African outbreak and at the time of the meeting was in Phase 1 clinical trials. In addition to vaccine development, 8 EBOV glycoprotein (EBOVGP) specific mAbs have been generated. ZMab, a cocktail of 3 of these mAbs, has shown 67 or 100% efficacy in guinea pigs when administered 3 and 2 days post infection, respectively.Citation4 It also resulted in 50 or 100 % survival in cynomologous monkeys when the cocktail was administered 48 and 24 hours after exposure, respectively.Citation5 Current efforts focus on extending the window for effective treatment further, with progress toward demonstrating efficacy in humans, by humanising and expressing in the NRC CHO cell line. ZMab was administered to 8 people during the current West African outbreak.
Industry Panel
A panel of industry speakers shared their experiences with vaccine biomarkers. First to speak was Dr. Heather Davies (Pfizer), who highlighted the roles of vaccine biomarkers with regard to efficacy, safety, immunogenicity, the animal rule, and therapeutic B and T cell markers. Biomarkers for vaccine safety are important to indicate potential adverse reactions or lack thereof.Citation6 Such biomarkers could be standard indicators, such as for local and systemic reactogenicity events, or specific assays, such as anti-DNA antibodies with a DNA vaccine. It is important to note that biomarkers of vaccine efficacy may differ between prophylactic and therapeutic vaccines ().
Table 2. Comparison of biomarkers for prophylactic and therapeutic vaccines. For prophylactic vaccines, it is often not possible to monitor efficacy directly (post exposure or challenge), and so a surrogate may be established to demonstrate efficacy. In contrast, for therapeutic vaccines, surrogates are not used for regulatory approval since it is necessary to show direct efficacy in the disease state
Dr. Marianne Stanford of Immunovaccine presented a talk entitled, ‘DepoVax™ in Cancer Immunotherapy and Infectious Disease Applications and The Challenge of Biomarker Identification Employing Novel Vaccine Platforms.’ Immunvaccine focuses on the development of vaccine formulation, and licenses their platform to third parties for various applications. Their DepoVax™ product, as an off the shelf vaccine formulation, prolongs the interaction between the antigen and the immune system. The role of biomarkers in DepoVax™ includes adverse event monitoring, aiding in the selection of appropriate targets, and supporting clinical trial decision making. As an example, Immunovaccine is currently working with NRC to identify potential biomarkers for efficacy and safety from Phase I/Ib ovarian cancer trials.
Dr. Stéphane Pillet of Medicago spoke about ‘The Use of Immunomarker Technology in the Development of Plant-Based Influenza Vaccines.’ Medicago offers a vaccine preparation technology based on Virus-like particles (VLPs) produced in Tobacco plants. VLPs are viruses that lack a genetic core, but still stimulate the immune system because of their resemblance to a real virus, while the shell acts as a vector for an antigen. This production system offers an alternative to traditional egg based vaccine production that is amenable to rapid scale up. There is a growing trend to increase knowledge of cell mediated immunity (CMI) markers for the development of vaccines, particularly for influenza which is a focus for Medicago. A quadrivalent influenza vaccine has been shown to induce cross reactivity that persists after 6 months. Immune markers can help us to understand these complex immune responses and immune subpopulations, which will aid influenza vaccine development.
Dr. Yoav Peretz of Caprion spoke about ‘Immune Profiling During Pre-Clinical Development and in Phase I/II Clinical Trials.’ In 2004, the National Immune Monitoring Laboratory was created which later became Immunecarta, and is now currently part of Caprion. Dr. Peretz discussed the functional signal of immune response obtained by intracellular cytokine staining assay, and then elaborated on the current work being pursued in collaboration with Immunovaccine for the Phase I Study Case of DPX-Survivac.
European Non-Governmental Organization Perspective
Dr. Nathalie Garçon of BIOASTER spoke about ‘Biomarkers for Adjuvanted Vaccine Formulations.’ BIOASTER is a not-for- profit organization, and aims to partner academics, industries, hospitals, and small and medium sized enterprises (SME) around technology platforms to solve biological problems. BIOASTER currently partners with over 40 SMEs and has dedicated collaborative laboratories. Dr. Garçon highlighted the many challenges of adjuvanted vaccine development, including the increase in the number of adjuvants, the complexity of molecular interaction networks of immune stimulation (for example, route of administration), and population level versus individual evaluations. Dr. Garçon also noted that biomarkers are critical for areas such as susceptibility and efficacy, to establish predictability of our animal models, to help develop new models, and to evaluate individual vs. population level effects. To bring biomarkers to the next level will require integration of biology with other fields, particularly technology and data integration.
Regulatory Landscape
The first speaker representing a regulatory body was Dr. Karen Elkins from the Center for Biologics Evaluation and Research, US. Food and Drug Administration (US FDA). In her talk, ‘Bioassays of Cell-Mediated Immunity to Support Vaccine Development and Licensure,’ she first outlined the different US FDA Centers and Regulatory Divisions of Labor (). Challenges in the vaccine field include the lack of validated assays applied to vaccine studies to derive clinical biomarkers per se and bioassays of cell-mediated immunity to support vaccine development and licensure. Only ELISPOTs and related methods have been validated and used to support licensure, while other research methods commonly used to study T cell functions, such as intracellular cytokine staining or “omics”-based signatures, have not yet been used during pivotal efficacy studies. There is a significant unmet need for validated bioassays to support vaccine studies.
Table 3. US FDA Centers and Regulatory Divisions of labor. These centers are involved in both biologics and vaccines approval
Representing Health Canada, Dr. Sean Li spoke about ‘Highly Conserved Epitopes in Influenza Virus: Implications on Vaccine Analyses.’ There are many challenges facing the development of influenza vaccines, including searching for highly conserved epitopes to target in influenza virus, and the generation of antibodies toward such epitopes. Dr. Li gave an overview of the influenza virus, focusing on a highly conserved epitope in the hemagglutinin (HA) protein that has been identified: GL(I)FGAIAGFIEG(N)G. When used as a fusion peptide, this has been investigated as a “universal vaccine.”Citation7,8 Despite some promising steps forward, there remain significant challenges in this field, including the lack of detection of antibodies again this universal epitope in nature (only in vitro) so design of vaccine needs to be improved.
European Perspectives on Industry Partnership Models in Vaccine Innovation in Biomarkers
In a session focused upon European activities, Dr. Maria Teresa De Magistris spoke of the ‘Innovative Medicines Initiative’ (IMI). IMI is the largest public-private partnership in Life Sciences research and development, including a consortium of public (academics, hospitals, regulators, SMEs, patient organizations) and private (companies that typically provide “in kind” contributions) parties. The second program phase (IMI2, 2014-2020), which is receiving € 3 billion, aims to create safer and more effective vaccines by removing barriers to large scale collaboration. IMI2 includes an IMI Vaccines section with 3 overlapping projects: BioVacSafe - Immunosafety of vaccines through biomarkers associated with adverse events (2012-2016; 30.2 M Euro); ADVANCE - Accelerated development of vaccine benefit-risk collaboration in Europe (2013-2018; 10.7 M Euro); and Flu vaccines - Immunological assay standardization and development for assessment of correlates of protection for influenza vaccines (begins 2014; 12.2 M Euro).
‘Biomarkers for Enhanced Vaccine Immunosafety,’ was the title of Dr. David Lewis' presentation. He represented the BioVacSafe project, intended to investigate immunosafety of vaccines, with a focus on conducting clinical trials to define new biomarkers of vaccine-induced inflammation. “Training” clinical trials are used to select vaccines, select sampling times, and identify candidate biomarkers, while “Confirmatory” trials are used to confirm biomarkers using one or 2 of the vaccines. The ultimate goal is to enable the transition of identified biomarkers through qualification and translation into guidance/regulatory documents. On closing, Dr. Lewis noted that BioVacSafe plans to work collaboratively with the NIH and the FDA.
Pre-Clinical Vaccine Biomarkers of Safety and Efficacy
The second session of the workshop focused upon Pre-Clinical Vaccine Biomarkers of Safety and Efficacy and was chaired by Dr. Sue Twine of the NRC.
The first speaker was Dr. Wayne Conlan of the NRC, who shared progress in seeking ‘Correlates of Protection to Screen Vaccine Efficacy through the Animal Rule.’ Dr. Conlan's work is focused on vaccines for tularemia, a disease caused by the bacterium Francisella tularensis. The ΔclpB mutant is the current lead vaccine candidate, demonstrating 100% protection against respiratory challenge in mice.Citation9 Despite extensive investigation and a well-established vaccination model, there were no antibodies (or group of antibodies) uniquely reactive with ΔclpB vaccination that could serve as a correlate of protection. An in vitro macrophage killing assayCitation10 has demonstrated that IFNγ is critical for host immunity, but only higher levels of IL17 in the lungs after vaccination and challenge was observed as a significant difference.Citation11 In the macrophage killing assay, a combination of IFNγ, IL17a, and TNFα were demonstrated to be most effective. The next step is to widen the search for biomarkers by using serum proteomics, expanding the repertoire of proteins detected beyond the limited panel targeted by the Luminex® approach.
Dr. Lakshmi Krishnan of the NRC gave an overview of ‘Predictive Biomarkers for Mechanism of Adjuvant Action.’ Dr. Krishnan shared the NRC portfolio of immune potentiators and delivery systems, which includes archaeosomes, recombinant vectors (bacterial and viral), and saponins. At the Canadian Adjuvant Initiative Workshop (March 26-27, 2013),Citation2 participants identified that adjuvants will not be approved as individual entities, but rather always as a constituent of an adjuvanted vaccine formulation. At NRC, biomarker analysis consists of several assays and tools including solute analysis, immunophenotyping (18-color flow cytometry), single cell analysis (6 way sorting), monitoring of innate, humoral, and cell-mediated immunity, and functional assays. The NRC's premier adjuvant platform comprises archaeosomes which are liposomal vesicles formed with total polar lipids of archaea.Citation12-14 The CD8+ T-cell response is monitored by enumerating antigen specific T-cells, monitoring IFNγ produced by ELISPOT assay, functional cytotoxicity assays, and assessment of memory phenotype, all serving as biomarkers of efficacy.
Dr. Volker Gerdts, of VIDO-InterVac, University of Saskatchewan, spoke about ‘Biomarkers in Animal Vaccine Development.’ He began by noting that animal health budgets can be very small, and can depend on the socioeconomic impact, resulting in distinct differences in vaccinating animals versus humans. Key considerations for animal vaccinations include cost, route of administration, storage, and DIVA (Differentiating Infected and Vaccinated Animals). There are a variety of animal models,Citation15,16 and many are used to help advance human vaccine development (assessing pathogenesis, vaccine delivery, efficacy, safety) and veterinary vaccinology can therefore support human vaccine development.Citation17 Historically, biomarkers for veterinary vaccines have included survival, clinical score, and adverse reactions, with current veterinary biomarkers trending toward immunological readouts, systemic vs local immunity, difference in age groups, and injection site reactions. Dr. Gerdts noted challenges facing veterinary vaccine development, including a lack of validated assays, a lack of validated correlates of protection, and a limited knowledge of vaccine-specific biomarkers.
Clinical Biomarkers of Vaccination
Session 3 of the workshop focused on Clinical Biomarkers and was co-chaired by Dr. Luis Barreto (NRC) and Dr. John Spika (PHAC).
Systems Biology Approaches
The first talk of the session was entitled ‘Predictors of Efficacy and Immune Responses to Vaccines,’ by Dr. Rafick Sékaly from the Department of Pathology, Case Western Reserve University. Dr. Sékaly presented the advantages of using a systems biology approach to produce an integrated database to refine and validate correlates for clinical trial monitoring.Citation18 He spoke about the concept of bioage as a predictor of vaccine response, citing the EM131 study to develop a statistical model that would predict vaccine hyporesponse (VHR) in the elderly using a panel of biomarkers. This study used 3 commercially available vaccines (Twinrix, Dukoral/rBS, and Td) on a cohort of 200 subjects >65 years of age. The result was a bioage signature made up of 20 metagenes. A second bioinformatics approach also found high numbers of B-cell indicates good response and high T-cell count at baseline predicts poor response.
Next in this session, Dr. Roger Brookes of Sanofi Pasteur spoke about an ‘in vitro Human Whole Blood Assay to Screen Biomarkers.’ Dr. Brookes discussed the challenge in linking vaccine formulation to immune functionality. The human whole blood assay (hWB) plates fresh human blood over the vaccine formulation and measures pro-inflammatory cytokines in the supernatant after 24 hours of incubation.Citation19 This assay can be used to demonstrate adjuvant modulated responses in vaccine formulations. He presented the IC31® modulated H4 response (Sanofi adjuvant) and demonstrated a strong IFNγ response. This assay demonstrated that the mechanism of action for TLR4 and IC31® are distinct and that an excess of IC31® is necessary for immunomodulation.Citation20
Dr. Mark Loeb of McMaster University spoke about ‘Vaccine Biomarkers in Aging Canadian Populations.’ Immunosenescence, or the waning of the immune system over time, is a strong risk factor for infections.Citation21 Through longitudinal studies performed by Swedish OCTO/NONA, the concept of an immune risk profile (IRP) was developed, where a high risk profile indicates a greater risk of death. This phenotype includes the expansion of CMV-specific, inverted CD4:CD8 ratio (i.e. <1), CMV seropositivity, low B-cell number, and poor T-cell response.Citation22,23 High regulatory T-cell counts were associated with reduced risk for viral respiratory infection. Conversely, high CMV-reactive CD4+ T-cells were associated with an increased risk of viral respiratory infection.Citation24 This is the first study to find predictive immune biomarkers in the elderly for respiratory viral infection.
Dr. Brian Ward of McGill University gave an overview of the ‘The Relevance of Clinical Biomarkers.’ He observed that biomarkers are important and it is important to move them forward to influence clinical development programs. Correlates of adverse reactions are more challenging, however, due to the rarity (perhaps one per million). Dr. Ward cautioned that the current expectation placed upon discovery and validation of clinical biomarkers may be too high, and that it is important to be conservative and realistic about how much can be achieved with biomarkers.
Dr. Scott Halperin of Dalhousie University spoke about the Canadian Immunization Research Network (CIRN, ). CIRN is a vaccine network sponsored by CIHR and PHAC that offers a 3 year grant opportunity over 2014-2017, at $2.2 million per year. The research structure is made up of 5 core networks. The Serious Outcomes Surveillance Network (SOS) is a hospital based surveillance for burden of disease in health care workers, adults, and immunocompromised individuals. The Clinical Trials Network (CTN) focuses on safety and immunogenicity evaluation in early/late phase clinical trials in specialized groups (elderly, aboriginal, pediatric, etc.). The Special Immunization Clinics Network (SICN) monitors adverse effects following immunization (AEFI). Other networks include the Canadian National Vaccine Safety Network (CANVAS), and the Provincial Collaborative Network (PCN). Several partners offer clinical sampling opportunities for biomarker evaluation as well as evaluation of safety, efficacy, and immunogenicity.
Biomarkers for the Development of Cancer Vaccines
The fourth session of the workshop was chaired by Dr. Marc Ouellette (CIHR) and Dr. Heather Davis (Pfizer) and focused upon Biomarkers for the Development of Cancer Vaccines.
Dr. Fernando Ulloa Montoya of GSK started the session with a talk entitled ‘Predictive Biomarkers to MAGE-A3 Cancer Immunotherapy.’ MAGE-A3 is a tumor specific recombinant protein antigen in GSK clinical development. A gene signature was identified and confirmed in 2 Phase II studies, with genes in the IFNλ connectivity network being overrepresented for responders to the MAGE-A3 immunotherapy.Citation25,26 A Phase III adjuvant study designated MAGE-A3 as Adjuvant Non-Small Cell LunG CanceR ImmunoTherapy (MAGRIT) demonstrated an absence of treatment effect, while the results of the second, ADuvant ImmunothERapy with MAGE-A3 melanomA (DERMA), are expected in 2015.
Dr. Jonathan Bramson of McMaster University spoke about ‘Transcriptional Profiling of Tumors for Immune Monitoring.’ He noted that although immune monitoring is performed using blood because acquiring samples is minimally invasive, responses in blood are not always indicative of patient outcomes. Recent work concluded that the the tumor environment has a level of complexity that cannot be determined from peripheral blood samples and consequently, transcriptional analysis can reveal much more about the local tumor environment. Proof of concept work was performed using cancer vaccines in a mouse model.Citation27,28 A cocktail of AdhCDT + α4 −1BB + αPD-1 results in tumor regression and 100% survival of mice by increasing local immune activity.
Dr. Réjean Lapointe of Université de Montréal gave a talk entitled ‘Burning down the house: Capturing and Combing Through the Human Immune Response to Cancer and Burn.’ He spoke about the importance of tissue banking and also standardized procedures for tissue banking, which will depend upon standard operating procedures (SOPs), quality control and management, links and communication with clinicians, funding, clinical/demographic information, and the collection of normal and patient samples. Ideally, an “immune card,” including biomarkers, could be created for each patient, detailing all of their information (T-cells, B-cells, dendritic cells, monocytes, adaptive responses, etc.). The Immune Assay Proficiency Panel Program (Cancer Research Institute) aims to facilitate and harmonize immune monitoring approaches for caner immunotherapy.
Vaccine Biomarkers, Canadian Context and Opportunities Moving Forward
Chaired by Dr. Jim Richards (NRC), representatives of funding organizations were invited to comment on opportunities to support biomarker research and development in session 5.
Dr. Marc Ouellette of Canadian Institutes of Health Research gave an overview of CIHR support for vaccine biomarker research, with $1 billion/year in research funding for academic or other eligible institutions. One of the 13 CIHR institutes is Infection and Immunity, which invest $25 million per year in vaccines alone. For CIHR, there will be a focus on human immunology and biomarkers going forward.
Dr. Gilles Patry of the Canada Foundation for Innovation (CFI) introduced the foundation, which was created in 1997 to enhance the capacity of research communities by funding research infrastructure (eg. facilities, instruments, databases, etc.). $1 billion per year is invested across the country, with the latest competition focused upon cyber infrastructure, to upgrade national platforms in data storage, cloud computing, and data management.
Dr. Cindy Bell spoke of Genome Canada an organization that began as a grass roots movement at the end of the 1990s with a vision to harness the transformative power of genomics to deliver benefits to Canadians. The organization has received $1 billion over 14 years to support “omics” research in Canada. As a result of the data overload from “omics” research, they are now funding data integration solutions as well.
Dr. Dan Trudeau of National Research Council Canada Industrial Research Assistance Program (NRC-IRAP) spoke about the need to support technological innovation and the NRC-IRAP mission is to accelerate the growth of SMEs by providing them with a comprehensive suite of innovation services and funding (). Their advisors frequently work on-site and in person with the SME clients.
Table 4. National Research Council Canada Industrial Research Assistance Program services. The services offered by this program support technological development for Canadian SMEs. Their suite of services, including funding and advisors, aim to help translate ideas into commercial success
Business-Led Networks of Centers of Excellence/ Centers of Excellence for Commercialization and Research
‘CQDM: Canadian Perspective,’ was presented by Dr. Mario Chevrette. CQDM is a pre-competitive pharma consortium in Canada, with a mission to fund and support breakthrough technologies that will enhance biopharmaceutical R&D productivity and accelerate the development of safer and more efficacious drugs. There are currently 34 research projects totaling $31.8 million in funding. CQDM can fund SMEs, universities, and government organizations anywhere in the world.
Dr. Edie Dullaghan of the Center for Drug Research and Development (CDRD) introduced the mandate to de-risk promising discoveries stemming from publicly funded academic health research and transform them into commercially viable opportunities for the private sector. CDRD is not-for-profit organization that houses scientific expertise and is affiliated with academic partners. The CDRD Biomarker projects seek biomarker signatures of disease progression and predictors of responses to therapy, but a challenge lies in a need for quality biospecimens.
A general discussion session was chaired by Dr. Patricia Beckmann (CDRD) and Dr. Jim Richards (NRC) asking the question, ‘What is needed to move biomarkers forward?’ Comments from the audience included the need for biomarker validation, a national facility, increased funding, more exchange of staff between industry and academia, and better access to information for academics about the transfer process, licensing, and commercialization. Mentorship to support the progression of vaccine research from concept to trials is critical for most researchers. There is also a need for validated assays to support regulation, particularly in validating biomarkers discovered in early stage research. Often, SMEs do not have capacity for this, therefore funding or collaboration is essential to translate biomarkers.
Conclusions
This workshop brought together representatives from industry, academia, government, and regulatory bodies to discuss the challenges and path forward for vaccine biomarkers. Several themes emerged from the 2 day discussions, including the need for vaccine specific biomarkers of vaccine safety and immunogenicity, biomarkers of vaccine efficacy, predictive biomarkers, improved immune monitoring, and biomarker assay validation. In conclusion, efforts to bridge gaps between researchers, industry, government, mentors, partners, and regulators are pivotal to bringing validated biomarkers to support vaccine safety and efficacy studies. Collaborative efforts toward next generation vaccine biomarkers will ultimately accelerate vaccine development and licensure ().
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all of the workshop participants for their informative presentations, thoughtful discussions, and their valued contributions to this meeting report. Finally, we wish to express our appreciation to the entire organization committee and secretariat for all of their efforts facilitating this workshop.
Funding
We thank Pfizer and Medicago for generously sponsoring this workshop, as well as the National Research Council Canada, Canadian Institutes of Health Research, and the Public Health Agency of Canada for their contributions.
References
- Virot P. 10 Facts on Immunization (World Health Organization). 2012
- Krishnan L, Twine S, Gerdts V, Barreto L, Richards JC. Canadian Adjuvant Initiative Workshop, March 26-27, 2013–Ottawa, Canada. Hum Vaccin Immunother 2014; 10:519-26; PMID:24192752; http://dx.doi.org/10.4161/hv.26972
- Qiu X, Fernando L, Alimonti JB, Melito PL, Feldmann F, Dick D, Stroher U, Feldmann H, Jones SM. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. Plos One 2009; 4:e5547; PMID:19440245; http://dx.doi.org/10.1371/journal.pone.0005547
- Qiu X, Fernando L, Melito PL, Audet J, Feldmann H, Kobinger G, Alimonti JB, Jones SM. Ebola GP-specific monoclonal antibodies protect mice and guinea pigs from lethal Ebola virus infection. PLoS Negl Trop Dis 2012; 6:e1575; PMID:22448295; http://dx.doi.org/10.1371/journal.pntd.0001575
- Qiu X, Audet J, Wong G, Pillet S, Bello A, Cabral T, Strong JE, Plummer F, Corbett CR, Alimonti JB, et al. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med 2012; 4:138ra81; PMID:22700957; http://dx.doi.org/10.1126/scitranslmed.3003876
- Katz R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx 2004; 1:189-95; PMID:15717019; http://dx.doi.org/10.1602/neurorx.1.2.189
- Chun S, Li C, Van Domselaar G, Wang J, Farnsworth A, Cui X, Rode H, Cyr TD, He R, Li X. Universal antibodies and their applications to the quantitative determination of virtually all subtypes of the influenza A viral hemagglutinins. Vaccine 2008; 26:6068-76; PMID:19007587; http://dx.doi.org/10.1016/j.vaccine.2008.09.015
- Li C, Jaentschke B, Song Y, Wang J, Cyr TD, Van Domselaar G, He R, Li X. A simple slot blot for the detection of virtually all subtypes of the influenza A viral hemagglutinins using universal antibodies targeting the fusion peptide. Nature Protocols 2010; 5:14-9; PMID:20010723; http://dx.doi.org/10.1038/nprot.2009.200
- Ryden P, Twine S, Shen H, Harris G, Chen W, Sjostedt A, Conlan W. Correlates of protection following vaccination of mice with gene deletion mutants of Francisella tularensis subspecies tularensis strain, SCHU S4 that elicit varying degrees of immunity to systemic and respiratory challenge with wild-type bacteria. Mol Immunol 2013; 54:58-67; PMID:23201853; http://dx.doi.org/10.1016/j.molimm.2012.10.043
- Cowley SC, Elkins KL. Multiple T cell subsets control Francisella tularensis LVS intracellular growth without stimulation through macrophage interferon gamma receptors. J Exp Med 2003; 198:379-89; PMID:12885873; http://dx.doi.org/10.1084/jem.20030687
- Twine S, Shen H, Harris G, Chen W, Sjostedt A, Ryden P, Conlan W. BALB/c mice, but not C57BL/6 mice immunized with a DeltaclpB mutant of Francisella tularensis subspecies tularensis are protected against respiratory challenge with wild-type bacteria: association of protection with post-vaccination and post-challenge immune responses. Vaccine 2012; 30:3634-45; PMID:22484348; http://dx.doi.org/10.1016/j.vaccine.2012.03.036
- Whitfield DM, Eichler EE, Sprott GD. Synthesis of archaeal glycolipid adjuvants-what is the optimum number of sugars? Carbohydrate Res 2008; 343:2349-60; http://dx.doi.org/10.1016/j.carres.2008.06.021
- Sprott GD, Dicaire CJ, Cote JP, Whitfield DM. Adjuvant potential of archaeal synthetic glycolipid mimetics critically depends on the glyco head group structure. Glycobiol 2008; 18:559-65http://dx.doi.org/10.1093/glycob/cwn038
- Krishnan L, Gurnani K, Dicaire CJ, van Faassen H, Zafer A, Kirschning CJ, Sad S, Sprott GD. Rapid clonal expansion and prolonged maintenance of memory CD8+ T cells of the effector (CD44highCD62Llow) and central (CD44highCD62Lhigh) phenotype by an archaeosome adjuvant independent of TLR2. J Immunol 2007; 178:2396-406; PMID:17277146; http://dx.doi.org/10.4049/jimmunol.178.4.2396
- Gerdts V, Babiuk LA, van Drunen Littel-van den H, Griebel PJ. Fetal immunization by a DNA vaccine delivered into the oral cavity. Nature Med 2000; 6:929-32; PMID:10932233; http://dx.doi.org/10.1038/78699
- Gerdts V, Snider M, Brownlie R, Babiuk LA, Griebel PJ. Oral DNA vaccination in utero induces mucosal immunity and immune memory in the neonate. J Immunol 2002; 168:1877-85; PMID:11823522; http://dx.doi.org/10.4049/jimmunol.168.4.1877
- Levast B, Schulz S, Hurk S, Gerdts V. Animal models for neonatal diseases in humans. Vaccine 2013; 31:2489-99; PMID:23238328; http://dx.doi.org/10.1016/j.vaccine.2012.11.089
- Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity 2010; 33:516-29; PMID:21029962; http://dx.doi.org/10.1016/j.immuni.2010.10.006
- Brookes RH, Hakimi J, Ha Y, Aboutorabian S, Ausar SF, Hasija M, Smith SG, Todryk SM, Dockrell HM, Rahman N. Screening vaccine formulations for biological activity using fresh human whole blood. Hum Vaccin Immunother 2014; 10:1129-35; PMID:24401565; http://dx.doi.org/10.4161/hv.27657
- Aboutorabian S, Hakimi J, Boudet F, Montano S, Dookie A, Roque C, Ausar SF, Rahman N, Brookes RH. A high ratio of IC31 adjuvant to antigen is necessary for H4 TB vaccine immunomodulation. Hum Vaccin Immunother 2015; 11(6):1449-55; PMID:25997147
- Bula CJ, Ghilardi G, Wietlisbach V, Petignat C, Francioli P. Infections and functional impairment in nursing home residents: a reciprocal relationship. J Am Geriatr Soc 2004; 52:700-6; PMID:15086648; http://dx.doi.org/10.1111/j.1532-5415.2004.52205.x
- Vasto S, Colonna-Romano G, Larbi A, Wikby A, Caruso C, Pawelec G. Role of persistent CMV infection in configuring T cell immunity in the elderly. Immun Ageing 2007; 4:2; PMID:17376222; http://dx.doi.org/10.1186/1742-4933-4-2
- Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Reviews Medical Virol 2009; 19:47-56; http://dx.doi.org/10.1002/rmv.598
- Johnstone J, Parsons R, Botelho F, Millar J, McNeil S, Fulop T, McElhaney J, Andrew MK, Walter SD, Devereaux PJ, et al. Immune biomarkers predictive of respiratory viral infection in elderly nursing home residents. PloS One 2014; 9:e108481; PMID:25275464; http://dx.doi.org/10.1371/journal.pone.0108481
- Ulloa-Montoya F, Louahed J, Dizier B, Gruselle O, Spiessens B, Lehmann FF, Suciu S, Kruit WH, Eggermont AM, Vansteenkiste J, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol 2013; 31:2388-95; PMID:23715562; http://dx.doi.org/10.1200/JCO.2012.44.3762
- Kruit WH, Suciu S, Dreno B, Mortier L, Robert C, Chiarion-Sileni V, Maio M, Testori A, Dorval T, Grob JJ, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organization for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol 2013; 31:2413-20; PMID:23715572; http://dx.doi.org/10.1200/JCO.2012.43.7111
- McGray AJ, Hallett R, Bernard D, Swift SL, Zhu Z, Teoderascu F, Vanseggelen H, Hassell JA, Hurwitz AA, Wan Y, et al. Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumor. Mol Therapy 2014; 22:206-18; http://dx.doi.org/10.1038/mt.2013.255
- McGray AJ, Bernard D, Hallett R, Kelly R, Jha M, Gregory C, Bassett JD, Hassell JA, Pare G, Wan Y, et al. Combined vaccination and immunostimulatory antibodies provides durable cure of murine melanoma and induces transcriptional changes associated with positive outcome in human melanoma patients. Oncoimmunology 2012; 1:419-31; PMID:22754760; http://dx.doi.org/10.4161/onci.19534