abstract
The introduction of combination vaccines plays a significant role in increasing vaccine acceptance and widening vaccine coverage. Primary vaccination against diphtheria, tetanus, pertussis, poliomyelitis and Haemophilus influenza type b (Hib) diseases has been implemented in Vietnam. In this study we evaluated the safety and reactogenicity of combined diphtheria-tetanus-pertussis-inactivated polio (DTPa-IPV)/Hib vaccine when administered as a booster dose in 300 healthy Vietnamese children <2 years of age (mean age: 15.8 months). During the 4-day follow-up period, pain (31.7%) and redness (27.3%) were the most frequent solicited local symptoms. Pain (2%) was also the most frequent grade 3 local symptom. One subject reported 2 serious adverse events that were not causally related to the study vaccine. DTPa-IPV/Hib conjugate vaccine was well tolerated as a booster dose in healthy Vietnamese children aged <2 years.
Combination vaccines represent a solution to the challenge of implementing elaborate childhood immunization schedules. The development of combination vaccines targeting multiple diseases in a single injection affords better compliance and coverage rates than separate single antigen vaccines.Citation1,2 The Expanded Program on Immunization (EPI) includes vaccination against diphtheria, tetanus, pertussis, poliomyelitis and Haemophilus influenza type b (Hib) diseases in most countries, including Vietnam. However, despite wide vaccine coverage, the disease burden remains high, particularly in developing countries.Citation2 In accordance with the EPI recommendations,Citation3 the Vietnamese schedule comprises primary vaccination with 3 doses of diphtheria, tetanus, pertussis, inactivated polio and Hib vaccines in the first year of life followed by a booster dose in the second year of life. Although pertussis vaccination in Vietnam has traditionally been delivered using whole cell pertussis vaccines (Pw), acellular pertussis vaccines (Pa) are known to be better tolerated than Pw.Citation4 A regulatory application for a combined diphtheria-tetanus-acellular pertussis-inactivated polio/Hib vaccine (DTPa-IPV/Hib) is being made in Vietnam. The reactogenicity profile of DTPa-IPV/Hib has already been established in other locations, including Europe,Citation5 Canada,Citation6 TaiwanCitation7 and Singapore.Citation8 Our study, which was designed to support registration, evaluated the safety and reactogenicity of DTPa-IPV/Hib (Infanrix™-IPV+Hib, GSK, Belgium) when administered as a booster dose in healthy Vietnamese children aged less than 2 years.
This was a Phase III, open label, single center study at the Hai Phong Center of Preventive Medicine, Hai Phong, Vietnam, conducted between December 2012 and April 2013 (www.clinicaltrials.gov; NCT01577732). Healthy children aged 12–24 months, inclusive, who had previously received 3 doses of DTPa and polio vaccines within the first 6 months of life, and were due to return for their routine booster vaccinations, were enrolled. Children were excluded if they had received any investigational drug/vaccine 30 days before vaccination, or immunoglobulins/blood products 90 days before the vaccination. Children with known hypersensitivity to the study vaccine components, confirmed/suspected immunosuppressive/immunodeficient condition, any neurological disorder/seizures, chronic illness or symptoms of acute illness at the time of enrolment were also excluded. Children who previously received booster vaccination against, or who had been exposed to, diphtheria, tetanus, pertussis, poliomyelitis and/or Hib disease were also excluded. Children in care (child under protection of an organization/ court/ government body/ foster parents/ living in care home or institution but not including an adopted child or child with an appointed legal guardian) were excluded from the study. Vaccination was postponed if the child had axillary temperature of ≥37.5°C or a rectal temperature of ≥38°C, or minor illness without fever.
Our study was conducted according to Good Clinical Practice, Declaration of Helsinki and all applicable regulatory requirements, and was approved by an Independent Review Board and Independent Ethics Committee. All study procedures were performed only after obtaining written informed consent from parents/guardians.
A single booster dose of the study vaccine was administered intramuscularly into the anterolateral side of the right thigh. Each 0.5 ml dose of reconstituted DTPa-IPV/Hib (developed and manufactured by GSK, Belgium) contained: 25 Lf diphtheria toxoid, 10 Lf tetanus toxoid, 25 µg pertussis toxoid, 25 µg filamentous haemagglutinin, 8 µg pertactin, 40D antigen units of poliovirus type 1, 8D antigen units of poliovirus type 2, 32D antigen units of poliovirus type 3, 0.5 mg aluminum as salts, 10 µg purified Hib capsular polysaccharide conjugated with approximately 25 µg tetanus toxoid, 10.08 mg lactose and 4.5 mg sodium chloride.
Solicited local adverse events (AEs; injection site pain, redness and swelling) and general symptoms (drowsiness, fever, irritability/fussiness and loss of appetite) were recorded by parents/guardians on diary cards, for 4 days (Days 0–3) after the booster dose. Unsolicited AEs and the use of concomitant drugs or vaccines were recorded for 31 days after the booster dose for each child. In addition, parents were instructed to contact the investigator immediately if their child showed any signs or symptoms that they considered to be serious. Investigators recorded serious AEs (SAEs) throughout the whole trial.
AEs were assessed according to a 3-point grading scale, where Grade 3 symptoms were defined as: pain at injection site and discomfort when the child's limb was moved; >20 mm diameter of redness and swelling at the injection site or fever of ≥39°C axillary temperature; irritability/fussiness if the child cried and could not be comforted or if it prevented normal activity; drowsiness if it prevented normal activity; loss of appetite if the child did not eat at all.
The presence of large injection site swelling (>50 mm diameter), noticeable diffuse swelling or noticeable increases in limb circumference were reported to the investigator for further evaluation as soon as possible.
The analysis of safety and reactogenicity was performed on the total vaccinated cohort that included all vaccinated subjects. The percentage of subjects with at least one solicited/unsolicited symptom with exact 95% confidence intervals (CI) were tabulated. All statistical analyses were performed using the Statistical Analysis Systems (SAS) version 9.2.
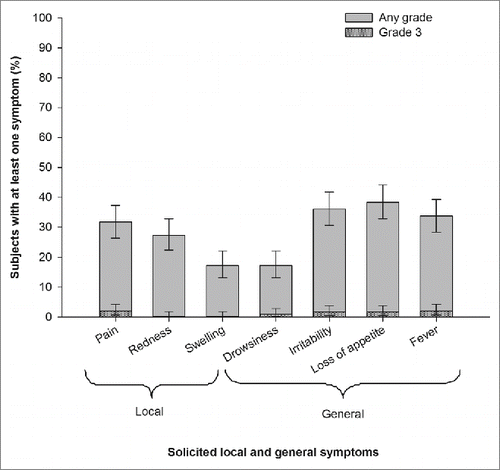
We enrolled a total of 300 children, of whom 143 (47.7%) were female. The mean age of the subjects at the time of DTPa-IPV/Hib booster dose was 15.8 months (standard deviation [SD]; 2.96). During the 4 days (Days 0–3) follow-up period after the booster dose, 192 (64.0%) subjects had at least one solicited and unsolicited symptom, and 17 (5.7%) subjects had at least one grade 3 solicited and unsolicited symptom. Nineteen (6.3%) subjects sought medical attention on account of the incidence of solicited and unsolicited symptoms during the first 4 days post vaccination, most commonly for loss of appetite or raised temperature. Pain (31.7%) and redness (27.3%) were the most frequently reported solicited local symptoms. Pain (2%) was also the most frequently reported grade 3 local symptom. Loss of appetite (38.3%) and irritability/fussiness (36%) were the most frequently reported solicited general symptoms. Fever (axillary temperature ≥37.5°C) was reported in 101 (33.7%) subjects ().
Figure 1. Incidence of solicited local and general symptoms during the 4-day (Day 0–3) after DTPa-IPV/Hib booster dose (Total vaccinated cohort).
Within the 31-day (Days 0–30) post-vaccination period, cough (16%) was the most frequently reported unsolicited symptom. Two SAEs were reported for one subject (pneumonia at Day 26 and convulsion at Day 32); both resolved and were assessed to be not causally related to the study vaccine. No fatal SAEs were reported. No subjects withdrew from the study due to AE or SAE.
The use of vaccines that combine more than one antigen is a widely accepted immunization method for children.Citation9 Besides simplification of the increasingly crowded childhood vaccination schedule, combination vaccines have the potential to widen coverage, provide greater convenience, improve compliance, reduce health clinic visits and increase cost effectiveness.Citation9,10 Establishing vaccination awareness, reducing vaccine wastage rates and maintaining high immunization coverage in Vietnam is highly important.Citation11,12 To date, vaccination against diphtheria, tetanus, pertussis, IPV and Hib has been separately implemented in Vietnam.Citation13 The implementation of combination vaccines would play a crucial role in achieving the vaccine coverage targets set by WHO and EPI.Citation11,14
In our study, the safety and reactogenicity of DTPa-IPV/Hib vaccine was evaluated for the first time among healthy Vietnamese children younger than 2 years. Despite being limited by a small sample size and lack of a control group, we demonstrated that the DTPa-IPV/Hib vaccine had an acceptable safety profile when administered as a booster dose. Solicited and unsolicited local and general symptoms are generally reported more frequently after a booster dose compared with the primary schedule. In our study, the incidence of solicited local and general symptoms after the booster dose of DTPa-IPV/Hib was lower, and the incidence of grade 3 symptoms was consistent with previous reports.Citation2,5,6,15 It is unclear if these minor variations reflect different cultural reporting thresholds, or real differences between populations in these small studies. The childhood vaccination schedule in Vietnam currently includes a booster dose with DTPw at 18 months, as part of the EPI. Although data from global studiesCitation4 have established that Pa-containing vaccines provide better tolerability than Pw-containing vaccines, to our knowledge, there are no published data on the tolerability of Pa vaccines in the Vietnamese population. Nevertheless, a study of DTPw booster administration in healthy toddlers from the Philippines, showed a total incidence of grade 3 solicited symptoms ranging from 33–41%,Citation16 which compared with 5.7% in the present study. Although there could be AE under-reporting because the diary cards were being completed by parents/guardians, the differences in incidence are sufficiently large between the 2 studies, to support the greater tolerability of Pa-containing vaccines.
In conclusion, this study suggests that DTPa-IPV/Hib combination vaccine administered as a booster dose allows the targeting of multiple antigens using a single injection and with an acceptable safety and reactogenicity profile in healthy Vietnamese children younger than 2 years.
Abbreviations
| AE | = | Adverse event |
| CI | = | Confidence intervals |
| DTPa-IPV | = | Diphtheria-tetanus-acellular pertussis-inactivated polio vaccine |
| DTwP | = | diphtheria; tetanus and whole cell pertussis vaccine |
| EPI | = | Expanded Program on Immunization |
| Hib | = | Haemophilus influenza type b |
| Lf | = | Limits of flocculation |
| SAE | = | Serious adverse event |
| SD | = | Standard deviation |
| WHO | = | World Health Organization |
Disclosure of potential conflicts of interest
HHH, SK, NK and OVDM are employees of GSK group of companies and HHH and OVDM declare having GSK stocks. DA and T-WY were employees of the GSK group of companies at the time of the study. DDA has no conflicts to declare.
Trademark
Infanrix™ is a trademark of the GlaxoSmithKline group of companies.
Acknowledgments
The authors acknowledge: Karin Hardt (employee of the GlaxoSmithKline group of companies) for her role in developing the protocol; Murtaza Shipchandler, Chua Tran Thuy and Greg Bewick (all employees of the GlaxoSmithKline group of companies), and Supriya Chauhan and Janice Tan (Quintiles on behalf of GSK Vaccines) for study coordination and project management; Prachee Panda for providing writing support and Jesse Quigley-Jones for editorial support and coordination of this publication (both employees of the GlaxoSmithKline group of companies).
Funding
GlaxoSmithKline Biologicals SA sponsored and funded the study conduct, analyses of data and the development and publication of the manuscript.
References
- Baldo V, Bonanni P, Castro M, Gabutti G, Franco E, Marchetti F, Prato R, Franco E. Combined hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type B vaccine; Infanrix hexa: twelve years of experience in Italy. Human Vacc Immunother 2014; 10:129-37; http://dx.doi.org/10.4161/hv.26269
- Lim FS, Phua KB, Lee BW, Quak SH, Teoh YL, Ramakrishnan G, Han HH, Van Der Meeren O, Jacquets JM, Bock HL. Safety and reactogenicity of DTPa-HBV-IPV/Hib and DTPa-IPV/Hib vaccines in a post-marketing surveillance setting. Southeast Asian J Trop Med Publ Health 2011; 42:138-47
- WHO recommendations for routine immunization - summary tables. Available at: http://www.who.int/entity/immunization/policy/Immunization_routine_table1.pdf?ua=1, accessed on 10th March 2015.
- Jefferson T, Rudin M, DiPietrantonj C. Systematic review of the effects of pertussis vaccines in children. Vaccine 2003; 16:2003-14; http://dx.doi.org/10.1016/S0264-410X(02)00770-3
- Dagan R, Igbaria K, Piglansky L, Melamed R, Willems P, Grossi A, Kaufhold A. Safety and immunogenicity of a combined pentavalent diphtheria, tetanus, acellular pertussis, inactivated poliovirus and Haemophilus influenzae type b-tetanus conjugate vaccine in infants, compared with a whole cell pertussis pentavalent vaccine. Pediatr Inf Dis J 1997; 16: 1113-21; http://dx.doi.org/10.1097/00006454-199712000-00004
- Halperin SA, King J, Law B, Mills E, Willems P. Safety and immunogenicity of Haemophilus influenzae-tetanus toxoid conjugate vaccine given separately or in combination with a three-component acellular pertussis vaccine combined with diphtheria and tetanus toxoids and inactivated poliovirus vaccine for the first four doses. Clin Inf Dis 1999; 28:995-1001; http://dx.doi.org/10.1086/514741
- Lin TY, Wang YH, Chang LY, Chiu CH, Huang YC, Tang H, Bock H. Safety and immunogenicity of a diphtheria, tetanus, and acellular pertussis-inactivated poliovirus vaccine/Haemophilus influenzae type B combination vaccine administered to Taiwanese infants at 2, 4, and 6 months of age. Chang Gung Med J 2003; 26:315-22; PMID:12934847
- Phua KB, Quak SH, Lim FS, Goh P, Teoh YL, Datta SK, Han HH, Bock HL. Immunogenicity, reactogenicity and safety of a diphtheria-tetanus-acellular pertussis-inactivated polio and Haemophilus influenzae type b vaccine in a placebo-controlled rotavirus vaccine study. Ann Acad Med 2008; 37:546-53
- Gabutti G, Trucchi C, Conversano M, Zivelonghi G, Zoppi G. Booster Vaccination: The Role of Reduced Antigen Content Vaccines as a Preschool Booster. BioMed Res Int 2014; 2014:541319; PMID:24678509; http://dx.doi.org/10.1155/2014/541319
- Le CT. Combination vaccines: choices or chaos? A practitioner's perspective. Clin Inf Dis 2001; 33 Suppl 4: S367-71; http://dx.doi.org/10.1086/322575
- UNICEF: National EPI Review Report: Review of Expanded Program of Immunization Vietnam 2009. Available at: http://www.unicef.org/vietnam/EPI_NATIONAL_Review_Report_Vietnam_2009_Final.pdf. Accessed on 11th May 2015.
- Hoang MV, Nguyen TB, Kim BG, Dao LH, Nguyen TH, Wright P. Cost of providing the expanded programme on immunization: findings from a facility-based study in Vietnam, 2005. Bull WHO 2008; 86: 429-34; PMID:18568271
- The national expanded programme on immunization - Vietnam. Available at: http://www.nihe.org.vn/new-en/chuong-trinh-tiem-chung-mo-rong-quoc-gia-703261429/1034/General-Information.vhtm. Accessed on 11th May 2015.
- World Health Organization. Report of the seventh meeting of the Global Technical Consultative Group for Poliomyelitis Eradication. Geneva, Switzerland: Department of Vaccines and Biologicals, World Health Organization; 2002. Report WHO/V&B/02.12.
- Kim CH, Cha SH, Shin SM, Kim CS, Choi YY, Hong YJ, Chey MJ, Kim KN, Hur JK, Jo DS, et al. Immunogenicity, reactogenicity and safety of a combined DTPa-IPV vaccine compared with separate DTPa and IPV vaccines in healthy Korean infants. Korean J Pediatr Infect Dis 2010; 17:156-168
- Quiambao B, Van Der Meeren O, Kolhe D, Gatchalian S. A randomized, dose-ranging assessment of the immunogenicity and safety of a booster dose of a combined diphtheria-tetanus-whole cell pertussis-hepatitis B-inactivated poliovirus-Hemophilus influenzae type b (DTPw-HBV-IPV/Hib) vaccine vs. co-administration of DTPw-HBV/Hib and IPV vaccines in 12 to 24 months old Filipino toddlers. Human Vacc Immunother 2012; 8:347-54; http://dx.doi.org/10.4161/hv.18630
