Abstract
The introduction of pneumococcal conjugate vaccine (PCV) into the South African public immunization program since 2009 adopted a novel vaccination schedule of 3 doses at 6, 14 and 40 weeks of age. Over the past 5 y it has been shown that infant PCV immunization in South Africa is effective in reducing the burden of invasive pneumococcal disease (IPD) among HIV-infected and HIV-uninfected children. Furthermore, indirect protection of unvaccinated age-groups (including high risk groups such as HIV-infected adults) against IPD was demonstrated despite the absence of any substantial catch-up campaign of older children. This indirect effect against IPD is corroborated by the temporal reduction in vaccine-serotype colonization among age-groups targeted for PCV immunization as well as unvaccinated HIV-infected and HIV-uninfected adults, which was evident within 2 y of PCV introduction into the immunization program. Vaccine effectiveness has also been demonstrated in children against presumed bacterial pneumonia. The evaluation of the impact of PCV in South Africa, however, remains incomplete. The knowledge gaps remaining include the evaluation of PCV on the incidence of all-cause pneumonia hospitalization among vaccinated and unvaccinated age-groups. Furthermore, ongoing surveillance is required to determine whether there is ongoing replacement disease by non-vaccine serotypes, which could offset the early gains associated with the immunization program in the country.
Introduction
The estimated annual number of global under-5 childhood deaths attributed to Streptococcus pneumoniae has reportedly declined between the years 2000 (n = 826,000, approximately 90% due to pneumonia) and 2010 (n = 411,000 pneumococcal pneumonia deaths).Citation1,2 Nevertheless, pneumococcal disease still remains a leading vaccine-preventable cause of childhood mortality, with the majority (43%) of deaths in 2010 occurring in Africa, despite it constituting only 23% of the global under-5 childhood population.Citation2 Whereas invasive pneumococcal disease (IPD) has a higher case fatality ratio than non-bacteraemic pneumococcal pneumonia, they account for approximately 10% and 90% of all pneumococcal deaths, respectively.Citation1
The recommendation by the World Health Organization (WHO) in 2003 for the inclusion of pneumococcal conjugate vaccines (PCV) into public immunization programs of all countries with a high infant mortality rate (>50 per 1000 live births), or those with greater than 50,000 childhood deaths per year or in settings with a high prevalence of pediatric HIV infection,Citation3 were largely premised on the evidence generated in 2 vaccine efficacy trials of an investigational 9-valent PCV (PCV9) undertaken in South Africa and The Gambia.Citation4,5 These studies evaluated a 3 dose primary series scheduled at 6, 10 and 14 weeks of age in the absence of a booster dose. The observed vaccine efficacy (VE) among HIV-uninfected children in these studies against PCV9-serotype IPD was 78%–83% (Table 1) and 20%–37% against radiologically confirmed pneumonia per WHO recommended interpretation criteria (CXR-AC).Citation4,5 Furthermore, the study from South Africa demonstrated a 65% reduction of PCV9-serotype IPD in vaccinated HIV-infected children followed for 2.3 y This vaccine efficacy, however, declined to 38% (P > 0.05) by 5 y of age among the HIV-infected children (Table 1), who generally were not treated with antiretroviral therapy (ART).Citation6 Although the efficacy trial did not show any decline in CXR-AC in PCV9-vaccinated HIV-infected children,Citation4 possibly due to the lower specificity of this endpoint as a marker of pneumococcal pneumonia in HIV-infected children,Citation7 there was a 15% (95%CI: 5%–24%) reduction in all-cause clinical pneumonia hospitalization among these children.Citation8 Furthermore, the vaccine attributable reduction (per 100,000 vaccinated children) for clinical pneumonia hospitalizations was approximately 9-fold greater among HIV-infected (2,302) compared to HIV-uninfected children (267).Citation8
The Gambian PCV9 efficacy trial also reported 16% (95%CI: 2%–38%) reduction in all-cause mortality.Citation5 In South Africa, 66% of all deaths were attributable to lower-respiratory tract infection (LRTI) and there were fewer overall deaths (5% difference) among PCV9-recipients, however, this difference was not significant.Citation4 Furthermore, 73% of deaths in South Africa involved HIV-infected children, among whom PCV9-recipients experienced 6% (P > 0.05) fewer deaths.Citation4 In a post hoc analysis, the vaccine efficacy against CXR-AC and all-cause death in The Gambia, did not differ based on the first dose being administered at 6 or 10 weeks of age and/or whether there was a one or 2 month interval between PCV doses.Citation9
Although the roll-out of PCV into immunization programs of low-middle income countries has lagged behind high-income countries, this delay is reduced compared to the previous roll-out of Haemophilus influenzae type b conjugate vaccine.Citation10 By 2015, 50 of 72 low-income countries had either introduced or had been approved to introduce PCV into their public immunization programs, supported financially by GAVI and with a nominal country co-payment for vaccine procurement.Citation11 The sustainability of PCV immunization in low-income countries in the absence of GAVI financial support, will largely depend on convincing policy-makers regarding the effectiveness of PCV in reducing childhood morbidity and mortality.
The financial strain that the continuation of childhood PCV immunization might have on low-income countries in the absence of external funding support is evident from cost-effectiveness studies undertaken in some of these countries. In Kenya, the introduction of either the 10-valent (PCV10) or 13-valent PCV (PCV13) was considered cost-effective at the GAVI procurement price of $3.50 USD per dose, although the break-even price for the introduction of PCV10 and PCV13 were $0.41 USD and $0.51 USD, respectively. This break-even cost could, however, be higher (i.e. improved cost-effectiveness ratios) by 43%–56% should indirect protection be observed among the unvaccinated population.Citation12 Importantly, such cost-effectiveness analysis also need to be contextualized to the actual country expenditure on health. As an example, in The Gambia where the per capita expenditure on health was $32 USD in 2009,Citation13 the introduction of a 3 dose series of PCV (at $7 USD per dose) would increase the overall vaccination cost per fully-vaccinated child from $25 USD to $46 USD.Citation14 As such, the corroborating public-health impact of PCV immunization is warranted in at least some low-middle income countries, to support its ongoing inclusion when faced with competing funding priorities.
PCV effectiveness evaluation, in addition to corroborate the evidence from the efficacy trials, could also unmask vaccine effects which are not necessarily apparent in well controlled vaccine clinical trials that span relatively short time periods and are focussed primarily on study-participants. These issues include: whether there is an increase in non-vaccine serotype pneumococcal disease beyond that observed in the efficacy trials, evaluation of the indirect effect of PCV in the unvaccinated population and the impact of vaccination against pneumonia (which accounts for the majority of severe and fatal pneumococcal disease). To comprehensively address these public-health issues, the evaluation of the impact of PCV against pneumococcal disease needs to be multi-faceted.
Whereas evaluation of vaccine effectiveness through case-control studies is useful for measuring the performance of the vaccine under real-life programmatic settings compared to efficacy evaluation in controlled environments, such studies are prone to potential confounders. Included among such potential confounders are factors that may affect case ascertainment such as healthcare seeking behavior, barriers to care, determinants of hospitalization and diagnostic capacities. Also, different potential sources of control groups should be considered to identify the group least likely to lead to bias in the study and issues such as differences in barriers for vaccination in the cases and controls, as well as the ability to obtain valid, complete data on vaccination status is essential. Furthermore, case-control studies do not necessarily address the overall public-health impact of the vaccines, e.g. impact of PCV in reducing overall incidence of pneumococcal disease or indirect effect of vaccine at the community level. The impact of PCV on the public-health burden of pneumococcal disease has been evaluated by ecological studies, using available health care utilization administrative registries and other vital statistics data, in many high-income settings and a few middle-income countries in Latin America.Citation15-18 These types of data source are seldom available in the majority of low-middle income countries, consequently; alternate approaches are warranted to evaluate the impact of PCV in these countries. This could include ecological studies on pneumococcal nasopharyngeal colonization among vaccinated and unvaccinated (including high risk groups such as HIV-infected adults) populations comparing pre- and post-PCV eras, which could be used to impute the direct and indirect effects of PCV against IPD.Citation19
This manuscript reviews the evaluation of the impact of PCV immunization, and the challenges faced in quantifying the public-health impact in South Africa.
Introduction of PCV into the South African public immunization program
South Africa was the first African country to introduce PCV into its public immunization program in April 2009. Since South Africa is ineligible for financial support from GAVI, the introduction of PCV (and very soon thereafter of rotavirus vaccine) was State funded. Based on national administrative data, the coverage of 3 doses of PCV (using a vaccination schedule of 6, 14 and 40 weeks of age [2+1 schedule]) was estimated to be 10% in 2009, 64% in 2010, 90% in 2011 and 99% in 2012.Citation20 The 7-valent PCV (PCV7) was introduced in 2009, without any catch-up campaign for children older than 6 weeks of age who had already received the other vaccines scheduled at that age. The program transitioned to utilization of PCV13 since May 2011, which was coupled with a limited catch-up campaign for children less than 30 months of age.Citation20
The evaluation of the impact of vaccines against some syndromes in South Africa is assisted by the Government providing free health care to all children, hence reducing impediments to health care access and the fact that all public-health facilities, which provide a service to 80% of the population (and possibly close to 100% of hospitalizations for those not on private medical insurance) are serviced by a single integrated laboratory (National Health Laboratory Service). This enables country wide laboratory based surveillance which has been central to the National Institute for Communicable Diseases tracking national and regional trends in select invasive bacterial diseases since 1999, including IPD.Citation21 This system is, however, sensitive to differences in physician practices for investigating suspected invasive bacterial disease, and the absence of standardised diagnostic algorithms in the country; which could contribute to the regional and temporal differences observed in disease rates.Citation22-25
In addition to the laboratory-based surveillance for invasive bacterial disease, other national collaborative research activities between South African institutions have been undertaken for the evaluation of PCV-effectiveness, including case-control studies against IPD and probable bacterial pneumonia.Citation26,27 Unfortunately, as in most low-middle income countries, there is no electronic registry of hospitalizations in the public sector, which prevents an in-depth analysis of the effect of selected interventions against specific disease syndromes, such as all-cause pneumonia, at a national or regional level. Such a system dating back to 2006 has, however, been established in at least one large urban area of the country (Soweto, Johannesburg). In this area a population of 1.4 million, including 125,000 children under-5 y of age was serviced until 2014 by a single public hospital, i.e., Chris Hani Baragwanath Academic Hospital. Much of the research on PCV, including the initial vaccine efficacy trial, studies on immunogenicity of PCV and the effect of PCV introduction on community-wide nasopharyngeal colonization has been undertaken in this setting.Citation4,28,29
Furthermore, although South Africa is categorized as a middle-income country, Soweto, which is inhabited almost exclusively by Black-Africans, has a high unemployment rate (45%) and high prevalence of HIV-infection (12% in adults nationally, including 29% in pregnant women in Soweto), which is a major contributor to the high burden of IPD in South Africa.Citation30
South African PCV schedule at 6, 10 and 40 weeks of age and comparative immunogenicity studies
The WHO recommendation for the introduction of PCV into public immunization programs advocated for a 3 dose primary series during early infancy, as had been evaluated in the African efficacy trials, or alternately a 2 dose primary series and a booster dose in the second year of life.Citation31 Based on the local epidemiology of IPD coupled with the experience from the PCV9 efficacy trial in South Africa, the National Advisory Group for Immunization in South Africa recommended a 3 dose series during the first year of life, including 2 doses scheduled at 6 and 14 weeks of age and a third (booster) dose at 9 months of age. The rational for this dosing schedule included evidence from the United Kingdom that during infancy a 2 dose primary series spaced at least 2 months apart was more immunogenic than when spaced one month apart,Citation32 and that the 2 dose primary series spaced 2 months apart had similar immunogenicity compared to 3 doses at 2, 3 and 4 months of age.Citation33 Due to cost-constraints a schedule based on 3 dose primary series followed by a booster dose was not considered in South Africa.
In South Africa, the need to offer a booster dose of PCV was specifically aimed at addressing the waning of protection against vaccine-serotype IPD which had been observed among HIV-infected children.Citation6 The lower efficacy of PCV against IPD in HIV-infected compared to HIV-uninfected children, was in agreement with the poorer immunogenicity, and particularly opsonophagocytic activity (OPA), in the HIV-infected children.Citation34 Furthermore, the HIV-infected children at 6 y of age, who were generally naive to ART, also had less persistence of vaccine-serotype capsular antibody and absence of an anamnestic response when re-vaccinated at this stage.Citation6 A further motivation for including a booster dose of PCV, was related to the observation in HIV-uninfected children of possible waning of protection against serotype 1 (included in the investigational PCV9) in South Africa and The Gambia.Citation35 The importance of serotype 1 in Africa is evident with it being the third most common serotype (10% of all) causing IPD in this regionCitation36 including a leading cause of meningitis in some African countries.Citation37
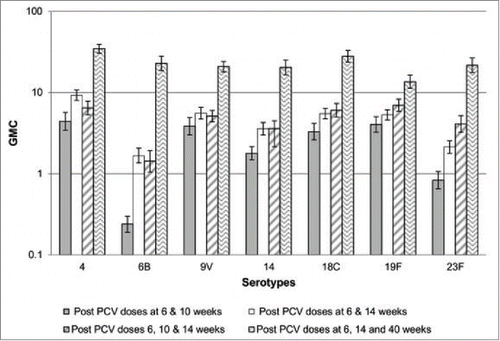
The immunogenicity of the PCV schedule implemented in South Africa was evaluated among HIV-uninfected children subsequent to its introduction and was compared to a historical cohort of children who received 3 doses of PCV7 at 6, 10 and 14 weeks of age.Citation38 The proportion of infants with capsular antibody ≥0.35 microg/ml following the 2nd PCV7-dose on the current schedule ranged from 84% for 6B to ≥89 % for other serotypes, in addition to which robust anamnestic responses (both quantitative and measured by OPA) were observed following the 3rd dose at 40 weeks of age. Furthermore the geometric mean concentrations (GMCs) following the 2nd PCV7-dose in the 6+14 weeks schedule were similar for most serotypes compared to after the 3rd PCV7-dose at 14 weeks of age in the historical cohort; and following the 3rd PCV7 dose given at 40 weeks of age in the 2+1 schedule GMCs were higher for all analyzed serotypes compared to following the 3rd dose at 14 weeks of age in the historical cohort, .Citation38 Theoretically this heightened immunogenicity of the South African dosing schedule could confer longer antibody persistence and duration of protection against pneumococcal disease.
Figure 1. Comparison of Geometric Mean Concentrations following 2 and 3 doses of PCV7 using different immunization schedules in HIV-uninfected children Comparison of antibody GMCs following vaccination with PCV7 administered either at 6, 10 and 14 weeks of age or at 6 and 14 weeks of age with a booster at 40 weeks of age. GMCs were assessed post-second and post-third dose in the series. GMCs: Geometric Mean Concentrations, PCV7: 7-valent Pneumococcal Conjugate Vaccine. Figure taken from.Citation38
A caveat of the South African immunogenicity study was that it had not included the immunogenicity evaluation of the novel schedule in HIV-infected children, the primary group of interest. The reason for the absence of this assessment was that enrolment of HIV-infected infants into clinical studies has become increasingly challenging in South Africa with the successful implementation of strategies aimed at HIV prevention of mother-to-child transmission (PMTCT) in the country.Citation39 Furthermore, there were changes in HIV management, with initiation of ART immediately upon diagnosis of HIV-positivity when screened at approximately 6 weeks of age, was also likely to affect the immunogenicity of PCV in these children compared to those included in earlier studies who generally had ongoing immunological deterioration in the absence of ART. Corroborating the effect of these nuances of HIV management on vaccine immunogenicity, was an evaluation of the immunogenicity of a 3 dose primary series of PCV in the CIPRA-04 study, which showed that HIV-infected children initiated on early ART compared to HIV-unexposed children had similar quantitative and qualitative antibody responses following either the 2nd or 3rd PCV7 dose (PCV7 given at 6, 10 and 14 weeks of age), .Citation40,41 HIV-infected children with CD4+ cell counts ≥25 % at enrolment and in whom ART initiation was delayed until they progressed to clinical AIDS or developed immunosuppression (based on CD4+ cells declining to <20 %) were also evaluated in the CIPRA-04 study; although quantitative antibody response was similar between these children and the HIV-infected children initiated on early ART, , the OPA geometric mean titres were significantly lower (for all 3 analyzed serotypes P ≤ 0.015) if ART initiation was deferred.Citation41 Also, a lower proportion of children in whom ART initiation was delayed had OPA titres ≥8 for 2 of the 3 studied serotypes (88%) compared to those in whom ART was initiated early (>98%; p = 0.003 for both).Citation41
Figure 2. Comparison of Geometric Mean Concentrations following 2 and 3 doses of PCV7 in HIV-uninfected and HIV-infected children (A) Comparison of antibody GMCs following vaccination with PCV7 administered at 6 and 10 weeks of age in HUU, HEU, ART+ and ART- children. (B) Comparison of antibody GMCs following vaccination with PCV7 administered at 6, 10 and 14 weeks of age in HUU, HEU, ART+ and ART- children. GMCs: Geometric Mean Concentrations, PCV7: 7-valent Pneumococcal Conjugate Vaccine; HUU: HIV-uninfected children born to HIV-uninfected mothers; HEU: HIV-uninfected children born to HIV-infected mothers; ART+: HIV-infected children with CD4+ ≥ 25% at enrolment randomized to initiate anti-retroviral treatment (ART) immediately; ART−: HIV-infected children with CD4+ ≥ 25% at enrolment randomized to deferred ART.
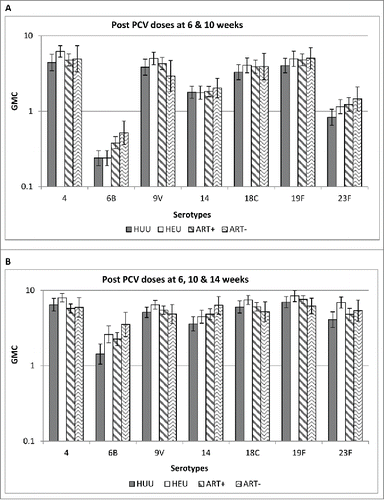
Accordingly, PCV effectiveness against IPD is likely to be higher in HIV-infected children initiated on early ART than was observed in the efficacy trial among ART-naive HIV-infected children and likely to be comparable to that observed among HIV-unexposed children even with the current vaccination schedule stablished in South Africa. Delaying ART initiation among HIV-infected children, in relation to the timing of vaccination might, however, reduce vaccine effectiveness in this group; and lower vaccine effectiveness may be evident in those in whom ART is not initiated and who progress to immune suppression. This illustrates the need for enhancing management of HIV through early ART initiation, preferably started before vaccination to optimise the effectiveness of PCV in preventing IPD among HIV-infected children, who remain at high risk of IPD even when on ART.Citation23
Another group of children evaluated in CIPRA-04 were HIV-uninfected children born to HIV-infected women (i.e. HIV-exposed, uninfected children; HEU). This group also has an increased risk of invasive bacterial disease especially during infancy, including 2.7-fold greater risk of IPD than HIV-unexposed children.Citation42 Although HEU compared to HIV-unexposed children had lower capsular antibody levels prior to the first PCV7-dose, they had higher antibody levels to most serotypes and similar OPA responses following the third PCV7-dose.Citation41 These data suggest that vaccine effectiveness against IPD was likely to be similar in HEU compared to HIV-unexposed children, although this had not been evaluated for in the PCV9 South African efficacy trial.
The CIPRA-04 children and those who participated in the 2+1 schedule study are currently being evaluated for anamnestic responses following a booster dose at 15–18 months of age, as well as persistence of antibodies until 5 y of age (Clinical trials Gov.: NCT00099658). This will inform on the anticipated long-term effectiveness of PCV in these high-risk population groups.
Impact of PCV against IPD in children targeted for vaccination
The effectiveness of PCV against IPD in South Africa has been evaluated in a multi-centered case-control study and an ecological time-series analysis. The multi-centered case-control study, which leveraged on an existing enhanced surveillance structure for invasive disease, relied on attending physician discretion for the investigation for invasive bacterial disease. This study was initiated in March 2010, approximately one year after the introduction of PCV7, and enrolled through November 2012, by when South Africa had transitioned to PCV13.Citation26
Among HIV-uninfected children the vaccine effectiveness against PCV7-serotype IPD was 74% following at least 2 PCV doses in children ≥16 weeks of age (Table 1) and 90% (95% CI: 14%–99%) following 3 doses in children ≥40 weeks of age. Furthermore, there was 96% (95%CI: 62%–100%) reduction of all-serotype multidrug-resistant IPD among HIV-uninfected children who received at least 2 doses of PCV. The vaccine effectiveness against IPD irrespective of serotype among the HIV-uninfected children was, however, not statistically significant with a trend showing increase in non-vaccine-serotype IPD among the vaccinated children following at least 2 PCV doses (Table 1).Citation26
Vaccine effectiveness among HEU was 92% (95% CI: 47%–99%) against PCV7-serotype IPD following at least 2 doses of PCVCitation26 which was consistent with the heightened immunogenicity of PCV observed in these children in the CIPRA-04 study.Citation41 Despite the case-control study enrolling the targeted number (N = 42) of vaccine-serotype IPD cases among HIV-infected children (109 of which 43 [39%] were PCV7-serotype), which provided adequate power to detect 65% vaccine effectiveness against PCV7-serotype IPD, the study was unable to demonstrate significant protection against this endpoint following either ≥2 doses or ≥3 doses of PCV (VE: 57%; 95%CI: −371%–96%) (Table 1). Furthermore, there was no demonstrable vaccine effectiveness against all-serotype IPD, while there was a trend toward increase in non-vaccine serotype IPD following at least 2 doses of PCV in HIV-infected children (Table 1). Notably, the majority of HIV-infected children with IPD in this study were severely immunocompromised, including 90% with stage III/IV Clinical AIDS, and only 26% had been initiated on ART at the time of their IPD episode (compared to 53% of the controls). This highlights the challenges in implementing the recommendations for early ART initiation in HIV-infected infants even in settings such as South Africa, which was shown to be required for optimal vaccine immunogenicity and protection.
More compelling than the case-control study in evaluating the public-health impact of PCV against IPD was a nationwide ecological study using the laboratory-based surveillance program in South Africa.Citation25 The interpretation of ecological studies on the impact of PCV in South Africa needs to be contextualized within the framework of the interplay between IPD and HIV infection. This was illustrated in a study from Soweto, in which the burden of IPD among HIV-infected children had been on a downward trend since implementation of the ART program in 2006, which was temporally associated with a 51% (95%CI: 42%–59%) reduction in overall IPD between 2006 to 2009 among HIV-infected children, with similar magnitude of change for PCV7-serotype and non-PCV7 serotype IPD, while this remained unchanged among HIV-uninfected children.Citation23 Furthermore, a reduction in vertical transmission of HIV from pregnant women to their newborns from 8% in 2006 to <2% since 2009 is likely to have resulted in further decline of overall IPD cases in South Africa,Citation39 where HIV-infected children contributed to 65% of all IPD cases prior to the implementation of the new HIV-targeted interventions.Citation43
During evaluation of the impact of PCV7 on IPD in South Africa, the pre-vaccine period 2005–2008 was compared to 2012 (3 y post-vaccine introduction) and a 69% (95%CI: 65%–72%) decline in the overall IPD rate was observed in children <2 years of age, including 89% (95%CI: 86%–92%) reduction in PCV7-serotypes causing IPD. Furthermore, there was 85% (95%CI: 76%–91%) reduction in serotype 6A IPD and 57% (95%CI: 42%–58%) reduction in IPD due to the other five serotypes included in PCV13. The analysis also revealed a decline in serotype 19A (70%; 95%CI: 55%–88%) and serotype 1 (57%; 95%CI: 16%–79%) IPD within one year of having transitioned from PCV7 to PCV13, whereas there were very few cases of serotypes 3, 5 and 7F for meaningful interpretation.Citation25
Stratification by HIV-infection status in children <2 years of age, showed 85% and 86% reduction in PCV7-serotype IPD among HIV-uninfected and HIV-infected children, respectively (Table 1). The incidence of PCV7-serotype IPD, however, remained 25-fold greater in HIV-infected compared to HIV-uninfected children by 2012. Analyzing whether changes in HIV management might have contributed to the reduction in IPD among the HIV-infected children, detected that there was no significant decline in non-vaccine serotype IPD among HIV-infected children from 2006–2008 compared to 2012 (31%, non-significant reduction) with the absolute difference in point percent reduction in IPD due to PCV7- and non-vaccine serotype being 55 percentage points. This suggests that PCV was likely to have contributed to a 55% reduction in PCV7-serotype IPD among the HIV-infected children, which was similar to that observed in the PCV9 efficacy trial.Citation4 It is, however, possible that the attributable reduction due to PCV might be lower if improved management of HIV might have masked an increase in non-vaccine serotype IPD among the HIV-infected population.
Among the HIV-uninfected children, despite a reduction in serotype 19A disease, a 33% increase in non-PCV13 serotype IPD was observed between the pre-vaccine era and 2012 (Table 1). Although there was no significant increase due to any specific non-vaccine serotype, the total number of non-vaccine serotypes in 2012 (N = 205) exceeded those due to PCV13 serotypes (N=164 cases) in children <2 years of age.Citation25
Together, the case-control and the ecological study support that PCV immunization in South Africa has had a marked effect on reducing the incidence of vaccine-serotype IPD among HIV-uninfected children <2 years of age, which is similar to that observed from many high-income settings with different dosing schedules.Citation16 Ongoing surveillance, however, is required to track the increase in non-vaccine serotype IPD, as the full magnitude of replacement disease due to non-vaccine serotypes may lag behind the more immediate effect of vaccination in reducing vaccine-serotype disease.
The evidence of the impact of PCV immunization in reducing IPD among HIV-infected children is, however, less clear with the inconclusive findings from the case-control study and the limitations in interpreting the decline of vaccine-serotype IPD observed in the ecological study. Furthermore, despite the decline in vaccine-serotype IPD among HIV-infected children, 1115 (78%) of the 1423 IPD cases in children <2 years of age occurred among HIV-infected children in 2012 in the ecological study, including 89% of PCV13-serotypes and 87% of non-PCV13 serotypes.Citation25 Consequently, the residual burden of childhood IPD in the PCV-era in South Africa is fuelled by the ongoing HIV problem, with a greater proportion of IPD cases being HIV-infected compared to prior PCV introduction (65%).Citation23 Notably, the majority of the IPD cases among HIV-infected children in 2012 occurred in those who were less likely to be on ART and who had progressed to severe immune suppression, which as shown above is likely to lessen the efficacy of PCV. This underscores the need for strengthening the ART management of HIV-infected children in South Africa, which could reduce the burden of IPD through reduced susceptibility and improved immunogenicity of the vaccine. Also, further reduction in the rate of vertical HIV transmission due to PMTCT programs in the first instance, would have decrease the size of the at-risk population and contributed to the control of IPD in settings with a high prevalence of maternal HIV infection as in South Africa. An alternate mechanism of protection of HIV-infected children against vaccine-serotype IPD could be through reduced acquisition of vaccine-serotypes, resulting from widespread PCV immunization of young children interrupting transmission of the vaccine-serotypes in the general population.Citation44
Experience of the impact of PCV on pneumonia
The impact of PCV on pneumonia hospitalizations in South Africa has been evaluated using a case-control study,Citation27 in addition to which there is an ongoing ecological study in Soweto that will analyze the temporal association of PCV on all-cause pneumonia hospitalizations from 2006 until 2014. The case-control study was designed to evaluate the effectiveness of at least 2 doses of PCV against presumed bacterial pneumonia. This endpoint was an expanded definition to CXR-AC,Citation45 to include pneumonia hospitalization associated with a CXR infiltrate other than CXR-AC and C-reactive protein (CRP) concentration ≥40 g/dl. This modified case definition was used based on earlier experience from the PCV9 efficacy trial in South Africa, which indicated that the CXR-AC measure underestimated the burden of vaccine-preventable pneumonia in by as much as 63%.Citation46,47 This expanded case-definition was also used in the COMPAS study which evaluated the efficacy of PCV10 in Latin America.Citation48
The vaccine effectiveness in the South African case-control was 20% (95%CI: −9%–42%) in children aged ≥8 weeks who were up-to-date for their age regarding PCV vaccination status, and 39% (95%CI: 9%–60%) among children 16–103 weeks of age (i.e., eligible to have received their second PCV dose). The 95% confidence intervals of the vaccine effectiveness estimates overlapped with that observed in the PCV9 efficacy trial against CXR-AC (VE: 20%; 95%CI: 3%–35%) among HIV-uninfected South African children.Citation4 Although the case-control study included a broader case definition, the majority of cases (78.4%) were nevertheless diagnosed by the CXR-AC criteria, and for which vaccine effectiveness estimates were similar to that of the expanded case-definition. The case-control study was initiated within one year (April 2010) of PCV7 introduction and enrolment was continued until August 2012 during which the program had transitioned to PCV13. Although the study was not powered to address the vaccine effectiveness of either specific formulation, an exploratory analysis did not show much difference in the adjusted vaccine effectiveness of the 2 formulations.Citation27
While the case-control study provides corroborating data to the vaccine efficacy trials for PCV against pneumonia in Africa, and provided the first African vaccine effectiveness estimates of PCV7/13 against pneumonia, questions remain with regard to the public-health impact of PCV against all-cause pneumonia morbidity and mortality in African settings. The CXR-AC endpoint while useful for improving the specificity for identifying pneumococcal pneumonia episodes, only constituted approximately 17%–19% of all-cause pneumonia hospitalizations in the African efficacy trials.Citation4,5 Furthermore, in The Gambian efficacy trial, although pneumonia episodes with CXR-AC were associated with higher case fatality ratio (3.0%) compared to pneumonia cases without CXR-AC features (case fatality ratio 1.1%), 64% of all deaths associated with clinically diagnosed pneumonia occurred among the latter.Citation49,50 Also, although a higher proportion of CXR-AC cases in The Gambia had confirmed pneumococcal etiology (8.3%; which included cases diagnosed from positive blood or lung aspirate cultures), pneumococcus was also identified in pneumonia cases without CXR-AC (8.3% vs. 0.2%, respectively) despite lung aspirates having not been undertaken in these cases.Citation5,50 The majority of the pneumonia cases without CXR-AC in The Gambia with microbiological confirmed bacterial etiology were due to non-typhoid Salmonella sp or bacteria other than pneumococcus.
The Gambian study highlights the challenges faced in addressing the public-health impact of PCV against the syndrome of pneumonia in low-income settings, and specifically in the absence of a sensitive and specific measure for attributing causality to pneumococcus. Although a 16% reduction in all-cause mortality was observed in The Gambian efficacy trial,Citation5 there is limited evidence from any low-income country to demonstrate a temporal associated reduction in all-cause pneumonia mortality and PCV immunization. The multiple causes of pneumonia, including bacterial and/or viral pathogens, might in part contribute to this lack of association, more so since respiratory viruses with or without bacterial co-infections are likely to be responsible for the majority of severe pneumonia cases.Citation51,52
The absence of data on the impact of PCV against pneumonia mortality is in contrast to the experience with rotavirus vaccine introduction into immunization programs. Rotavirus was established to be the major pathogen responsible for cases of severe diarrhea (33%–49% of all hospitalized cases) prior to the introduction of rotavirus immunization.Citation53 The importance of rotavirus to childhood diarrheal associated-mortality has now been corroborated by a number of studies from middle-income Latin American countries which demonstrated a 22%–50% reduction in all-cause diarrhea mortality, in addition to a 49%–92% reduction in rotavirus confirmed-diarrhea hospitalization that was temporally associated with rotavirus vaccine immunization.Citation54 Hence although PCV immunization in some middle-income Latin American countries and high-income settings has been associated with an approximately one-third (15%–65%) decline in all-cause pneumonia hospitalization,Citation15-19 which itself exceeds the expectations from the more modest 6%–9% vaccine efficacy against clinical pneumonia observed in the efficacy trials, the impact of PCV on pneumonia mortality once implemented into public immunization programs remains to be established. Although a study from Nicaragua reported a 23% decline in all-cause infant mortality which was temporally associated with PCV introduction, the greatest reduction occurred during the neonatal period which is unlikely to have been due to pneumococcal disease.Citation55
Another gap in evidence of the effect of PCV immunization against pneumonia in Africa is its effectiveness against pneumonia in HIV-infected children, who have a 6.5-fold greater risk for all-cause pneumonia hospitalization than HIV-uninfected children.Citation56 Although the South African case-control study aimed to evaluate the effectiveness of PCV against pneumonia in HIV-infected children, the study was unsuccessful in recruiting sufficient numbers of pneumonia cases to evaluate vaccine effectiveness. Moreover the ecological study currently underway in Soweto will also be unlikely to address the prevention of all-cause pneumonia among HIV-infected children, in whom there is a broader repertoire of pathogens causing pneumonia than in HIV-uninfected children,Citation57 which too will be affected by changes in HIV management that have occurred concurrently with the roll-out of PCV in South Africa.Citation58
Indirect effect of PCV in HIV-infected and HIV-uninfected individuals
From a public-health perspective, the direct effect of preventing pneumococcal disease among children targeted for vaccination has been overwhelmingly surpassed by the indirect effect of vaccination in reducing pneumococcal disease among the unvaccinated population in high-income countries.Citation59 This indirect effect of PCV is mediated through immunization of young children resulting in them having a reduced risk of acquisition of vaccine-serotypes with a resultant interruption in transmission of these serotypes in the community. Consequently PCV immunization of children has been temporally associated with a reduction in vaccine-serotype IPD and pneumonia hospitalization in adults commonly in contact with children, as well as other unvaccinated age-groups.Citation59,60 In the USA, up to 10 times more cases of IPD and 2–3 times more cases of pneumococcal pneumonia hospitalizations are being prevented from the indirect effect of PCV compared to cases directly averted in children targeted for vaccination.Citation59
The indirect effect of PCV in low-middle income countries was recently corroborated by the ecological study from South Africa. Included among this were reductions in overall-IPD among children too old to have been vaccinated aged 5–9 y (reduction: 44%; 95%CI: 33%–54%) and adults 15–24 y old (reduction: 30%; 95%CI: 15%–42%), 25–44 y old (reduction: 34%; 95%CI: 29%–39%) and those 45–64 y old (reduction: 14%; 95%CI: 3%–23%). Among the adults 25–44 y of age, the decline was driven predominantly by a reduction in PCV7-serotype disease (reduction: 57%; 95%CI: 50%–63%), as well as reductions in the additional serotypes included in PCV13, whereas non-vaccine serotype IPD remained unchanged between 2006–2008 compared to 2012. These reductions in PCV-serotype IPD were observed in HIV-infected and HIV-uninfected adults 25–44 y old. By 2012 there was a 40 percentage point difference in decline of PCV7-serotype compared to the decline of non-vaccine serotype IPD among the HIV-infected adults suggesting that much of the overall decline in IPD among the HIV-infected adults was due to an indirect effect of infant PCV immunization. The South African study also showed an indirect effect in infants <10 weeks of age, who at most would have only received a single dose of PCV. Whereas the incidence of non-PCV13 serotype IPD in these children remained unchanged from 2006–2008 compared to 2012, there was an approximately 80% reduction in PCV7 and serotype 6A IPD in 2012 compared to the pre-vaccine period.Citation23 Even though non-PCV13 serotype IPD had increased in children <2 years of age by 2012, and particularly so among HIV-uninfected children, the incidence of non-PCV13 serotype IPD remained unchanged among adults age 25–44 y (including among HIV-infected adults). In contrast, over a similar observation period in the USA (3 y post-PCV7 introduction), whereas there was a 62% reduction in PCV7-serotype disease, a 44% increase in non-vaccine serotype IPD was observed among HIV-infected adults, resulting in a net reduction in overall IPD of a more modest 19%.Citation61
PCV and nasopharyngeal colonization in South Africa
Pneumococcal nasopharyngeal acquisition is a precursor for disease and therefore a decrease in the acquisition of new virulent serotypes reduces the risk of disease at an individual level. The measurement of the effects of PCV on pneumococcal colonization has been proposed as a proxy for the expected direct protection against IPD and mucosal infections and also to provide information on PCV-induced protection at the population level by indirect effects.Citation19 Cross-sectional studies evaluating the effect of PCV on carriage require smaller samples sizes than when disease is used as an endpoint, and to approach this issue both community-randomized trials or pre- and post-PCV observational studies evaluating the rates of pneumococcal carriage in targeted and non-targeted groups have been performed.Citation62
The first evidences that PCV vaccination reduced the risk of nasopharyngeal acquisition of vaccine-serotype in children emanated from studies in The Gambia and South Africa, which showed approximately 50% lower prevalence in vaccine-serotype colonization at 9 months of age among PCV-vaccinated children.Citation29,63 A number of clinical trials and ecological studies have since been undertaken in high and some low-middle income countries, which have corroborated the association between PCV immunization of children and the decline in vaccine-serotype colonization among the age-groups targeted for vaccination. Furthermore, a reduction in vaccine-serotype colonization has also been observed among the general population in age-groups not targeted for PCV immunization. The majority of these ecological studies have been undertaken in settings where there was a catch-up campaign of PCV vaccination of older children or as part of demonstration projects.Citation62
Evaluations of the impact of PCV on colonization and different immunization strategies from Africa include studies from The Gambia, Kenya and South Africa; where different dosing schedules, different immunization strategies and a diversity of populations have been evaluated.Citation28,64–66 In a Gambian study all children <30 months of age received PCV7, in addition to which villages in which they lived were randomized for all other older children and adults to receive either PCV7 (vaccinated villages) or meningococcal serogroup C conjugate vaccine (control villages).Citation65 Twenty months post-vaccine introduction, a significant decrease was observed in PCV7-serotype colonization prevalence among all age-groups in both vaccinated and control villages. This included reductions in PCV7-serotype colonization among children 2–5 y old (vaccinated age-group) of 56% (from 54% at baseline to 24% at 22 months post-vaccination) in children from control villages and 74% (from 50% to 13%) in those from the vaccinated villages. Among older age-groups living in the control villages the reductions at 22 months post-vaccination in PCV7-serotype colonization were 57% (from 34.7% to 14.8%) for those 5-<15 years old and 54% (from 16.7% to 7.6%) for those 15 y old or older. The corresponding reductions for the individuals from the vaccinated villages were 78% (from 28.0% to 6.1%) for older children and from 15.9% to 0% for adults. These data indicated that community-wide vaccination of older individuals had an additional effect in interrupting transmission of PCV7-serotypes in a setting such as The Gambia where there was a high prevalence of PCV7-serotype colonization even among older individuals. The findings of this study may, however, have been confounded by a mass campaign of azithromycin administration targeting trachoma elimination to all individuals older than 6 months of age (excluding pregnant women). This could also have led to the unexpected reduction observed in non-vaccine serotype colonization in individuals ≥15 years of age (from 41% at baseline to 29.2%) although non-PCV7 serotype colonization prevalence remained unchanged in the other age-groups.Citation65
In Kenya, PCV10 immunization included a 3 dose primary series (6, 10, 14 weeks of age) targeting infants and additional outreach campaigns of up to 2 doses for children 12–59 months old.Citation64 Annual cross-sectional carriage studies were performed in the 2 y before and 2 y after PCV10 introduction. The prevalence of PCV10-serotype colonization among children <5 years old declined from 34% in the pre-vaccination period to 13% (64% reduction; 95%CI: 49%–74%) in the PCV10 period, and from 8% to 4% among individuals aged 5 y or older (66% reduction; 95%CI: 38%–82%). Significant increases in non-vaccine serotype colonization from pre-PCV10 (41%) to post-PCV10 (57%) were only detected in the <5 years old. The observed reductions in PCV10-serotype colonization in children and in older individuals were detected within one-year of PCV10 introduction, when vaccine coverage with at least one dose in children <5 years age was 69%, indicating substantial indirect effect with only 2-thirds of the targeted population being vaccinated.Citation64
Differing from the Kenyan and The Gambian studies, PCV immunization in South Africa was limited only to infants using the 2+1 vaccine schedule (6, 14 and 40 weeks of age), without any catch-up campaign of older children. In South Africa, 2 cross-sectional surveys were undertaken in a rural community from May to October in 2009 (Period-1) which coincided with the introduction of PCV7 and from May to October in 2011 (Period-2).Citation66 In this high HIV prevalence setting, nasopharyngeal swabs were collected from the index child and other household members in households with at least 1 child <2 years of age. From Period-1 to Period-2 the prevalence of PCV7-serotype colonization declined by 50% (95%CI: 41%–58%) in the age-group <2 years (from 45.1% to 23.5%), by 21% (95%CI: 1%–37%) in those 2–5 y old (from 35.5% to 28.7%), by 34% (95%CI: 8%–52%) in those 6–12 y old (from 19.0% to 12.6%) and by 64% (95%CI: 26%–82%) in adults (from 3.0% to 1.1%). The prevalence of PCV7-serotype colonization also decreased from 10.2% to 5.4% (P ≤ 0.001) by Period-2 among the primary caregivers of the young children. The reduction in PCV7-serotype colonization observed in this setting, occurred while only 51.5% of the targeted population (children <1 year age) being fully vaccinated, indicating that despite a high prevalence of PCV7-serotype colonization among older children, the children <2 years of age are the primary source of vaccine serotypes transmission in the community.Citation67 These results are in agreement with the decline of vaccine-serotype IPD observed in the ecological study performed in the country.Citation25
Another observation from this study included the expected increase (35%; 95%CI: 17%–56%) in the prevalence of non-PCV7 serotype colonization among <2 year old children, although there was an unexpected declined (45%–54% reduction) among adolescents and adults.Citation66 This reduction in non-vaccine serotype colonization among the adults, could be due to a lag in replacement colonization by the non-vaccine serotypes in these individuals, as well as possibly related to changes in HIV management which may affect pneumococcal colonization. Further surveillance is underway to address this trend in non-vaccine serotype colonization in this population.
A further study in South Africa involved specifically addressing the effect of childhood PCV immunization against colonization in HIV-infected and HIV-uninfected mother-child pairs.Citation28 The focus on HIV-infected women, rather than the general population of HIV-infected adults, was premised on them being particularly susceptible to PCV7-serotype IPD.Citation68 Taking advantage of the transition from PCV7 to PCV13 in 2011, the temporal changes in colonization in PCV13-serotypes were evaluated between the PCV7-era (2010) and the PCV13-era (2012).Citation28 This study undertaken in Soweto showed that PCV13-serotype colonization decreased from 37% to 13% within one-year of transitioning to PCV13 (reduction: 68%; 95%CI: 60%–75%). These reductions were observed among HIV-infected (from 38.3% to 19.8%) and HIV-uninfected children (from 37% to 13%), as well as in individual age-groups of <9 months (significant in HIV-uninfected children), 9–24 months, >24-48 months and >48-144 months (significant in HIV-infected children). Furthermore, among the mothers reductions in PCV13-serotype colonization were observed in HIV-uninfected women from 5.4% to 2.0% (reduction of 66%) and in the HIV-infected women from 8.7% to 4.8% (reduction of 37%). However, there was an increase from 4.5% to 7.7% in non-PCV13 serotype colonization in HIV-uninfected women (adjusted OR: 1.6; 95%CI: 1.0–2.5), there was a decline in HIV-infected women from 12.4% to 9.0% (adjusted OR: 0.7; 95%CI: 0.5–1.0). This difference in the reduction of colonization by non-PCV13 serotypes could be due to a differential impact of childhood PCV13 immunization on the dynamics of pneumococcal colonization, including a modest effect on PCV13 colonization in HIV-infected women, or due to changes in HIV management of these women.Citation28
The CIPRA-04 cohort in South Africa was also used to evaluate the relative effect of PCV7 immunization on pneumococcal colonization among HIV-unexposed, HEU and HIV-infected children. In this study, which included 8 sampling points from 6 weeks until 24 months of age, PCV7-immunized HIV-infected (independent of when ART was initiated) and HEU children had similar prevalence of PCV7-serotype colonization compared to HIV-unexposed children throughout the study.Citation69 The prevalence of non-PCV7 serotype colonization (and consequently overall pneumococcal colonization) were, however, consistently lower among the HIV-infected children throughout the study.Citation69 This suggests that in the presence of cotrimoxazole prophylaxis and timeous ART management of HIV-infected children, there may be less replacement disease by non-vaccine serotypes attributable to increase in nasopharyngeal acquisition. This is in part corroborated in the South African ecological study, where among children <2 years of age there was an increase in non-vaccine serotype IPD following PCV introduction in HIV-uninfected children, while the incidence of non-vaccine serotype IPD remained unchanged in HIV-infected children.Citation25
Safety of PCV in South Africa
One of the major gaps in immunization practices in low-middle income countries, including South Africa, is the absence of robust surveillance systems to monitor for safety of vaccines post-licensure. Initial safety evaluation of PCV9 in South Africa was undertaken in the immunogenicity studies, in which the vaccine local and systemic adverse event profile was modest.Citation29 This was corroborated in the subsequently PCV9 efficacy trial.Citation4 The large (n = 39,836) phase III trial which included surveillance for all-cause hospitalization, however, identified a greater incidence of generalized seizures in PCV9 (n = 35) than placebo recipients (n = 19; p = 0.04), but lower incidence of “unspecified” seizures among PCV9-recipients (9 vs 21 cases, p = 0.04). Overall, there was, however, no net significant difference in any seizure type between PCV9- and placebo-recipients. A further observation was a greater incidence of hospitalization for viral (especially respiratory syncytial virus) associated pneumonia within 8 d of vaccination (p = 0.03), and higher rate of hospitalization for hyper-reactive airway disease/asthma (Relative risk 1.79, p = 0.009) among PCV9-recipients. The heightened risk of asthma persisted in PCV9-recipients older than one-year of age, with an overall absolute risk of 2.96 per 1000 compared to 1.66 per 1000 in placebo recipients.
Conclusion
Vaccination of infants with a novel 2+1 immunization schedule in South Africa has been associated with direct and indirect protection against IPD among HIV-uninfected and HIV-infected individuals. This is corroborated by the temporal decline in vaccine-serotype colonization associated with the PCV immunization program, including in HIV-infected adults. The indirect effect against IPD is likely to improve the cost-benefit ratio of PCV immunization and support the Government's ongoing funding of PCV in the country. Furthermore, this immunization schedule has also now been established to protect against presumed bacterial pneumonia.
The evaluation of the impact of PCV in South Africa, however, remains incomplete. Included among the knowledge gaps is the impact of vaccination on incidence of all-cause pneumonia hospitalization among vaccinated and unvaccinated age-groups. This is unfortunate, since the majority of severe pneumococcal disease averted through the indirect effect in a country such as the USA has been shown to be non-bacteremic pneumonia.Citation59 Furthermore, continued surveillance on community-wide colonization studies, as well as IPD is required to determine whether the initial gains of PCV in reducing vaccines-serotype IPD may be offset by an increase in non-vaccines-serotype IPD. This should include molecular studies to determine whether such replacement disease from non-vaccine serotypes are due to capsular switch induced under immunological pressure of vaccination, or the emergence of novel strains encapsulated by non-vaccine serotypes. Also, currently under investigation in South Africa is to determine whether by measuring changes in vaccine-serotype and non-vaccine serotype colonization among vaccinated and unvaccinated age-groups, could be used as a proxy measure for imputing the effectiveness of PCV in preventing IPD as proposed by Weinberger et al.Citation19 Furthermore, studies are also underway to determine what effect childhood PCV vaccination could have on colonization by other potentially pathogenic bacteria such as Haemophilus influenzae, Staphylococcus aureus, Moraxella catarrhalis and Neisseria sp. in PCV-vaccinated and –unvaccinated age-groups. Also, greater emphasis needs to be placed on establishing sentinel surveillance sites to monitor for vaccine safety, including exploring the associations of PCV vaccination possibly increasing susceptibility to asthma and whether this effect is transient or enduring into later life.
Table 1. Vaccine efficacy and effectiveness of pneumococcal conjugate vaccine in South African HIV-infected and HIV-uninfected children against invasive pneumococcal disease
Disclosure of Potential Conflicts of Interest
SAM has received grant support, an honorarium and has been a clinical investigator on studies related to pneumococcal vaccine from Pfizer and GSK.
Funding
The authors have partial support from the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation in Vaccine Preventable Diseases; and the Medical Research Council: Respiratory and Meningeal Pathogens Research Unit. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of their institutions organizations or their sponsors.
References
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374(9693):893-902; PMID:19748398; http://dx.doi.org/10.1016/S0140-6736(09)61204-6
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet 2013; 381(9875):1405-16; PMID:23582727; http://dx.doi.org/10.1016/S0140-6736(13)60222-6
- Progress in introduction of pneumococcal conjugate vaccine–worldwide, 2000-2008. MMWR Morb Mortal Wkly Rep 2008; 57(42):1148-51; PMID:18946462
- Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 2003; 349(14):1341-8; PMID:14523142; http://dx.doi.org/10.1056/NEJMoa035060
- Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, Oluwalana C, Vaughan A, Obaro SK, Leach A, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 2005; 365(9465):1139-46; PMID:15794968; http://dx.doi.org/10.1016/S0140-6736(05)71876-6
- Madhi SA, Adrian P, Kuwanda L, Jassat W, Jones S, Little T, Soininen A, Cutland C, Klugman KP. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine 2007; 25(13):2451-7; PMID:17023095; http://dx.doi.org/10.1016/j.vaccine.2006.09.019
- Madhi SA, Cumin E, Klugman KP. Defining the potential impact of conjugate bacterial polysaccharide-protein vaccines in reducing the burden of pneumonia in human immunodeficiency virus type 1-infected and -uninfected children. Pediatr Infect Dis J 2002; 21(5):393-9; PMID:12150175; http://dx.doi.org/10.1097/00006454-200205000-00009
- Madhi SA, Kuwanda L, Cutland C, Klugman KP. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Infect Dis 2005; 40(10):1511-8; PMID:15844075; http://dx.doi.org/10.1086/429828
- Mackenzie GA, Bottomley C, van Hoek AJ, Jeffries D, Ota M, Zaman SM, Greenwood B, Cutts F. Efficacy of different pneumococcal conjugate vaccine schedules against pneumonia, hospitalisation, and mortality: re-analysis of a randomised trial in the Gambia. Vaccine 2014; 32(21):2493-500; PMID:24631086; http://dx.doi.org/10.1016/j.vaccine.2014.02.081
- Ojo LR, O'Loughlin RE, Cohen AL, Loo JD, Edmond KM, Shetty SS, Bear AP, Privor-Dumm L, Griffiths UK, Hajjeh R. Global use of Haemophilus influenzae type b conjugate vaccine. Vaccine 2010; 28(43):7117-22; PMID:20691265; http://dx.doi.org/10.1016/j.vaccine.2010.07.074
- http://www.gavi.org/support/nvs/pneumococcal/. Accessed 15 June 2105.
- Ayieko P, Griffiths UK, Ndiritu M, Moisi J, Mugoya IK, Kamau T, English M, Scott JA. Assessment of health benefits and cost-effectiveness of 10-valent and 13-valent pneumococcal conjugate vaccination in Kenyan children. PLoS One 2013; 8(6):e67324; PMID:23826268; http://dx.doi.org/10.1371/journal.pone.0067324
- http://data.worldbank.org/indicator/SH.XPD.PCAP/countries. Accessed 1 June 2105.
- Usuf E, Mackenzie G, Lowe-Jallow Y, Boye B, Atherly D, Suraratdecha C, Griffiths UK. Costs of vaccine delivery in the Gambia before and after, pentavalent and pneumococcal conjugate vaccine introductions. Vaccine 2014; 32(17):1975-81; PMID:24503271; http://dx.doi.org/10.1016/j.vaccine.2014.01.045
- Loo JD, Conklin L, Fleming-Dutra KE, Deloria Knoll M, Park DE, Kirk J, Goldblatt D, O'Brien KL, Whitney CG. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on prevention of pneumonia. Pediatr Infect Dis J 2014; 33(Suppl 2):S140-51; PMID:24336056; http://dx.doi.org/10.1097/INF.0000000000000082
- Conklin L, Loo JD, Kirk J, Fleming-Dutra KE, Deloria Knoll M, Park DE, Goldblatt D, O'Brien KL, Whitney CG. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type invasive pneumococcal disease among young children. Pediatr Infect Dis J 2014; 33(Suppl 2):S109-18; PMID:24336053; http://dx.doi.org/10.1097/INF.0000000000000078
- Afonso ET, Minamisava R, Bierrenbach AL, Escalante JJ, Alencar AP, Domingues CM, Morais-Neto OL, Toscano CM, Andrade AL. Effect of 10-valent pneumococcal vaccine on pneumonia among children, Brazil. Emerg Infect Dis 2013; 19(4):589-97; PMID:23628462; http://dx.doi.org/10.3201/eid1904.121198
- Pirez MC, Algorta G, Cedres A, Sobrero H, Varela A, Giachetto G, Montano A. Impact of universal pneumococcal vaccination on hospitalizations for pneumonia and meningitis in children in Montevideo, Uruguay. Pediatr Infect Dis J 2011; 30(8):669-74; PMID:21407145; http://dx.doi.org/10.1097/INF.0b013e3182152bf1
- Weinberger DM, Bruden DT, Grant LR, Lipsitch M, O'Brien KL, Pelton SI, Sanders EA, Feikin DR. Using pneumococcal carriage data to monitor postvaccination changes in invasive disease. Am J Epidemiol 2013; 178(9):1488-95; PMID:24013204
- Madhi SA, Bamford L, Ngcobo N. Effectiveness of pneumococcal conjugate vaccine and rotavirus vaccine introduction into the South African public immunisation programme. S Afr Med J 2014; 104(3 Suppl 1):228-34; PMID:24893498
- Huebner RE, Klugman KP, Matai U, Eggers R, Hussey G. Laboratory surveillance for Haemophilus influenzae type B meningococcal, and pneumococcal disease. Haemophilus surveillance working group. S Afr Med J 1999; 89(9):924-5; PMID:10554623
- von Gottberg A, Cohen C, de Gouveia L, Meiring S, Quan V, Whitelaw A, Crowther-Gibson P, Madhi SA, Whitney CG, Klugman KP. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: South Africa, 2003-2008. Vaccine 2013; 31(38):4200-8; PMID:23684826; http://dx.doi.org/10.1016/j.vaccine.2013.04.077
- Nunes MC, von Gottberg A, de Gouveia L, Cohen C, Moore DP, Klugman KP, Madhi SA. The impact of antiretroviral treatment on the burden of invasive pneumococcal disease in South African children: a time series analysis. AIDS 2011; 25(4):453-62; PMID:21178754; http://dx.doi.org/10.1097/QAD.0b013e328341b7f1
- Nunes MC, von Gottberg A, de Gouveia L, Cohen C, Kuwanda L, Karstaedt AS, Klugman KP, Madhi SA. Persistent high burden of invasive pneumococcal disease in South African HIV-infected adults in the Era of an antiretroviral treatment program. PLoS One 2011; 6(11):e27929; PMID:22140487; http://dx.doi.org/10.1371/journal.pone.0027929
- von Gottberg A, de Gouveia L, Tempia S, Quan V, Meiring S, von Mollendorf C, Madhi SA, Zell ER, Verani JR, O'Brien KL, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med 2014; 371(20):1889-99; PMID:25386897; http://dx.doi.org/10.1056/NEJMoa1401914
- Cohen C, von Mollendorf C, de Gouveia L, Naidoo N, Meiring S, Quan V, Nokeri V, Fortuin-de Smit M, Malope-Kgokong B, Moore D, et al. Effectiveness of 7-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in HIV-infected and -uninfected children in South Africa: A matched case-control study. Clin Infect Dis 2014; 59(6):808-18; PMID:24917657; http://dx.doi.org/10.1093/cid/ciu431
- Madhi SA, Groome MJ, Zar HJ, Kapongo CN, Mulligan C, Nzenze S, Moore DP, Zell ER, Whitney CG, Verani JR. Effectiveness of pneumococcal conjugate vaccine against presumed bacterial pneumonia hospitalisation in HIV-uninfected South African children: a case-control study. Thorax 2015; 70(12):1149-55; PMID:26092924; http://dx.doi.org/10.1136/thoraxjnl-2014-206593
- Nzenze SA, von Gottberg A, Shiri T, van Niekerk N, de Gouveia L, Violari A, Nunes MC, Madhi SA. Temporal changes in pneumococcal colonization in HIV-infected and HIV-uninfected mother-child pairs following transitioning from 7-valent to 13-valent pneumococcal conjugate vaccine, Soweto, South Africa. J Infect Dis 2015; (7) 1082-1092; PMID:25784729
- Mbelle N, Huebner RE, Wasas AD, Kimura A, Chang I, Klugman KP. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis 1999; 180(4):1171-6; PMID:10479145; http://dx.doi.org/10.1086/315009
- http://countryoffice.unfpa.org/southafrica/2013/05/03/6675/hiv/. Accessed 1 June 2105.
- Pneumococcal vaccines WHO position paper - 2012 - recommendations. Vaccine 2012; 30(32):4717-8; PMID:22621828; http://dx.doi.org/10.1016/j.vaccine.2012.04.093
- Goldblatt D, Southern J, Ashton L, Andrews N, Woodgate S, Burbidge P, Waight P, Miller E. Immunogenicity of a reduced schedule of pneumococcal conjugate vaccine in healthy infants and correlates of protection for serotype 6B in the United Kingdom. Pediatr Infect Dis J 2010; 29(5):401-5; PMID:20010312; http://dx.doi.org/10.1097/INF.0b013e3181c67f04
- Goldblatt D, Southern J, Ashton L, Richmond P, Burbidge P, Tasevska J, Crowley-Luke A, Andrews N, Morris R, Borrow R, et al. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis J 2006; 25(4):312-9; PMID:16567982; http://dx.doi.org/10.1097/01.inf.0000207483.60267.e7
- Madhi SA, Kuwanda L, Cutland C, Holm A, Kayhty H, Klugman KP. Quantitative and qualitative antibody response to pneumococcal conjugate vaccine among African human immunodeficiency virus-infected and uninfected children. Pediatr Infect Dis J 2005; 24(5):410-6; PMID:15876939; http://dx.doi.org/10.1097/01.inf.0000160942.84169.14
- Klugman KP, Madhi SA, Adegbola RA, Cutts F, Greenwood B, Hausdorff WP. Timing of serotype 1 pneumococcal disease suggests the need for evaluation of a booster dose. Vaccine 2011; 29(18):3372-3; PMID:21396901; http://dx.doi.org/10.1016/j.vaccine.2011.02.089
- Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Muenz LR, O'Brien KL. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med 2010; 7(10); PMID:20957191; http://dx.doi.org/10.1371/journal.pmed.1000348
- Mueller JE, Yaro S, Ouedraogo MS, Levina N, Njanpop-Lafourcade BM, Tall H, Idohou RS, Sanou O, Kroman SS, Drabo A, et al. Pneumococci in the African meningitis belt: meningitis incidence and carriage prevalence in children and adults. PLoS One 2012; 7(12):e52464; PMID:23285051; http://dx.doi.org/10.1371/journal.pone.0052464
- Jones SA, Groome M, Koen A, Van Niekerk N, Sewraj P, Kuwanda L, Izu A, Adrian PV, Madhi SA. Immunogenicity of seven-valent pneumococcal conjugate vaccine administered at 6, 14 and 40 weeks of age in South african infants. PLoS One 2013; 8(8):e72794; PMID:24015277; http://dx.doi.org/10.1371/journal.pone.0072794
- Barron P, Pillay Y, Doherty T, Sherman G, Jackson D, Bhardwaj S, Robinson P, Goga A. Eliminating mother-to-child HIV transmission in South Africa. Bull World Health Organ 2013; 91(1):70-74; PMID:23397353; http://dx.doi.org/10.2471/BLT.12.106807
- Madhi SA, Izu A, Violari A, Cotton MF, Panchia R, Dobbels E, Sewraj P, van Niekerk N, Jean-Philippe P, Adrian PV. Immunogenicity following the first and second doses of 7-valent pneumococcal conjugate vaccine in HIV-infected and -uninfected infants. Vaccine 2013; 31(5):777-83; PMID:23228814; http://dx.doi.org/10.1016/j.vaccine.2012.11.076
- Madhi SA, Adrian P, Cotton MF, McIntyre JA, Jean-Philippe P, Meadows S, Nachman S, Kayhty H, Klugman KP, Violari A. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J Infect Dis 2010; 202(3):355-61; PMID:20583920; http://dx.doi.org/10.1086/653704
- von Mollendorf C, von Gottberg A, Tempia S, Meiring S, de Gouveia L, Quan V, Lengana S, Avenant T, du Plessis N, Eley B, et al. Increased risk for and mortality from invasive pneumococcal disease in hiv-exposed but uninfected infants aged. Clin Infect Dis 2015; 60(9):1346-56; PMID:25645212
- Madhi SA, Petersen K, Madhi A, Wasas A, Klugman KP. Impact of human immunodeficiency virus type 1 on the disease spectrum of Streptococcus pneumoniae in South African children. Pediatr Infect Dis J 2000; 19(12):1141-7; PMID:11144373; http://dx.doi.org/10.1097/00006454-200012000-00004
- Klugman KP. Efficacy of pneumococcal conjugate vaccines and their effect on carriage and antimicrobial resistance. Lancet Infect Dis 2001; 1(2):85-91; PMID:11871480; http://dx.doi.org/10.1016/S1473-3099(01)00063-9
- Cherian T, Mulholland EK, Carlin JB, Ostensen H, Amin R, de Campo M, Greenberg D, Lagos R, Lucero M, Madhi SA, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ 2005; 83(5):353-9; PMID:15976876
- Madhi SA, Klugman KP. World Health Organisation definition of “radiologically-confirmed pneumonia” may under-estimate the true public health value of conjugate pneumococcal vaccines. Vaccine 2007; 25(13):2413-9; PMID:17005301; http://dx.doi.org/10.1016/j.vaccine.2006.09.010
- Madhi SA, Kohler M, Kuwanda L, Cutland C, Klugman KP. Usefulness of C-reactive protein to define pneumococcal conjugate vaccine efficacy in the prevention of pneumonia. Pediatr Infect Dis J 2006; 25(1):30-36; PMID:16395099; http://dx.doi.org/10.1097/01.inf.0000195787.99199.4a
- Tregnaghi MW, Saez-Llorens X, Lopez P, Abate H, Smith E, Posleman A, Calvo A, Wong D, Cortes-Barbosa C, Ceballos A, et al. Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: A double-blind randomized controlled trial. PLoS Med 2014; 11(6):e1001657; PMID:24892763; http://dx.doi.org/10.1371/journal.pmed.1001657
- Enwere G, Biney E, Cheung YB, Zaman SM, Okoko B, Oluwalana C, Vaughan A, Greenwood B, Adegbola R, Cutts FT. Epidemiologic and clinical characteristics of community-acquired invasive bacterial infections in children aged 2-29 months in the Gambia. Pediatr Infect Dis J 2006; 25(8):700-5; PMID:16874169; http://dx.doi.org/10.1097/01.inf.0000226839.30925.a5
- Enwere G, Cheung YB, Zaman SM, Akano A, Oluwalana C, Brown O, Vaughan A, Adegbola R, Greenwood B, Cutts F. Epidemiology and clinical features of pneumonia according to radiographic findings in Gambian children. Trop Med Int Health 2007; 12(11):1377-85; PMID:18045264; http://dx.doi.org/10.1111/j.1365-3156.2007.01922.x
- Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med 2004; 10(8):811-3; PMID:15247911; http://dx.doi.org/10.1038/nm1077
- Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, Stockmann C, Anderson EJ, Grijalva CG, Self WH, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372(9):835-45; PMID:25714161; http://dx.doi.org/10.1056/NEJMoa1405870
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12(2):136-41; PMID:22030330; http://dx.doi.org/10.1016/S1473-3099(11)70253-5
- Tate JE, Parashar UD. Rotavirus vaccines in routine use. Clin Infect Dis 2014; 59(9):1291-301; PMID:25048849; http://dx.doi.org/10.1093/cid/ciu564
- Becker-Dreps S, Amaya E, Liu L, Moreno G, Rocha J, Briceno R, Aleman J, Hudgens MG, Woods CW, Weber DJ. Changes in childhood pneumonia and infant mortality rates following introduction of the 13-valent pneumococcal conjugate vaccine in Nicaragua. Pediatr Infect Dis J 2014; 33(6):637-42; PMID:24445827
- Theodoratou E, McAllister DA, Reed C, Adeloye DO, Rudan I, Muhe LM, Madhi SA, Campbell H, Nair H. Global, regional, and national estimates of pneumonia burden in HIV-infected children in 2010: a meta-analysis and modelling study. Lancet Infect Dis 2014; 14(12):1250-8; PMID:25455992
- Punpanich W, Groome M, Muhe L, Qazi SA, Madhi SA. Systematic review on the etiology and antibiotic treatment of pneumonia in human immunodeficiency virus-infected children. Pediatr Infect Dis J 2011; 30(10):e192-202; PMID:21857264
- Gona P, Van Dyke RB, Williams PL, Dankner WM, Chernoff MC, Nachman SA, Seage GR, 3rd. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. Jama 2006; 296(3):292-300; PMID:16849662; http://dx.doi.org/10.1001/jama.296.3.292
- Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: a time series analysis. Lancet Respirat Med 2014; 2(5):387-94; PMID:24815804; http://dx.doi.org/10.1016/S2213-2600(14)70032-3
- Poehling KA, Talbot TR, Griffin MR, Craig AS, Whitney CG, Zell E, Lexau CA, Thomas AR, Harrison LH, Reingold AL, et al: Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. Jama 2006; 295(14):1668-74; PMID:16609088; http://dx.doi.org/10.1001/jama.295.14.1668
- Flannery B, Heffernan RT, Harrison LH, Ray SM, Reingold AL, Hadler J, Schaffner W, Lynfield R, Thomas AR, Li J, et al. Changes in invasive Pneumococcal disease among HIV-infected adults living in the era of childhood pneumococcal immunization. Ann Intern Med 2006; 144(1):1-9; PMID:16389249; http://dx.doi.org/10.7326/0003-4819-144-1-200601030-00004
- Loo JD, Conklin L, Fleming-Dutra KE, Knoll MD, Park DE, Kirk J, Goldblatt D, O'Brien KL, Whitney CG. Systematic review of the indirect effect of pneumococcal conjugate vaccine dosing schedules on pneumococcal disease and colonization. Pediatr Infect Dis J 2014; 33(Suppl 2):S161-71; PMID:24336058; http://dx.doi.org/10.1097/INF.0000000000000084
- Obaro SK, Adegbola RA, Banya WA, Greenwood BM. Carriage of pneumococci after pneumococcal vaccination. Lancet 1996; 348(9022):271-2; PMID:8684225; http://dx.doi.org/10.1016/S0140-6736(05)65585-7
- Hammitt LL, Akech DO, Morpeth SC, Karani A, Kihuha N, Nyongesa S, Bwanaali T, Mumbo E, Kamau T, Sharif SK, et al. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob health 2014; 2(7):e397-405; PMID:25103393; http://dx.doi.org/10.1016/S2214-109X(14)70224-4
- Roca A, Hill PC, Townend J, Egere U, Antonio M, Bojang A, Akisanya A, Litchfield T, Nsekpong DE, Oluwalana C, et al. Effects of community-wide vaccination with PCV-7 on pneumococcal nasopharyngeal carriage in the Gambia: a cluster-randomized trial. PLoS Med 2011; 8(10):e1001107; PMID:22028630; http://dx.doi.org/10.1371/journal.pmed.1001107
- Nzenze SA, Shiri T, Nunes MC, Klugman KP, Kahn K, Twine R, de Gouveia L, von Gottberg A, Madhi SA. Temporal changes in pneumococcal colonization in a Rural-African community with high HIV-prevalence following routine infant pneumococcal-immunization. Pediatr Infect Dis J 2013; 32(11):1270-8; PMID:24061271
- Nzenze SA, Shiri T, Nunes MC, Klugman KP, Kahn K, Twine R, de Gouveia L, von Gottberg A, Madhi SA. Temporal association of infant immunisation with pneumococcal conjugate vaccine on the ecology of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus nasopharyngeal colonisation in a rural South African community. Vaccine 2014; 32(42):5520-30; PMID:25101982; http://dx.doi.org/10.1016/j.vaccine.2014.06.091
- Buie KA, Klugman KP, von Gottberg A, Perovic O, Karstaedt A, Crewe-Brown HH, Madhi SA, Feldman C: Gender as a risk factor for both antibiotic resistance and infection with pediatric serogroups/serotypes, in HIV-infected and -uninfected adults with pneumococcal bacteremia. J Infect Dis 2004; 189(11):1996-2000; PMID:15143465; http://dx.doi.org/10.1086/386548
- Madhi SA, Izu A, Nunes MC, Violari A, Cotton MF, Jean-Philippe P, Klugman KP, von Gottberg A, van Niekerk N, Adrian PV. Longitudinal study on Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus nasopharyngeal colonization in HIV-infected and -uninfected infants vaccinated with pneumococcal conjugate vaccine. Vaccine 2015; 33(23):2662-9; PMID:25910923; http://dx.doi.org/10.1016/j.vaccine.2015.04.024
