ABSTRACT
Pancreatic adenocarcinoma is notoriously lethal, and despite improvements in systemic chemotherapy approaches bringing survival rates for metastatic disease to almost 1 year, by 2030 it is expected to become the second leading cause of cancer death. Pancreatic cancer (PC) prognosis has been associated with both the presence of intratumoral helper and cytotoxic T lymphocytes, as well as humoral immune responses to tumor associated antigens like mesothelin. It is well described that the PC microenvironment is characterized by a fibroinflammatory and immunosuppressive stroma. On these premises several immune-targeted strategies have been developed to harness the adaptable immune system with a goal of improving survival with little toxicity. Cancer vaccines involve the administration of tumor-associated antigens with the goal of inducing an endogenous anti-tumor response. Among several strategies discussed, we will focus on the algenpantucel-L (HyperAcute™ Pancreas) immunotherapy. Algenpantucel-L is a whole cell immunotherapy consisting of irradiated allogeneic PC cells genetically engineered to express the murine enzyme α(1,3)-galactosyltransferase (αGT), which ultimately leads to hyperacute rejection with complement- and antibody-dependent cytotoxicity. While phase III data in the adjuvant treatment of pancreatic cancer are pending, phase II results have been encouraging, particularly for patients who demonstrated humoral immunologic responses. Novel strategies using immune checkpoint inhibitors, costimulatory antibodies, and combinations with cancer vaccines may overcome immunotolerance and improve treatment success.
Introduction
Approximately 277,000 new cases of pancreatic cancer (PC) are diagnosed each year worldwide,Citation1 including 49,000 cases per year in both the United States,Citation2 and Europe.Citation3 PC has the highest mortality rate of all major cancers and is predicted by 2030 to be to the 2nd leading cause of cancer death in the United States.Citation2
Surgery followed by adjuvant chemotherapy represents the only potentially curative treatment. However, only 15 to 20% of patients present with potentially resectable PC, and among those who undergo complete surgical resection and receive adjuvant chemotherapy, relapse is common with a 5-year survival rate of only 20%.Citation4 For those with advanced PC, gemcitabine has long been considered the standard of care but survival rates were only 6 months.Citation5 More recently, FOLFIRINOX (folinic acid, 5-FU, irinotecan, oxaliplatin) and the combination of gemcitabine and nab-paclitaxel were shown in large, randomized, phase III studies to be superior to gemcitabine alone.Citation6,7 However, the median overall survival (OS) for FOLFIRINOX and gemcitabine/nab-paclitaxel was only 11.1 and 8.5 months, respectively. As these statistics highlight, the outcomes achieved with standard treatments remain suboptimal, and better strategies are desperately needed.
PC is notoriously treatment resistant, owing to a multitude of factors. In a comprehensive analysis of the PC genome, Jones et al. found pancreatic cancers have an average of 60 mutations per tumor involving 12 core signaling pathways, with substantial inter-patient variability.Citation8 Because of this genetic complexity and heterogeneity, treatments targeting a single pathway, or applied indiscriminately are unlikely to have meaningful clinical efficacy. A frequently invoked reason for the aggressiveness and chemoresistance associated with pancreatic cancer has been its immunosuppressive tumor microenvironment. Immunotherapy in general aims to elicit an anti-tumor immune response specific for an individual patient's tumor, with all of its unique genetic and epigenetic changes. This approach holds tremendous potential for multiple cancer types, including PC, and is a research priority rapidly moving forward. The present paper will review recent advances in immunotherapy for PC, focusing on algenpantucel-L and other anti-cancer vaccines.
Pancreatic cancer and the immune system
While not generally considered an immunogenic tumor, there is evidence to suggest that pancreatic tumors are capable of inducing an anti-tumor immune response, which can impact the disease course. Patients with PC have detectable tumor-specific T cells in their bone marrow and peripheral blood.Citation9 Moreover, the presence of tumor-infiltrating CD8+ and CD4+ T cells has been associated with lower tumor stage and improved prognosis.Citation10
Pancreatic tumors appear to induce an immunosuppressive environment early in their development. Using mice genetically engineered to express oncogenic Kras (KrasG12D) in pancreatic epithelial cells (iKras*), Zhang et al. showed that mutant Kras is an important mediator of the response to acute pancreatic injury.Citation11 Specifically, following induction of pancreatitis, control mice were able to repair injured tissue relatively quickly, while iKras* mice developed a fibrotic stroma with acinar-ductal metaplasia, and ultimately, tissue-wide pancreatic intraepithelial neoplasms (PanINs), known precursors to pancreatic ductal adenocarcinoma. In addition, the development of PanINs in iKras* mice could be prevented by depleting the CD4+ T cells (iKras*;CD4−/− mice). In a different mouse model, Hiraoka et al. found that the prevalence of cytotoxic CD8+ T cells decreased and the prevalence of regulatory T cells (Tregs) increased during the progression from a premalignant to invasive lesion.Citation12 Analysis of the immune infiltrate in human pancreatic tumor specimens reveals a predominance of immune suppressor cells including Tregs, myeloid-derived suppressor cells (MDSCs), and macrophages, which in the tumor microenvironment acquire an M2 phenotype leading to tumor growth and progression.Citation12-15 Moreover, compared to healthy controls, patients with PC have increased numbers of circulating Tregs and MDSCs.Citation16,17
Pancreatic tumors create an immunosuppressive environment via several mechanisms. Cancer cells secrete factors such as TGF-β, IL-10, indoleamine 2,3-dioxygenase (IDO), and galectin-1 (Gal-1), which recruit and activate immune suppressor cells, and inhibit immune effector cells.Citation18,19 PC cells have also been shown to express ligands, such as PD-L1, which function to suppress the anti-tumor immune response.Citation20,21 In PC, PD-L1 tumor expression has been shown to be associated with fewer tumor infiltrating lymphocytes (TILs), particularly CD8+ T cells, and worse prognosis.Citation21
Non-immune cells in the microenvironment also play a role in inducing immune dysfunction. Pancreatic tumors are surrounded by a dense stroma composed of multiple cells types including cancer associated fibroblasts (CAF). In addition to directly promoting tumor progression,Citation22,23 peri-tumoral fibroblasts recruit M2 macrophages into the tumor microenvironment,Citation23 and secrete fibroblast activation protein (FAP-α), which suppresses effector T cell function.Citation24-26 In a PC mouse model, Feig et al. showed that chemokine (C-X-C motif) ligand 12 (CXCL12) secreted by FAP+ CAFs through interaction with its chemokine (C-X-C motif) receptor 4 (CXCR4) on T cells, is an essential mediator of immune evasion, preventing the infiltration of T cells into the tumor microenvironment.Citation25 Inhibition of the CXCR4 idecreased tumor growth, an effect that was enhanced by the co-administration of an immune checkpoint inhibitor.
In summary, there is evidence to suggest that patients can mount an immune response against pancreatic tumor cells, which may alter the course of the disease. However, it is also clear that competing local and systemic factors work to establish and maintain an immunosuppressive environment, which promotes PC immune evasion.
Vaccine immunotherapies
Cancer vaccines involve the administration of a tumor-specific antigen, with the goal of inducing an endogenous anti-tumor immune response. Multiple vaccination strategies have been evaluated in PC, including whole cell vaccines, peptide vaccines, dendritic cell (DC) vaccines, and recombinant virus-based vaccines ().
Table 1. Mechanisms of action of vaccine strategies in pancreatic cancer.
Whole-cell vaccine immunotherapies
Algenpantucel-L (HyperAcute™ Pancreas)
Algenpantucel-L is a whole-cell vaccine with a combination of 2 irradiated allogeneic PC cells lines (HAPa-1, HAPa-2) genetically engineered by retrovirus transduction to express the murine enzyme α(1,3)-galactosyltransferase (αGT).Citation27 αGT is responsible for the synthesis of the α-galactosyl (αGal) epitope, a carbohydrate present on cell surface glycoconjugates. While present on most mammalian cells, the αGal epitope is not expressed on humans due to inactivation of the αGT gene.Citation28 As a result of continuous antigenic stimulation by normal gut flora expressing αGal, humans produce large quantities of antibodies targeting this epitope. Anti-αGal antibodies, in fact, account for 1% of all circulating immunoglobulins, making them the most abundant antibody present in human sera.Citation29 These antibodies are clinically relevant because they mediate hyperacute rejection (HAR). HAR is a form of rejection occurring minutes to hours after transplantation of a non-human allograft (xenotransplantation), and is the result of anti-αGal antibodies binding to αGal epitopes causing complement- and antibody-dependent cell-mediated destruction of the transplanted allografts. The αGal hyperacute technology and its role to modulate and enhance potency of antiviral vaccines was originally published by Rother and Squinto in 1996,Citation30 and Link and colleagues described its anticancer activity.Citation31 Hyperacute αGal-mediated vaccine immunotherapy has been further researched for various cancer vaccines platforms including melanoma (dorgenmeltucel-L),Citation32 pancreatic (algenpantucel-L),Citation27 and prostate cancer (HyperAcute Prostate).Citation33 Dorgenmeltucel-L (HyperAcute Melanoma, HAM) combined with pegylated interferon was evaluated in a phase II study in 25 high risk melanoma patients (stages IIC = 1, IIIB = 6, IIIC = 2, IV=16).Citation34 Among 16 stage IV patients, 2 patients had a complete response (CR) lasting 28 months+, one had stable disease, and 4 patients had no evidence of disease (NED) after resection. Among stage II and III patients, 3/;9 remained NED, and 1 had disease progression Overall the median survival was 19 months. Treatment was well tolerated and 4/25 patients developed vitiligo, correlating with 2 CR and post-surgical NED in 2 patients. The individual role of HAM is difficult to discern in the context of surgical resection and concomitant pegylated interferon therapy. Dorgenmeltucel-L in conjunction with ipilimumab or PD-1 inhibitors (nivolumab or pembrolizumab) is currently being studied in randomized phase II study in 100 patients with advanced melanoma (NCT02054520).
The pancreatic algenpantucel-L vaccine works by harnessing a natural robust immune response against pancreatic cancer (). When algenpantucel-L cells are injected into humans, natural anti-αGal antibodies recognize the αGal epitopes on these vaccine tumor cells and mediate hyperacute rejection and phagocytosis. While the initial immune response targets the αGal epitopes, the immune effector cells are “educated” to recognize other epitopes and antigenic molecules through a process called epitope spreading. In this way, the immune response expands to include tumor-associated antigens expressed by the 2 PC cell lines components of algenpantucel-L immunotherapy. The HAPa-1 and HAPa-2 cell lines express antigens that are also expressed (shared) by the majority of PCs, therefore PC patients receiving this immunotherapy are expected to mount a response against their own pancreatic cancer cells.
Figure 1. Proposed mechanism of action of algenpantucel-L immunotherapy. Algenpantucel-L relies on preexisting anti-αGal antibody response; vaccine cells are destroyed by complement-mediated lysis, which results in cross-presentation of tumor antigens; tumor specific CD8+ cytotoxic T cells are generated which recognize patient's own tumor; humoral immunity relies on antitumor antibodies.
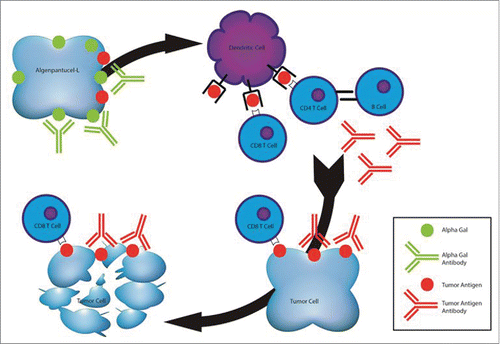
Algenpantucel-L was initially evaluated in patients with resectable PC. In a phase I study among 7 patients there were no dose-limiting toxicities and the maximum tolerated dose was not established (algenpantucel-L investigator brochure). A subsequent multi-institutional, open-label, dose-finding phase II study NLG0205 enrolled 70 patients with stages I and II PC who had undergone R0 or R1 resection, between 2008 and 2010.Citation27 Patients received algenpantucel-L in addition to standard adjuvant gemcitabine and 5FU-based chemoradiotherapy administered according to the RTOG-9704 study(5-FU vs gemcitabine chemotherapy before and after 5-FU-based chemoradiation following resection of pancreatic adenocarcinoma).Citation35 The immunotherapy was dosed at 100 or 300 million cells and was given by intradermal injection weekly 2 times prior to the start of chemotherapy. Patients then received 2 additional vaccinations on days 1 and 15 of cycle 2 of gemcitabine (1000 mg/m2 on days 1, 8, 15 of a 28-day cycle). During chemoradiotherapy with 5-FU continuous infusion 250 mg/m2/day, algenpantucel-L was administered on days 1, 15, 29 and 43. Finally, assuming restaging studies did not show evidence of relapse, patients received 3 additional cycles of gemcitabine in combination with algenpantucel-L. Overall patients received on average 12 vaccinations (range 1–14) during adjuvant therapy. The trial was to be considered a success if the 1-year disease free survival (DFS) was ≥ 56%, and ineffective if DFS was ≤50%. To provide context, the RTOG-9704 study reported 1-year DFS of 50%, 1-year OS of 69%, and 3-year OS of 30% in patients with similar stages of disease.
Algenpantucel-L was well tolerated. No grade 4 adverse events were described, and less than 12% of patients experienced grade 3 adverse events that were considered to be related to the vaccine (fatigue, injection site reaction, pain and lymphopenia). Overall, the most common toxicities were grade 1–2 vaccine site induration, fatigue and other injection site reactions (pain, erythema, rash, pruritus, swelling). After a median follow-up of 21 months, the primary endpoint, 1-year DFS, was reached by 62% of 69 evaluable patients, and the median DFS was 14.1 months. Pre-specified subgroup analyses noted that patients treated with 300 million cells/dose (n = 26) had a 1-year DFS of 81%, and those treated with 100 million cells/dose (n = 43) had a 1-year DFS of 51% (p = 0.02). The 1-year OS was 86% for all patients, 96% in the 300 million cells/dose, and 79% in the 100 million cells/dose subgroups (p = 0.053). More recent survival data among the 69 patients treated in NLG0205 showed a 3-year OS rate of 39% (). Compared to the RTOG-9704 trial, this study included more patients with locoregional lymph node involvement (81% vs. 68%), but other baseline characteristics (tumor size, tumor differentiation, and the R0/R1 resection status) were similar.
Defining immunological markers of benefit from immunotherapies and specifically, from vaccine therapies such as algenpantucel-L is of huge interest. Calreticulin (CALR) is a calcium-binding chaperone protein that normally resides in the endoplasmic reticulum (ER) where it affects calcium homeostasis assuring adequate protein folding, including that of the major histocompatibility complex (MHC) class I.Citation36,37 MHC class I alterations can influence antigen presentation to cytotoxic T cells, and therefore cellular immunogenicity. Certain cytotoxic drugs have been shown to induce CALR translocation from the ER to the surface of cancer cells, which can lead to immune-mediated rejection of these cells.Citation37 The HAPs cell lines components of algenpantucel-L immunotherapy express CALR.
Immunological biomarker data from the phase II NLG0205 adjuvant study described that increased titers of anti-calreticulin (anti-CALR) antibodies correlated with overall survival.Citation38 Among 64 evaluable patients, 31 (48%) had increased titers of anti-CALR antibodies (20% increased from baseline) by ELISA, and these patients had significantly improved median OS compared to those without increased antibody titers (35.8 vs. Nineteen.2 months; p = 0.03). Rossi and colleagues also analyzed anti-mesothelin (MSLN), anti-carcinoembryonic (CEA) antigen, and anti-αGal antibodies increases from baseline in the NLG0205 study.Citation39 Mesothelin is a commonly overexpressed tumor antigen by pancreatic tumorsCitation40 and is also expressed by the components of the algenpantucel-L vaccine. Twenty of 64 patients (31%) had ≥25 % increase in anti-MSLN antibody titers with corresponding median OS of 42 months, compared to 20 months for patients without immunologic response (p = 0.0273). In addition, 90% of patients demonstrated increased anti-αGal antibody titers which remained elevated for more than 200 d Data presented at ASCO 2013 noted that the 3-year OS and median OS for patients with no immunological response (n = 27) was 19% and 17 months respectively, while for patients with one antibody titer increase (n = 26) it was 42% and 26 months respectively, and for patients with multiple antibody titers increases (n = 13) it was 69% and >36 months (not reached), respectively ().Citation39, 35 It is evident that mounting humoral immunity to algenpantucel-L was associated with improved survival, but the data is limited by small patient numbers, and will need to be further confirmed in larger randomized trials like IMPRESS.
Table 2. NLG0205 algenpantucel-L phase II study summary and correlations with immunological biomarker response.
The phase III IMPRESS study (NCT01072981) of gemcitabine plus chemoradiotherapy with or without algenpantucel-L in patients with resected PC completed accrual in September 2013, and the results are anticipated shortly. IMPRESS enrolled 722 patients and will assess OS as the primary endpoint, and DFS and correlative biomarkers endpoints as secondary objectives. The study was powered to detect a 20% difference in survival, and was stratified for nodal status, radiotherapy administration, and CA19–9. Algenpantucel-L was administered at 300 million cells/dose every 2 weeks for 6 months, then monthly for an additional 6 months. The second interim analysis estimated a median overall survival of 28.5 months for the 2 study arms pooled together (NLG press release).
Algenpantucel-L is currently being evaluated for patients with borderline resectable (stage II) and locally advanced unresectable (stage III) PC in the randomized phase III trial PILLAR, which launched in October 2012 and is expected to complete enrollment in 2015. The study's main endpoint is overall survival. Algenpantucel-L is administered at the 300 million cells/dose every 2 weeks for up to 18 doses, in combination with modern chemotherapy regimens (i.e. FOLFIRINOX or gemcitabine/nab-paclitaxel) plus chemoradiation (capecitabine or 5-FU based) (NCT01836432). An additional study for borderline resectable PC (NCT02405585) testing algenpantucel-L with FOLFIRINOX and stereotactic body frame radiotherapy (SBRT) will open to accrual shortly.
Allogeneic GM-CSF secreting vaccines
Granulocyte macrophage colony-stimulating factor (GM-CSF) is a cytokine that promotes the growth and differentiation of dendritic cells (DCs), which are considered to be the most efficient antigen presenting cells (APCs). Dranoff et al. showed that when engineered to express GM-CSF, poorly immunogenic irradiated tumor cells were able to generate a robust, long-lasting, tumor-specific immune response.Citation41 These preclinical findings provided the rationale for the development of GVAX, a whole cell-based vaccine consisting of 2 irradiated allogeneic human PC cell lines engineered to express GM-CSF.
Following a phase I study confirming the safety and tolerability of GVAX,Citation42 Lutz et al. conducted a phase II study of GVAX in patients with resected PC (stages I/;II).Citation43 Patients received the GVAX vaccine 8 to 10 weeks following surgery, 3 additional treatments following 5-FU-based chemoradiation, and a final booster injection 6 months later. The primary endpoint, median DFS was 17.3 months, and the median OS was 24.8 months. At 1 year, 67% of patients were disease-free, and 85% were alive.
Two studies have evaluated different strategies to enhance the anti-tumor immune response elicited by GVAX. Cyclophosphamide (Cy) has previously been shown to inhibit TregsCitation44 which provided the basis for a pilot study of GVAX with Cy (Cy/GVAX) in patients with metastatic (stage IV) PC (treatment naïve or previously treated).Citation45 Compared to GVAX alone, the addition of Cy was associated with a trend toward improved OS (130 vs 69 days), and enhanced mesothelin-specific T cell responses.
GVAX has also been studied in combination with a Listeria monocytogenes (Lm)-based vaccine. Lm directly targets DCs, and activates both innate and adaptive cellular immunity. CRS-207 is a live-attenuated strain of Lm engineered to express mesothelin, a tumor-associated antigen (TAA) commonly expressed by PCs. The actA and internalin B (InlB) genes encoding 2 virulence factors of the Listeria bacteria have been inactivated. In a phase I study of CRS-207 in patients with multiple cancer types, 3 patients with metastatic (stage IV) PC previously treated with GVAX lived >15 months.Citation46 These intriguing findings prompted a phase II study, which randomized 90 patients with previously treated (50% had ≥2 prior chemotherapies) metastatic (stage IV) PC to 2 doses of Cy/GVAX (every 3 weeks) followed by 4 doses of CRS-207 (Arm A), or to 6 doses of Cy/GVAX alone (Arm B).Citation47 One treatment course was 6 vaccine doses (20 weeks), but after a 4 week rest, patients clinically stable were offered additional treatment courses. The addition of CRS-207 to Cy/GVAX significantly improved OS, the primary study endpoint (6.1 vs. Three.9 mo; HR 0.59; p = 0.02). The 1-year probability of survival was estimated at 24% (95% confidence interval (CI): 530%–), and 12% for the combination and GVAX alone arms, respectively. In a secondary per-protocol analysis, patients who received at least 3 doses of therapy (2 doses of Cy/GVAX and 1 dose of CRS-207 in Arm A, or 3 doses of Cy/GVAX in Arm B) were noted to have an even larger OS benefit (median OS 9.7 vs. Four.6 months, HR 0.53; p = 0.02). The superior results in Arm A were also noted in patients who received >2 prior chemotherapy regimens (median OS 5.1 vs. Three.7 months for Arm A vs. Arm B, respectively; HR 0.34; p = 0.001). The vaccines were well tolerated, and the most frequent adverse events were local injection reactions, and transient fevers, chills, nausea, vomiting and fatigue. Immunological biomarkers assessed in this study were mesothelin-specific CD8 T-cells, and higher levels after treatment (week 20) compared to baseline were only detected among patients treated with Cy/GVAX plus CRS-207 (Arm A) (p = 0.042). Overall 44 patients were evaluable for mesothelin-specific T cell responses (29 in Arm A, 15 in Arm B). The induction of mesothelin-specific CD8 T-cells after 2 Cy/GVAX injections were directly correlated with the likelihood of survival beyond 6 months (p = 0.0033), irrespective of treatment arm. This study is one of the first to demonstrate a meaningful survival benefit from immunotherapy in patients with metastatic PC. However, it is important to note that patients with ascites and pleural effusions, or a diagnosis of thromboembolism in the prior 2 months were excluded. In addition, among patients treated with the combination, 20% had stable disease at study entry, and 64% had an ECOG performance status of 0. Thus, this was a highly selected group of patients with metastatic PC, which may limit the generalizability of the findings to the general population.
A larger, randomized phase IIb study (“Safety and Efficacy of Combination Listeria/GVAX Pancreas Vaccine in the Pancreatic Cancer Setting (ECLIPSE))” (NCT02004262) is currently ongoing. This study includes patients with previously treated metastatic PC and randomizes them to Cy/GVAX plus CRS-207, CRS-207 alone, or chemotherapy alone.
Peptide and protein vaccines
Several peptide or protein-based vaccines have been studied in PC based on common tumor-associated antigens and epitopes shown to elicit cytotoxic or humoral immune responses in preclinical studies.
RAS
Mutant KRAS has been considered a promising vaccine target, as KRAS gene mutations are present in the majority of PCs, but are not found in normal cells.Citation8 In the first study to evaluate this strategy, 5 patients (unknown stage) with KRAS mutant PC were injected with autologous peripheral blood mononuclear cells (PBMCs) pulsed with a mutant ras peptide and 2 mounted an immune response.Citation48 A subsequent study by the same investigators evaluated a ras peptide vaccine in combination with intradermal GM-CSF in patients with all PC stages.Citation49 Patients with resected (stage I/;II) PC (n = 10) received a vaccine containing a single mutant ras peptide, while those with advanced disease (stages III/;IV) (n = 38) received a vaccine containing 4 of the most commonly identified ras peptides in their tumor cells. In this study, 25 of 43 evaluable patients with all disease stages (58%) developed a peptide-specific immune response, and immune responders lived significantly longer than non-responders (148 vs. 61 days; p = 0.0002). In a follow up report, including the 10 patients with resected (stages I/;II) PC and an additional 13 patients with PC treated with the ras vaccine plus GM-CSF after surgery, 17 out of 20 evaluable (85%) had a documented immune response to the vaccine.Citation50 Five of these patients survived for at least 5 years, all of whom were immune responders. Abou Alfa et al. conducted a single institution pilot study of a ras peptide vaccine given monthly for 3 months in combination with GM-CSF to 24 patients with KRAS mutant, resected (stage I/;II) PC, the majority of whom had also received adjuvant chemotherapy. Only 1 of 9 evaluable patients had an anti-KRAS immune response, and the median OS of the entire cohort was 20.3 months.Citation51
GI-4000 is a whole heat-killed recombinant Saccharomyces cerevisiae yeast vaccine that expresses mutated human ras proteins. Phase I data in 6 patients with pancreatic and colorectal cancers selected for KRAS HRAS or NRAS gene mutations, noted prolonged survival in several patients, associated with mutation-specific T cell responses.Citation52 A randomized phase II adjuvant trial tested gemcitabine with and without GI-4000 after surgery in 176 patients with stages I/;II PC.Citation53 Among 39 patients with margin-positive resection (R1) in this study, 47% percent of those treated with GI-4000 and 8% in the control arm developed a ras-specific immune response (p = 0.032). Moreover, the addition of GI-4000 was associated with a trend toward improved outcomes: median recurrence-free survival (RFS) 9.4 vs. Eight.4 months, 1 y survival 72% vs. 56%, and median OS 19.6 vs. Seventeen.2 months for the combination vs. gemcitabine alone, respectively. More recently, results presented at the 2014 AACR-RAS Oncogene conference showed differential immunological responses to mutated KRAS for R0 (margin negative resection) vs. R1 (microscopic margin positive resection) patients. While the overall study results have not yet been published, in general, R0 patients with glycine to arginine mutation (KRAS G12R) vs. other KRAS gene mutations, irrespective of treatment, had superior prognosis (39 vs 27.7 months). In addition, the median OS was 19 months longer among the GI-4000 treated patients in the KRAS G12R subgroup as compared to the control arm with gemcitabine alone.Citation54,55
Telomerase
Telomeres are repetitive nucleotide sequences located at the ends of chromosomes, which become progressively truncated with repeated rounds of DNA replication. Telomerase is a ribonucleotide enzyme which maintains telomeres and confers cancer cells immortality; it is mostly expressed in cancer but not normal cells, and its activation contributes to malignant transformation in PCs.Citation56,57
The GV1001 vaccine consists of fragments of the human telomerase reverse transcriptase (hTERT) protein, which is able to bind to multiple HLA class molecules and activates a CD4/;CD8 T-cell immune response. In a phase I/;II, dose-escalation study, GV1001 was administered in combination with GM-CSF to patients with treatment naïve advanced (stages III/;IV) PC. The vaccine was safe, and elicited an immune response in 24 of 38 (63%) patients. Median OS was 8.6 months for patients who received the intermediate vaccine dose vs. Four and 5.1 months for the low and high doses, respectively. Median OS was 7.2 months for immune responders vs. Two.9 months for non-immune responders.Citation58
Results from TeloVac, an open-label, phase III study of gemcitabine/capecitabine chemotherapy with or without GV1001, were recently reported.Citation59 In this study, 1062 patients with previously untreated locally advanced (stage III) or metastatic (stage IV) PC were randomized to chemotherapy with or without sequential or concurrent GV1001. All patients received gemcitabine 1000mg/m2 given on days 1, 8 and 15, and capecitabine 830 mg/m2 twice daily on days 1–21 every 28 d cycles. Those in the sequential arm also received GM-CSF and GV1001 on days 1, 3 and 5 during weeks 2 to 4, and every 6 months thereafter. Patients in the concurrent arm received GM-CSF and GV1001 on days 1, 3 and 5 with each cycle of chemotherapy. With a median follow-up of 6 months, median OS was 7.9, 6.9 and 8.4 months in the chemotherapy alone, sequential chemo-immunotherapy (HR 1.19, p = 0.05, vs. chemotherapy), and concurrent chemo-immunotherapy (HR = 1.05, p = 0.64, vs. chemotherapy) arms, respectively. Median time to disease progression was also similar for the 3 groups. Moreover, the development of an immune response, measured in a subgroup of patients and defined as a delayed type hypersensitivity response and/or T-cell proliferation, did not translate into improved outcomes.
PrimoVax was another phase III trial comparing gemcitabine alone to GV1001 plus GM-CSF followed by gemcitabine at the time of progression in patients with advanced stages III/;IV PC. This study was closed early due to lack of efficacy in the experimental arm.Citation60
Vascular Endothelial Growth Factor Receptor (VEGFR)
The profound immunoinflammatory tumor microenvironment encompasses VEGF and the VEGFRs which have been shown to play a role in PC oncogenesis.Citation61 VEGFR-2 is the main VEGFR involved in neovascularization, and preferentially expressed by newly formed tumor blood vessels. VEGFR-2 is an immunogenic TAA in pancreatic cancer.Citation62,63 In a phase I, dose-escalation study, patients with advanced (stages III/;IV) PC (treatment naïve or pretreated) received gemcitabine and a vaccine consisting of an epitope peptide for VEGFR-2 (VEGFR2–169).Citation64 Of the 18 patients who received at least 1 dose of treatment, 11 (61%) were found to have peptide-specific cytotoxic T cells, and the median OS of the population was 8.7 months. Another recent report noted OS of 7 months among refractory pancreatic cancer patients treated with a multi-peptide vaccine targeting cancer testis antigens and VEGFR-1 and −2.Citation62 VXM01 is a novel oral vaccine of live attenuated Salmonella bacteria expressing a VEGFR-2 encoding plasmid. Preliminary biomarker data from a phase I study among 30 patients treated with VXM01 and 15 patients treated with placebo, noted Salmonella specific humoral immune responses and VEGFR2 effector T cells responses in the study arm, together with pharmacodynamic markers of antiangiogenesis: decreased tumor perfusion and higher VEGF-A serum levels.Citation65 Clinical results are not yet available.
Survivin
Survivin is a member of the inhibitor of apoptosis family and functions to inhibit caspase, thereby negatively regulating apoptosis. Survivin is expressed by the majority of PC cells, but not by non-malignant tissue.Citation66 Kameshima et al. conducted a small study of a survivin-peptide based vaccine, HLA-A2-restricted in 6 patients with advanced (stages III/;IV) PC (treatment naïve or pretreated).Citation67 More than 50% of patients had evidence of immunologic response associated with clinical benefit. Wobser et al. reported on a patient with refractory stage IV PC treated with an HLA-A2 restricted survivin-based peptide vaccine who obtained a complete remission lasting for 8 months, associated with immune-reactivity against survivin.Citation68 Despite these preliminary interesting results, to our knowledge, survivin-based vaccines are not actively undergoing clinical trials in PC.
Gastrin
Gastrin, a hormone that stimulates acid production from gastric parietal cells is upregulated by pancreatic cancers, and has been shown to promote oncogenesis.Citation69 In a randomized, double-blind, placebo-controlled, multicenter trial, 154 advanced (stages III/;IV) PC patients who were not candidates for or were unwilling to receive chemotherapy were treated with a gastrin-based vaccine (G17DT), or placebo.Citation70 Median OS was significantly longer for patients who received the vaccine (151 vs. 82 d for vaccine vs. placebo, respectively; p = 0.03). Moreover, those who developed immune-reactivity against gastrin lived longer than nonresponders or those treated with placebo (median OS 176 vs. 63 vs. 83 days, respectively; p = 0.003).
Heat shock proteins
Heat shock proteins (HSPs) are present in all living organisms and help to stabilize and transport intracellular proteins. Some HSPs can bind intracellular peptides, including tumor-specific peptides, and present them to the immune system in the context of cell surface MHC class I molecules. In preclinical studies, tumor-derived HSP-peptide complexes have been shown to induce an anti-tumor immune response.Citation71 Maki et al. evaluated a vaccine comprised of HSP96-peptide complexes produced from resected tumor specimens. In this phase I study, 10 patients with resected stages I/;II PC were vaccinated with autologous HSP96-peptide complexes weekly for 4 weeks; these patients did not receive any other adjuvant therapy.Citation72 The vaccine was well-tolerated, and median OS was 2.2 y
Dendritic cell vaccines
Dendritic cells (DCs) are considered to be the most efficient antigen presenting cells (APCs). They can process and present antigens to both CD8+ and CD4+ T cells and can prime naïve T cells. While a promising technology, the production of a DC vaccine is a cumbersome process. DCs must be isolated from the patient's peripheral blood, loaded with TAAs or with TAA-coding or tumor-derived mRNA, and re-infused back into the patient. These manipulated DCs then travel to lymph nodes and present to immune effector cells while also providing appropriate co-stimulatory signals.
Several studies have evaluated DC-based vaccine strategies in patients with PC. In a phase I trial, peripheral blood DCs were collected via apheresis from 7 patients with advanced stages III/;IV PC and pulsed with a mucin (MUC1)-peptide.Citation73 MUC1 is a large, transmembrane glycoprotein that is aberrantly glycosylated and overexpressed by the majority of PCs, and has previously been shown to be capable of eliciting an immune response.Citation74,75 Patients received 3 to 4 doses of the vaccine, which were safe, and in 2 of 7 patients, resulted in a significant increase in interferon (IFN)-Υ and granzyme B production by peripheral blood mononuclear cells (PBMCs). In another phase I/;II trial, autologous DCs were transfected with mucin cDNA.Citation75 The vaccine was administered to 10 patients, including 2 with PC, both of whom demonstrated an increase in the frequency of post-vaccine mucin-specific IFN-Υ-secreting CD8+ T cells. Lepisto et al. treated 12 patients with resected (stages I/;II) pancreatic (n = 10) or biliary cancer (n = 2) with autologous DCs loaded with a MUC1-derived peptide.Citation76 With more than 4 y of follow-up 4 patients were alive without disease recurrence. Another study evaluated a DC-based vaccine with or without lymphokine-activated killer (LAK) cells combined with gemcitabine and/or S1 in patients with advanced (stages III/;IV) PC refractory to standard treatment.Citation77 Among 49 patients, there were 2 with a complete response, 5 with a partial response, and 10 patients with stable disease. The median OS overall was 360 d.
Carcinoembryonic antigen (CEA), an antigen expressed by many pancreatic cancers, has also been used in DC-based immune strategies. In a study of 3 patients with stages I/;II PC who had undergone neoadjuvant chemoradiotherapy and resection, autologous monocyte-derived DCs loaded with mRNA encoding for CEA resulted in all patients being alive disease-free more than 2.5 y from diagnosis.Citation78
Vaccines with microorganisms
Viral- and bacterial-based vaccines take advantage of the natural immunogenicity of most viruses and bacterium. These vaccine vectors can be engineered to express tumor-associated antigens, thereby eliciting a T cell response against the antigen(s) encoded by the vaccine vector.
In a phase I study, 10 patients with advanced stages III/;IV PC (treatment naïve or pretreated) were primed with a vaccinia virus expressing an HLA-A2 restricted CEA peptide and a MUC-1 peptide along with the co-stimulatory molecules B7.1, ICAM-1 and LFA-3 (PANVAC-v).Citation79 Patients then received booster vaccinations with a fowlpox virus expressing the same antigens and co-stimulatory molecules. The vaccinations were given in conjunction with GM-CSF. Of 8 evaluable patients, 5 (62.5%) had a significant increase in CEA- or MUC-1-specific immune responses. The median OS for the whole population was 6.3 months, and OS was significantly improved for patients who developed a vaccine-specific T cell response (15.3 vs.3.9 mo for responders vs. non-responders, respectively; p = 0.002).
Dalgleish et al recently reported on a heat-killed whole cell vaccine of Mycobacterium obuense, IMM-101 combined with gemcitabine for first line treatment of 110 patients with advanced stages III/;IV pancreatic cancer (n = 92 stage IV) in the IMAGE 1 study.Citation80 The observed OS was 7.2 months versus 5.6 months with gemcitabine alone (p = 0.022, HR = 0.60), with a similar improvement of PFS 4.4 vs 2.3 months (p < 0.001, HR = 0.40).Citation80 In an updated analysis, patients with stage IV disease demonstrated the largest survival advantage (OS 7 months with IMM-101 vs 4.4 months with gemcitabine alone, p = 0.01, HR = 0.54, and 1-year OS 22.4% vs 11.5% in the corresponding treatment arms.Citation81
Vaccine strategies in PC include many pilot studies with small numbers of patients who are mostly hypothesis generating. A few larger phase II studies in various disease stages (algenpantucel-L and GI-4000 for resected stages I/;II PC, and Cy/GVAX with CRS207, IMM-101, GV1001, and G17DT for advanced stages III/;IV PC, have showed mixed results, but all noted the overarching conclusion that patients who are able to mount humoral or cellular immune responses to the vaccines derive clinical benefit, with superior survival rates or prolonged responses/stable diseases.
Immune checkpoint blockade and novel immunotherapies
The T cell response is initiated when the T cell receptor recognizes an antigen presented by an APC in the context of an MHC molecule. In order to become activated, however, T cells require a second co-stimulatory signal. Examples of co-stimulatory molecules include CD80 and CD86, which are expressed by APCs, and bind to CD28 on the surface of T cells. These stimulatory signals are balanced by inhibitory signals (immune checkpoints), which function to maintain self-tolerance, and to dampen the amplitude and duration of an inflammatory response, thereby limiting collateral damage to healthy tissue. Immune checkpoint molecules include cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), programmed death ligand 1 and 2 (PD-L1 and PD-L2). While most malignant cells lack the co-stimulatory molecules required for T cell activation, many cancers express inhibitory molecules, and can co-opt certain immune checkpoints as a mechanism of immune resistance.
Strategies that target these immune checkpoints represent one of the most promising anti-cancer therapeutics, and are being evaluated in multiple cancer types, including PC. Royal et al. evaluated ipilimumab, a fully humanized antibody against CTLA-4, in 27 patients with LAPC (stage III) or metastatic (stage IV) PC, the majority (74%) previously treated.Citation82 Ipilimumab 3mg/kg was given every 3 weeks, with 4 doses per course, and a maximum of 2 courses. By standard RECIST criteria there were no objective responses, though 1 patient did have a clinically meaningful, delayed response (after initial progression) lasting 9 months. The majority of patients had to discontinue treatment early due to side effects related to cancer progression. As a result, only 7 (26%) patients received 4 doses (1 course), and only 2 (7%) received the planned 8 doses (2 courses) of treatment. This highlights the need to utilize immune therapies, which can have delayed efficacy, to patients without rapidly progressive disease.
Immune checkpoint blockade has also been tried in combination with chemotherapy. Tremelimumab, another fully humanized anti-CTLA-4 monoclonal antibody, was given in combination with gemcitabine to 28 patients with previously untreated, metastatic (stage IV) PC.Citation83 In this phase Ib study, patients received gemcitabine on days 1, 8, and 15 of each 28-day cycle, and tremelimumab escalating doses, on day 1 of each 84-day cycle. The combination was safe, but the antitumor activity was modest, with median overall survival of 7.4 months. Two patients (7%) had PR, and 7/28 (25%) had SD for more than 10 weeks. The 2 patients who responded attained PR at week 8, and received 2 cycles (12 weeks), and 4 cycles (24 weeks) of treatment, respectively. For patients who were treated with tremelimumab at the 10 mg/kg and 15 mg/kg dose levels, median OS was 8 and 7.5 months, respectively.
In an attempt to both induce and maintain an anti-tumor T cell response, Le et al. studied ipilimumab in combination with GVAX.Citation84 In this phase Ib study meant to generate only preliminary efficacy data, 30 patients with previously treated (1–4 prior chemotherapy regimens), advanced (stages III/;IV) PC were randomized 1:1 to ipilimumab with or without GVAX. Ipilimumab 10 mg/kg and GVAX were given on weeks 1, 4, 7 and 10, and every 12 weeks thereafter (maintenance) for those with SD or response at the conclusion of the treatment period. Because immunotherapy can be associated with initial tumor progression followed by a delayed response, investigators allowed patients with initial PD to continue on protocol if they did not have clinical deterioration. The addition of the vaccine to ipilimumab was safe and did not significantly increase toxicity. While the study was not powered to evaluate efficacy, there was a trend toward improved OS survival (5.7 vs. Three.6 months, HR 0.51, p = 0.072) and 1-year survival (27% vs. Seven%) for patients in the combination arm. Best response was SD in 2/15 patients (13%) in each arm. Among the 14 patients evaluable for mesothelin-specific T cell responses after 2 treatments, those with an immunological response (n = 6, 4 in the ipilimumab plus GVAX arm, and 2 with ipilumumab alone) had a median OS of 15.7 months compared to 4.1 months for those without an immunological response (n = 8) (p = 0.031). In this study, only 3 patients with localized disease (n = 2) or lung metastases (n = 1) were noted to have radiographic disease shrinkage or CA19–9 responses, and only 2 patients with liver metastases had long OS (10 and 15 months), underscoring the importance of immune sanctuaries such as the liver, in limiting the efficacy of immune-based therapies. In addition, the authors noted that it was necessary for patients to undergo prolonged treatment exposure (>7 weeks) to derive meaningful benefit from this combined immunotherapy approach. Building upon these results, the Johns Hopkins' group is leading a randomized phase II trial of FOLFIRINOX for 8–12 doses induction chemotherapy followed by GVAX vaccine plus ipilimumab every 3 weeks × 4, then every 8 weeks, vs continuing FOLFIRINOX until progression or toxicity (NCT01896869).
Novel immunotherapeutic combination approaches using Cy/GVAX followed by CRS-207 with or without the PD-1 inhibitor nivolumab are being studied in metastatic (stage IV) PC (NCT02243371). For resectable (stages I/;II) PC in study NCT02451982, patients are treated with neoadjuvant Cy/GVAX followed by surgery followed by adjuvant chemotherapy and Cy/GVAX with or without nivolumab.
Another immunologic approach explored in PC has been the manipulation of the co-stimulatory molecule, CD40. CD40 is a member of the tumor-necrosis factor (TNF) receptor family, and is expressed by APCs, B cells and non-immune cells.Citation85 CD40 stimulation promotes DC maturation and is critical to the generation of an effective anti-tumor immune response. Beatty et al. studied CP-870,893, an agonist CD40 monoclonal antibody, in combination with gemcitabine in 21 patients with previously untreated LAPC or metastatic PC.Citation86 Gemcitabine was administered according to standard schedule, and CP-870,893 was given on day 3 of each 28 day cycle. By FDG-PET-CT, 88% of patients had a response in both the primary and metastatic lesions; by standard RECIST criteria, 4 (19%) had a PR, 11 (52%) had SD. One patient with a PR had complete resolution of a 7.6 cm liver lesion, and significant reduction in the size of another liver lesion, which upon biopsy, showed only necrosis and no active cancer. Another patient who experienced complete resolution of all liver lesions and a significant reduction in the size of the primary tumor was taken for primary pancreatic cancer resection but subsequently progressed. Median PFS and OS were 5.6 months and 7.4 months, respectively. Studies with the CD40 agonist (RO7009789) are being planned in combination with chemotherapy for neoadjuvant treatment prior to surgery for stages I/;II PC patients.
Recently, Zippelius et al. demonstrated in a mouse model that CD40 agonists up-regulate PD-L1 expression.Citation87 Thus, combination strategies with agonistic CD40 antibodies and PD1/;PD-L1 inhibitors may be a novel way of inducing immunocompetent antitumor activity.
Finally, indoleamine 2,3-dioxygenase (IDO) is a cytosolic, heme-containing enzyme that catalyzes the first and rate-limiting step in the metabolism of L-tryptophan to kynurenine, leading to trypthophan depletion. Expressed in antigen presenting cells, IDO is induced by interferon-γ and other proinflammtory cytokines, and its main activity is to inhibit T cell responses to autoantigens and fetal alloantigens in vivo.Citation88,89 IDO exerts its immunomodulatory effects by inhibiting effector T-cell proliferation, preventing the formation of memory T-cells, and inducing regulatory T cells differentiation. The immunosuppressive effects of myeloid derived suppressor cells (MDSCs) may depend on IDO activity.Citation90 IDO is upregulated in PC and contributes to its immunosuppressive microenvironment.Citation91 Therapeutic inhibition of IDO therefore, represents a promising treatment strategy for many cancers, including PC. In a pancreatic cancer tumor model, Manuel et al demonstrated significant antitumor activity with a combination of Salmonella-based therapy targeting IDO combined with PEGPH20, an enzyme capable of depleting tumor hyaluronic acid, and potentially enhancing immune cells infiltration in the PC tumor stroma.Citation92 Other in vivo data in a melanoma model demonstrated synergism between CTLA-4 or PD1/;PDL1 inhibition and IDO blockade which can be further exploited in the clinic, and possibly, in other tumors.Citation93
Clinically, IDO inhibitors are undergoing clinical trials in several tumors types such as melanoma, breast, prostate, brain and pancreatic cancers. In PC, the IDO inhibitor indoximod (600 mg orally BID to 1200 mg orally BID) is being studied in a phase I/;II trial in combination with gemcitabine and nab-paclitaxel in the first line treatment of 80 patients with metastatic disease (NLG2104, NCT02077881). Another IDO1 enzyme inhibitor, GDC-0919 is being studied in solid tumors, and phase Ib clinical trials in combination with PDL1 inhibition (MPDL3280A) (NCT02471846), and with agonist anti-OX40 therapies are planned to begin shortly.
Inhibition of the JAK/STAT pathway
The Janus kinase (JAK) and the signal transducer and activator of transcription (STAT) pathway represent an important target in PC. This pathway is constitutively activated in many malignancies, mediates cytokine signaling,Citation94 and promotes tumorigenesis, including in PC.Citation95 Moreover, PC is characterized by a chronic inflammatory state, with cachexia and weight loss being prominent clinical features. Pre-clinical evidence suggests that aberrant activation of the JAK-STAT pathway promotes this inflammatory state.Citation96 A phase II, randomized study (RECAP) of capecitabine with or without ruxolitinib, an oral inhibitor of JAK1 and JAK2 kinases, was conducted in 127 patients with metastatic PC who had progressed on first-line therapy.Citation97 There was no significant difference in OS for the overall study population (median OS 137 vs 130 days, HR = 0.79, p = 0.25), but in a pre-specified subgroup of patients with an elevated C-reactive protein (CRP) >13 mg/L, OS favored ruxolitinib (83 vs 55 days, HR 0.473, p = 0.0053). Among patients with an elevated CRP, 42% vs. Eleven% (HR 0.47, p = 0.01) were alive at 6 months for the capecitabine/ruxolitinib arm vs control arm respectively. Janus 1 (NCT02117479) is a randomized phase III trial in second line treatment of stage III and IV pancreatic cancer patients with CRP levels >10 mg/L, randomizing patients to capecitabine with ruxolitinib vs capecitabine and placebo, which plans to enroll 310 patients by 2016. A similar study, Janus 2 (NCT02119663) plans to complete enrollment of 270 patients by 2017.
Conclusion
Several immunotherapy strategies, including cancer vaccines, have been tested in patients with early and advanced stage PC, but to date, studies have not shown uniform or robust long-term efficacy. The phase II study of the algenpantucel-L vaccine in combination with chemotherapy and radiation for patients with resected PC yielded encouraging results, particularly for patients who mounted humoral immunological responses against several tumor-associated epitopes. Final results from the subsequent phase III IMPRESS study are expected soon. Given the 28.5 months median OS in the pooled analysis of both study arms in IMPRESS, it is expected that algenpantucel-L will have a significant impact in the outcomes of resected pancreatic cancer patients. In addition, other trials (PILLAR) of algenpantucel-L in combination with FOLFIRINOX or nab-paclitaxel/gemcitabine and radiotherapy for patients with locally advanced and borderline resectable PC are currently ongoing. The hyperacute vaccine platform is advancing in other cancers as well, such as in melanoma, renal cell and lung cancer patients, with ongoing clinical trials.
Several obstacles in the success of immune targeted therapies have been identified, including the immune tolerance and immunosuppressive cancer microenvironment, and the fact that most vaccines are not personalized to the patient's own tumor antigenic repertoire. Many clinical trials are trying to optimize combinations with other immunotherapy agents, chemotherapies, radiation therapy, or other molecularly targeted agents, as it is unlikely that one immunotherapy alone would be able to have sufficient cytotoxic effect to control pancreatic cancer progression. Therapeutic depletion of the fibroinflammatory pancreatic tumor stroma may represent another way to facilitate infiltration with immune effector cells. We also learned that patients with PC who benefit most from immunotherapies, to date have been those with slowly progressive tumors and indolent biology, such as patients with localized disease or lung metastases only. In addition, patients whose tumors progress slowly are more likely to undergo sequential immunotherapy administration over weeks/months and able to develop humoral or cellular immunity response, with longer than expected survival rates. Despite these challenges, it seems that PC can be receptive to immune modulation as we continue to learn many lessons on how to best deliver multimodality immune targeted therapies.
Disclosure of potential conflicts of interest
EG Chiorean and AL Coveler received research grant support from NewLink Genetics; GR Rossi, N Vahanian, C Link are employees of NewLink Genetics.
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359-86; PMID:25220842; http://dx.doi.org/10.1002/ijc.29210
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65(1):5-29; PMID:25559415; http://dx.doi.org/10.3322/caac.21254
- Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, La Vecchia C. Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980. Ann Oncol 2013; 24(10):2657-71; PMID:23921790; http://dx.doi.org/10.1093/annonc/mdt301
- Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zulke C, Fahlke J, Arning MB, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013; 310(14):1473-81; PMID:24104372; http://dx.doi.org/10.1001/jama.2013.279201
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15(6):2403-13; PMID:9196156
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, et al. FOLFIRINOX vs. gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364(19):1817-25; PMID:21561347; http://dx.doi.org/10.1056/NEJMoa1011923
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369(18):1691-703; PMID:24131140; http://dx.doi.org/10.1056/NEJMoa1304369
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno, A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008; 321(5897):1801-6; PMID:18772397; http://dx.doi.org/10.1126/science.1164368
- Schmitz-Winnenthal FH, Volk C, Z'Graggen K, Galindo L, Nummer D, Ziouta Y, Bucur M, Weitz J, Schirrmacher V, Buchler MW, Beckhove P. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res 2005; 65:10079-87; PMID:16267034; http://dx.doi.org/10.1158/0008-5472.CAN-05-1098
- Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 2004; 28, e26-31; PMID:14707745; http://dx.doi.org/10.1097/00006676-200401000-00023
- Zhang Y, Yan W, Mathew E, Bednar F, Wan S, Collins MA, Evans RA, Welling TH, Vonderheide RH, Pasca di Magliano M. CD4+ T lymphocyte ablation prevents pancreatic carcinogenesis in mice. Cancer Immunol Res 2014; 2(5):423-35; PMID:24795355; http://dx.doi.org/10.1158/2326-6066.CIR-14-0016-T
- Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006; 12(18):5423-34; PMID:17000676; http://dx.doi.org/10.1158/1078-0432.CCR-06-0369
- Esposito I, Menicagli M, Funel N, Bergmann F, Boggi U, Mosca F, Bevilacqua G, Campani D. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol 2004; 57(6):630-636; PMID:15166270; http://dx.doi.org/10.1136/jcp.2003.014498
- Dodson LF, Hawkins WG, Goedegebuure P. Potential targets for pancreatic cancer immunotherapeutics. Immunotherapy 2011; 3(4):517-37; PMID:21463193; http://dx.doi.org/10.2217/imt.11.10
- Sideras K, Braat H, Kwekkeboom J, van Eijck CH, Peppelenbosch MP, Sleijfer S, Bruno, M. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev 2014; 40(4):513-22; PMID:24315741; http://dx.doi.org/10.1016/j.ctrv.2013.11.005
- Ikemoto T, Yamaguchi T, Morine Y, Imura S, Soejima Y, Fujii M, Maekawa Y, Yasutomo K, Shimada M. Clinical roles of increased populations of Foxp3+CD4+ T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas 2006; 33:386-90; PMID:17079944; http://dx.doi.org/10.1097/01.mpa.0000240275.68279.13
- Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011; 60(10):1419-30; PMID:21644036; http://dx.doi.org/10.1007/s00262-011-1028-0
- Bellone G, Turletti A, Artusio E, Mareschi K, Carbone A, Tibaudi D, Robecchi A, Emanuelli G, Rodeck U. Tumor-associated transforming growth factor-β and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Amer J Pathol 1999; 155:537-47; http://dx.doi.org/10.1016/S0002-9440(10)65149-8
- Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 2006; 176:6752-61; PMID:16709834; http://dx.doi.org/10.4049/jimmunol.176.11.6752
- Geng L, Huang D, Liu J, Qian Y, Deng J, Li D, Hu Z, Zhang J, Jiang G, Zheng S. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol 2008; 134:1021-27; PMID:18347814; http://dx.doi.org/10.1007/s00432-008-0364-8
- Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 2007; 13:2151-57; PMID:17404099; http://dx.doi.org/10.1158/1078-0432.CCR-06-2746
- Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-Associated Stromal Fibroblasts Promote Pancreatic Tumor Progression. Cancer Res 2008; 68(3):918-26; PMID:18245495; http://dx.doi.org/10.1158/0008-5472.CAN-07-5714
- Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010; 17:135-47; PMID:20138012; http://dx.doi.org/10.1016/j.ccr.2009.12.041
- Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Science 2010; 330:827-30; PMID:21051638; http://dx.doi.org/10.1126/science.1195300
- Feig C, Jones JO, Kraman M, Wells RJB, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PDL1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci 2013; 110:20212-7; http://dx.doi.org/10.1073/pnas.1320318110
- Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res 2014; 2(3):187-93; PMID:24778314; http://dx.doi.org/10.1158/2326-6066.CIR-14-0002
- Hardacre JM, Mulcahy M, Small W, Talamonti M, Obel J, Krishnamurthi S, Rocha-Lima CS, Safran H, Lenz HJ, Chiorean EG. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg 2013; 17:94-100; PMID:23229886; http://dx.doi.org/10.1007/s11605-012-2064-6
- Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (α-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta 2008; 1780:75-88; PMID:18047841; http://dx.doi.org/10.1016/j.bbagen.2007.11.003
- Galili U, Anaraki F, Thall A, Hill-Black C, Radic M. One percent of human circulating B lymphocytes are capable of producing the natural anti-Gal antibody. Blood 1993; 82:2485-93; PMID:7691263
- Rother RP, Squinto SP. The α-galactosyl epitope: a sugar coating that makes viruses and cells unpalatable. Cell 1996; 86:185-8; PMID:8706123; http://dx.doi.org/10.1016/S0092-8674(00)80090-2
- Link CJ Jr, Seregina T, Atchison R, Hall A, Muldoon R, Levy JP. Eliciting hyperacute xenograft response to treat human cancer: α(1,3) galactosyltransferase gene therapy. Anticancer Res 1998; 18:2301-8; PMID:9703870
- Rossi GR, Mautino MR, Unfer RC, Seregina TM, Vahanian N, Link CJ. Effective treatment of preexisting melanoma with whole cell vaccines expressing α(1,3)-galactosyl epitopes. Cancer Res 2005; 65:10555-61; PMID:16288048; http://dx.doi.org/10.1158/0008-5472.CAN-05-0627
- Hemstreet GP 3rd, Rossi GR, Pisarev VM, Enke CA, Helfner L, Hauke RJ, Tennant L, Ramsey WJ, Vahanian NN, Link CJ. Cellular immunotherapy study of prostate cancer patients and resulting IgG responses to peptide epitopes predicted from prostate tumor-associated autoantigens. J Immunother 2013; 36:57-65; PMID:23211622; http://dx.doi.org/10.1097/;CJI.0b013e3182780abc
- Riker AI, Rossi GR, Masih P, Alsfeld LC, Denham F, Tennant L, Ramsey WJ, Vahanian NN, Link CJ. Combination immunotherapy for high-risk resected and metastatic melanoma patients. Ochsner J 2014; 14(2):164-74; PMID:24940124
- Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Rich TA, Willett CG. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the US. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol 2011; 18:1319-26; PMID:21499862; http://dx.doi.org/10.1245/s10434-011-1630-6
- de Bruyn M, Wiersma VR, Helfrich W, Eggleton P, Bremer E. The ever-expanding immunomodulatory role of calreticulin in cancer immunity. Front Oncol 2015; 5:35; PMID:25750898; http://dx.doi.org/10.3389/fonc.2015.00035
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Med 2007; 13:54-61; PMID:17187072; http://dx.doi.org/10.1038/nm1523
- Rossi GR, Rocha Lima CM, Hardacre JM, Mulcahy MF, Talamonti MS, Obel JC, Safran H, Lenz HJ, Chiorean EG, Vahanian NN, et al. Anti-calreticulin antibody titers correlate with improved overall survival in a phase 2 clinical trial of algenpantucel-l immunotherapy for patients with resected pancreatic cancer. J Clin Oncol 2014; 32 5s Suppl: abstr 3029.
- Rossi GR, Hardacre JM, Mulcahy MF, Talamonti MS, Obel JC, Rocha Lima CM, Safran H, Lenz HJ, Chiorean EG, Vahanian NN, et al. Algenpantucel-L immunotherapy for pancreatic cancer induces anti-mesothelin antibody titers that positively correlate with improved overall survival. J Clin Oncol 2013; 31 Suppl: abstr 3007.
- Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Ca Res 2001; 7(12):3862-68.
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA 1993; 90:3539-43; PMID:8097319; http://dx.doi.org/10.1073/pnas.90.8.3539
- Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001; 19:145-56; PMID:11134207
- Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg 2011; 253:328-35; PMID:21217520; http://dx.doi.org/10.1097/;SLA.0b013e3181fd271c
- Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med 2005; 201:1591-602; PMID:15883172; http://dx.doi.org/10.1084/jem.20042167
- Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Ca Res 2008; 14:1455-63; http://dx.doi.org/10.1158/1078-0432.CCR-07-0371
- Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, Sterman DH, Hassan R, Lutz E, Moyer B, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Ca Res 2012; 18:858-68; http://dx.doi.org/10.1158/1078-0432.CCR-11-2121
- Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015; 33:1325-33; PMID:25584002; http://dx.doi.org/10.1200/;JCO.2014.57.4244
- Gjertsen MK, Bakka A, Breivik J, Saeterdal I, Solheim BG, Soreide O, Thorsby E, Gaudernack G. Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet 1995; 346:1399-400; PMID:7475823; http://dx.doi.org/10.1016/S0140-6736(95)92408-6
- Gjertsen MK, Buanes T, Rosseland AR, Bakka A, Gladhaug I, Soreide O, Eriksen JA, Moller M, Baksaas I, Lothe RA, et al. Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer 2001; 92:441-50; PMID:11291084; http://dx.doi.org/10.1002/ijc.1205
- Weden S, Klemp M, Gladhaug IP, Moller M, Eriksen JA, Gaudernack G, Buanes T. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer 2011; 128:1120-28; PMID:20473937; http://dx.doi.org/10.1002/ijc.25449
- Abou-Alfa GK, Chapman PB, Feilchenfeldt J, Brennan MF, Capanu M, Gansukh B, Jacobs G, Levin A, Neville D, Kelsen DP, O'Reilly EM. Targeting mutated K-ras in pancreatic adenocarcinoma using an adjuvant vaccine. Am J Clin Oncol 2011; 34:321-5; PMID:20686403; http://dx.doi.org/10.1097/;COC.0b013e3181e84b1f
- Cohn A, Morse MA, O'Neil B, et al. Treatment of ras mutation-bearing solid tumors using whole recombinant S cerevisiae yeast expressing mutated ras: preliminary safety and immunogenicity results from phase I trial. J Clin Oncol 2005; 23(16s):2571.
- Muscarella P, Wilfong LS, Ross SB, Richards DA, Raynov J, Fisher WE, Flynn PJ, Whiting SH, Rosemurgy A, Harrell FE, et al. A randomized, placebo-controlled, double blind, multicenter phase II adjuvant trial of efficacy, immunogenicity, and safety of GI-4000 plus gemcitabine versus gemcitabine alone in patients with resected pancreas cancer with activating RAS mutations/survival and immunology assays of the R1 subgroup. J Clin Oncol 2012; 30 suppl; abstr e14501.
- Coeshott C, Holmes T, Mattson A, et al. Immune responses to mutated ras – development of a yeast based immunotherapeutic. In AACR-RAS Oncogene Conference 2014. Mol Cancer Res 2014;12: abstract A28.
- Hartley ML, Bade NA, Prins PA, Ampie L, Marshall JL. Pancreatic cancer, treatment options, and GI-4000. Human Vaccines Immunother 2014; 10(11):3347-53.
- Hiyama E, Kodama T, Shinbara K, Iwao T, Itoh M, Hiyama K, Shay JW, Matsuura Y, Yokoyama T. Telomerase activity is detected in pancreatic cancer but not in benign tumors. Cancer Res 1997; 57:326-31; PMID:9000577
- Suehara N, Mizumoto K, Kusumoto M, Niiyama H, Ogawa T, Yamaguchi K, Yokohata K, Tanaka M. Telomerase activity detected in pancreatic juice 19 months before a tumor is detected in a patient with pancreatic cancer. Amer J Gastroenterol 1998; 93:1967-71; http://dx.doi.org/10.1111/j.1572-0241.1998.00557.x
- Bernhardt SL, Gjertsen MK, Trachsel S, Moller M, Eriksen JA, Meo M, Buanes T, Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/;II study. Br J Cancer 2006; 95:1474-82; PMID:17060934; http://dx.doi.org/10.1038/sj.bjc.6603437
- Middleton GW, Silcocks P, Cox T, Valle J, Wadsley J, Propper D, Coxon F, et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open label, randomized, Phase 3 trial. Lancet Oncol 2014; 15(8):829-40; PMID:24954781; http://dx.doi.org/10.1016/S1470-2045(14)70236-0
- Gunturu KS, Rossi GR, Saif MW. Immunotherapy updates in pancreatic cancer: are we there yet? Ther Adv Med Oncol 2013; 5:81-9; PMID:23323149; http://dx.doi.org/10.1177/1758834012462463
- Korc M. Pathways for aberrant angiogenesis in pancreatic cancer. Mol Cancer 2003; 2:8; PMID:12556241; http://dx.doi.org/10.1186/1476-4598-2-8
- Okuyama R, Aruga A, Hatori T, Takeda K, Yamamoto M. Immunological responses to a multi-peptide vaccine targeting cancer-testis antigens and VEGFRs in advanced pancreatic cancer patients. Oncoimmunology 2013; 2:e27010; PMID:24498547; http://dx.doi.org/10.4161/onci.27010
- Terashima T, Mizukoshi E, Arai K, Yamashita T, Yoshida M, Ota H, Onishi I, Kayahara M, Ohtsubo K, Kagaya T, et al. P53, hTERT, WT-1, and VEGFR2 are the most suitable targets for cancer vaccine therapy in HLA-A24 positive pancreatic adenocarcinoma. Cancer Immunol Immunother 2014; 63(5):479-89; PMID:24633336; http://dx.doi.org/10.1007/s00262-014-1529-8
- Miyazawa M, Ohsawa R, Tsunoda T, Hirono S, Kawai M, Tani M, Nakamura Y, Yamaue H. Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci 2010; 101:433-9; PMID:19930156; http://dx.doi.org/10.1111/j.1349-7006.2009.01416.x
- Schmitz-Winnenthal FH, Hohmann N, Niethammer AG, Friedrich T, Lubenau H, Springer M, Breiner KM, Mikus G, Ulrich A, Buechler MW, et al. Anti-angiogenic activity of VXM01, an oral T-cell vaccine against VEGF receptor 2, in patients with advanced pancreatic cancer: a randomized, placebo-controlled, phase 1 trial. Oncoimmunology 2015; 16: 4(4):e1001217; http://dx.doi.org/10.1080/;2162402X.2014.1001217
- Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer 2001; 92:271-8; PMID:11466679; http://dx.doi.org/10.1002/1097-0142(20010715)92:2%3c271::AID-CNCR1319%3e3.0.CO;2-0
- Kameshima H, Tsuruma T, Kutomi G, Shima H, Iwayama Y, Kimura Y, Imamura M, Torigoe T, Takahashi A, Hirohashi Y, et al. Immunotherapeutic benefit of α-interferon (IFNalpha) in survivin2B-derived peptide vaccination for advanced pancreatic cancer patients. Cancer Sci 2013; 104:124-9; PMID:23078230; http://dx.doi.org/10.1111/cas.12046
- Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother 2006; 55(10):1294-8; PMID:16315030; http://dx.doi.org/10.1007/s00262-005-0102-x
- Monstein HJ, Ohlsson B, Axelson J. Differential expression of gastrin, cholecystokinin-A, and cholecystokinin-B receptor mRNA in human pancreatic cancer cell lines. Scand J Gastroenterol 2001; 36:738-43; PMID:11444473; http://dx.doi.org/10.1080/003655201300192003
- Gilliam AD, Broome P, Topuzov EG, Garin AM, Pulay I, Humphreys J, Whitehead A, Takhar A, Rowlands BJ, Beckingham IJ. An international multicenter randomized controlled trial of G17DT in patients with pancreatic cancer. Pancreas 2012; 41, 374-9; PMID:22228104; http://dx.doi.org/10.1097/;MPA.0b013e31822ade7e
- Oki Y, Younes A. Heat shock protein-based cancer vaccines. Expert Rev Vaccines 2004; 3:403-11; PMID:15270645; http://dx.doi.org/10.1586/14760584.3.4.403
- Maki RG, Livingston PO, Lewis JJ, Janetzki S, Klimstra D, Desantis D, Srivastava PK, Brennan MF. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci 2007; 52:1964-72; PMID:17420942; http://dx.doi.org/10.1007/s10620-006-9205-2
- Rong Y, Qin X, Jin D, Lou W, Wu L, Wang D, Wu W, Ni X, Mao Z, Kuang T, Zang YQ. A phase I pilot trial of MUC1-peptide-pulsed dendritic cells in the treatment of advanced pancreatic cancer. Clin Exper Med 2012; 12:173-80; http://dx.doi.org/10.1007/s10238-011-0159-0
- Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res 1994; 54:2856-60; PMID:7514493
- Pecher G, Haring A, Kaiser L, Thiel E. Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/;II clinical trial. Cancer Immunol Immunother 2002; 51:669-73; PMID:12439613; http://dx.doi.org/10.1007/s00262-002-0317-z
- Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, Geller BA, Schmotzer A, Potter DP, Whiteside T, et al. A phase I/;II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther 2008; 6:955-64; PMID:19129927
- Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, Shimodaira S, Koido S, et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas 2012; 41:195-205; PMID:21792083; http://dx.doi.org/10.1097/;MPA.0b013e31822398c6
- Morse MA, Nair SK, Boczkowski D, Tyler D, Hurwitz HI, Proia A, Clay TM, Schlom J, Gilboa E, Lyerly HK. The feasibility and safety of immunotherapy with dendritic cells loaded with CEA mRNA following neoadjuvant chemoradiotherapy and resection of pancreatic cancer. Int J Gastrointest Cancer 2002; 32:1-6; PMID:12630764; http://dx.doi.org/10.1385/;IJGC:32:1:1
- Kaufman HL, Kim-Schulze S, Manson K, DeRaffele G, Mitcham J, Seo KS, Kim DW, Marshall J. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med 2007; 5:60; PMID:18039393; http://dx.doi.org/10.1186/1479-5876-5-60
- Dalgleish AG, The IMAGE I Trial Investigators. A multicenter randomized, open-label, proof-of-concept, phase II trial comparing gemcitabine with and without IMM-101 in advanced pancreatic cancer. J Clin Oncol 2015; 33 3 Suppl:abstr 336.
- Dalgleish AG, The IMAGE I Trial Investigators. Long term survival in IMAGE 1, a randomized, open-label phase II trial comparing gemcitabine with and without IMM-101 in advanced pancreatic cancer. J Clin Oncol 2015; 33 Suppl:abstr 3051.
- Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010; 33:828-33; PMID:20842054; http://dx.doi.org/10.1097/;CJI.0b013e3181eec14c
- Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH, Jr, Bagala C, Colombi F, Cagnazzo C, Gioeni L, Wang E, et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol 2014; 25:1750-5; PMID:24907635; http://dx.doi.org/10.1093/annonc/mdu205
- Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA Jr, Donehower RC, Jaffee EM, Laheru DA. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013; 36:382-9; PMID:23924790; http://dx.doi.org/10.1097/;CJI.0b013e31829fb7a2
- Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/;CD40L engagement in the immune system. Immunol Rev. 2009; 229(1):152-72; PMID:19426221; http://dx.doi.org/10.1111/j.1600-065X.2009.00782.x
- Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, O'Dwyer PJ. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Ca Res 2013; 19:6286-95; http://dx.doi.org/10.1158/1078-0432.CCR-13-1320
- Zippelius A, Schreiner J, Herzig P, Muller P. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol Res 2015; 3:236-44; PMID:25623164; http://dx.doi.org/10.1158/2326-6066.CIR-14-0226
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998; 281:1191-3; PMID:9712583; http://dx.doi.org/10.1126/science.281.5380.1191
- Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia AJ, Burgess R, Slingluff CL, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 2002; 297:1867-70; PMID:12228717; http://dx.doi.org/10.1126/science.1073514
- Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004; 103:4619-21; PMID:15001472; http://dx.doi.org/10.1182/blood-2003-11-3909
- Witkiewicz A, Williams TK, Cozzitorto J, Durkan B, Showalter SL, Yeo CJ, Brody JR. Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J Am Coll Surg 2008; 206:849-6; PMID:18471709; http://dx.doi.org/10.1016/j.jamcollsurg.2007.12.014
- Manuel ER, Chen J, D'Apuzzo M, Lampa MG, Kaltcheva TI, Thompson CB, Ludwig T, Chung V, Diamond DJ. Salmonella-based therapy targeting indoleamine 2,3-dioxygenase coupled with enzymatic depletion of tumor hyaluronan induces complete regression of aggressive pancreatic tumors. Cancer Immunol Res 2015; 3(9):1096-1107. PMID:26134178
- Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 2013; 210:1389-402; PMID:23752227; http://dx.doi.org/10.1084/jem.20130066
- Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 2009; 16:487-97; PMID:19962667; http://dx.doi.org/10.1016/j.ccr.2009.10.015
- Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, Yoshimura A, Reindl W, Sipos B, Akira S, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011; 19:456-69; PMID:21481788; http://dx.doi.org/10.1016/j.ccr.2011.03.009
- Gilabert M, Calvo E, Airoldi A, Hamidi T, Moutardier V, Turrini O, Iovanna J. Pancreatic cancer-induced cachexia is Jak2-dependent in mice. J Cell Physiol 2014; 229:1437-43; PMID:24648112; http://dx.doi.org/10.1002/jcp.24580
- Hurwitz H, Uppal N, Wagner SA, et al. A randomized double-blind phase 2 study of ruxolitinib or placebo with capecitabine as second-line therapy in patients with metastatic pancreatic cancer. J Clin Oncol 2014; 32(5s) Suppl:abstr 4000.
