ABSTRACt
Cancer progression depends on stepwise accumulation of oncogenic mutations and a select group of growth factors essential for tumor growth, metastasis and angiogenesis. Agents blocking the epidermal growth factor receptor (EGFR, also called HER1 and ERBB1) and the co-receptor called HER2/ERBB2 have been approved over the last decade as anti-cancer drugs. Because the catalytically defective member of the family, HER3/ERBB3, plays critical roles in emergence of resistance of carcinomas to various drugs, current efforts focus on antibodies and other anti-HER3/ERBB3 agents, which we review herein with an emphasis on drug combinations and some unique biochemical features of HER3/ERBB3.
Introduction
Despite significant progress in the development of new anti-cancer drugs, more than 14 million new cases of cancer were diagnosed globally in 2012, and approximately 8.2 million patients died in 2012 due to their disease.Citation1 Solid tumors are characterized by stepwise accumulation of oncogenic mutations, which present an immense pharmacological challenge. However, tumor cell survival, migration and ability to attract blood and lymph vessels depend on a multitude of growth factors, and some of these bind with cell surface localized receptor tyrosine kinases (RTKs).Citation2 Due to their accessibility and the ability to block the catalytic kinase function using small molecule, tyrosine kinase inhibitors (TKIs), RTKs have become a sustained source of attractive targets for cancer drugs.Citation3 For example, type 1 RTKs, a group containing the epidermal growth factor receptor (EGFR, also called ERBB1) and the co-receptor called HER2/ERBB2, is one of the most studied and targeted groups of molecules in cancer therapy.Citation4 Importantly, currently available drugs target only 2 members of the HER/ERBB family, namely EGFR and HER2. Nevertheless, many new agents targeting HER3 are in various stages of clinical development, and some mAbs are approaching phase 3 trials. Hence, it is both timely and important to overview the large efforts invested to develop novel pharmacological interceptors of HER3 (see ). This review provides a systematic description of the biology of HER3 and its family members, and critically relates to the multiple experimental interceptors, with an emphasis on potentially effective drug combinations.
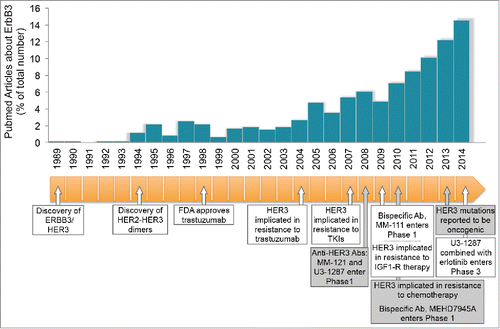
Figure 1. An avalanche of HER3 studies. The histogram depicts the yearly number of HER3/ERBB3 reports, which became available through Pubmed since the early 1990s. The timeline at the bottom identifies and dates some milestones in HER3 research since the discovery of the protein and transcript in 1989.
Targeted cancer therapy directed at the EGFR (HER/ERBB) family
All members of the EGF family of growth factors, which includes 11 ligands, bind with moderate or high affinity to type 1 RTKs.Citation2,4,5 The founding member of this first RTK sub-family was discovered in 1982 by Stanley Cohen and colleagues.Citation6 Three similar receptors have later been characterized. These are human EGFR 2, HER2 (also called ERBB2), HER3 (ERBB3) and HER4 (ERBB4). All four HER/ERBB proteins contain an extracellular ligand binding domain, a transmembrane region and an intracellular domain harbouring a catalytic tyrosine kinase function. The extracellular portion consists of 4 subdomains, referred to as domains I-IV, of which domains I and III (of EGFR) are necessary for ligand binding (see ).Citation7 Following ligand binding, a relatively large structural conformation converts an untethered, inactive form to a tethered/active form, thereby enables homo- or hetero-dimerization with a similar or different member of the EGFR/ERBB family.Citation8 In this context, one of the favorite partners for dimer formation is HER2, which binds no known EGF-like ligand and its conformation is constitutively tethered, ready for dimerization.Citation9 Once dimerization occurred, receptor activation and phosphorylation initiate, leading to a cascade of phosphorylation events, which activates 2 main signaling pathways, namely the mitogen-activated protein kinase (MAPK/ERK) pathway and the phosphatidylinositol 3-kinase (PI3K) to AKT pathway (). These pathways control numerous cellular responses, such as proliferation, cell cycle entry, survival, metabolic pathway activation, apoptosis and angiogenesis.Citation5,10-14
Figure 2. Key sites of HER3. (A) Shown are phosphotyrosine phosphorylation sites of HER3/ERBB3 able to dock binding proteins involved in MAPK (ERK) or PI3K/AKT signaling pathways, such as SHC, GRB2 or the α subunit of PI3K. (B) Reported sites of mutations within HER3/ERBB3 reported in different types of tumors. Note that hot spot mutations (red) are more frequently detected in cancer.
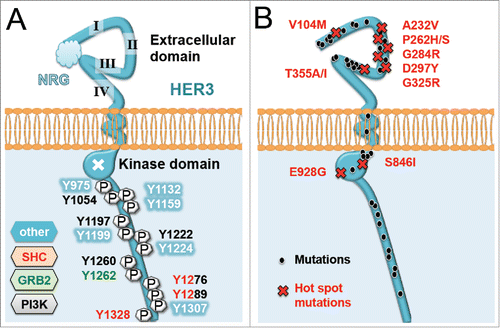
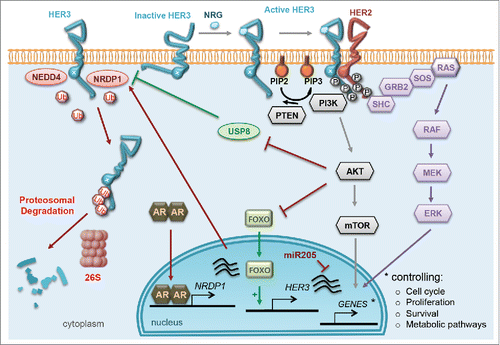
Figure 3. Regulation of HER3 and downstream signaling. HER3/ERBB3 adopts an active conformation following binding of a ligand, called neuregulin (NRG), in between domains I and III of the extracellular domain. Since the tyrosine kinase domain is impaired, HER3/ERBB3 can undergo only weak auto-phosphorylation. In addition, HER3/ERBB3 can form heterodimers with other receptor tyrosine kinases (RTKs), leading to efficient trans-phosphorylation of the cytoplasmic domain. The main dimerization partner of HER3 is HER2/ERBB2 . Both the MAPK pathway (ERK, shown in purple) and the AKT pathway (gray) are activated when such heterodimers form. Nuclear translocation of the downstream effectors of these pathways permits transcriptional and translational regulation of genes involved in numerous cellular responses, such as cell cycle control, proliferation, survival, metabolism, apoptosis and angiogenesis. On its own, HER3 is regulated at different levels: 2 ubiquitin ligases, NEDD4 and NRDP1, have been reported to mediate its ubiquitination and proteosomal degradation. The deubiquitinating enzyme USP8, which is regulated by AKT, negatively regulates NRDP1. The activated androgen receptor (AR) also controls HER3 levels by binding to the NRDP1 promoter regions and activating NRDP1 transcription. In addition, several miRNA molecules, such as miR205, miR125a and miR125b, have been reported to control HER3/ERBB3 expression.
Because EGFR is involved in survival of epithelial cells, including cancer cells, and both EGFR and HER2 are frequently overexpressed or mutated in cancer, several therapies have been developed with the aim of intercepting their signaling, and arresting tumor growth. Two major pharmacological strategies have been developed, and they are concisely reviewed below: these are low molecular weight compounds, called TKIs, which target the intracellular domain of the receptor, and monoclonal antibodies (mAbs) targeting the extracellular domain of the receptor. Multiple TKIs have been designed to target the EGFR family. These compounds inhibit the catalytic tyrosine kinase function by binding to the nucleotide-binding site of the target receptor.Citation15 Initially, mono-specific TKIs were developed. For example reversible, ATP-competitive EGFR inhibitors (e.g., gefitinib/Iressa and erlotinib/Tarceva) were found to be selective to mutant forms of EGFR found in 10–40% of non-small cell lung cancer (NSCLC).Citation16-18 In addition to lung cancer, since 2005 erlotinib is used in combination with chemotherapy for the treatment of metastatic pancreatic carcinoma.Citation19 Unlike erlotinib and gefitinib, lapatinib (Tykerb/Tyverb) is a dual tyrosine kinase inhibitor that forms relatively selective and unique complexes with both EGFR and HER2.Citation20 Several clinical trials established lapatinib's activity, together with chemotherapy, in the treatment of patients with HER2-positive advanced breast cancer previously treated with trastuzumab and chemotherapy.Citation21 Afatinib is the first clinically approved TKI of the second generation of HER/ERBB inhibitors, designed to have more potent inhibition of EGFR and to overcome the EGFR T790M resistance mutation. Afatinib is an oral, irreversible PanHER family blocker, which selectively and potently blocks signaling from the 3 catalytically active HER-family receptors, and also inhibits transphosphorylation of the inactive member, HER3/ERBB3.Citation22 In lung cancer patients with EGFR mutations, afatinib has been associated with prolonged progression-free survival, compared to chemotherapy. This led to approval of oral afatinib, in 2013, for the first-line treatment of patients with metastatic NSCLC who have tumors with EGFR mutations.Citation23 Third generation TKIs were developed to inhibit EGFR T790M while sparring wild type EGFR, consequently they show less toxicities. AZD9291,Citation24 CO-1686 (also called rociletinib),Citation25 and HM61713Citation26 are under clinical development, and early phase results are in general quite encouraging. An example is given by rocetinib, which has been awarded breakthrough therapy designation (BTD) by FDA and has been studied in phase 2 and 3.
The first mAbs to EGFR were generated by Mendelsohn and colleagues in the early 1980s.Citation27 Sela and colleagues later demonstrated that anti-EGFR antibodies can synergize with platinum-based chemotherapy, when administered to tumor-bearing animals.Citation28 Mendelsohn's murine antibody is the father of cetuximab (Erbitux™), a chimeric human/mouse mAb that inhibits binding of EGF and downstream signaling.Citation29 The antibody was clinically developed and eventually approved in 2004, together with chemotherapy, for the treatment of metastatic colorectal cancer (mCRC).Citation30 Panitumumab (Vectibix™), a fully human antibody, binds to the same site of EGFR. An acquired mutation in EGFR (S492R) prevents cetuximab binding but retains panitumumab binding.Citation31 Importantly, it was noted that the effects of both panitumumab and cetuximab were limited to patients with wild type KRAS tumors; antibody treatment did not benefit patients whose tumors expressed a mutant form of KRAS.Citation32–35 Multiple determinants of resistance to anti EGFR therapy have been described recently, among them, mutation in the receptor itself - changing its interactions with the drug molecule – or in downstream transducers and deregulation of parallel signaling pathways.Citation36 For example recent data suggest that the mutation status of KRAS, NRAS, BRAF and/or PIK3CA genes may be a signature for EGFR dependency in CRC.Citation37
Studying a mutant form of the rodent HER2 (also called NEU), Greene, Weinberg and colleagues showed that ectopic expression of the oncoprotein in rat fibroblast enabled them to grow as tumors, but a corresponding mAb reverted this transformed phenotype.Citation38 Another murine antibody, 4D5, specifically inhibited the growth of human breast tumor-derived cell lines overexpressing HER2/ERBB2.Citation39 Humanization of 4D5, the predecessor of trastuzumab (Herceptin™), along with genetic engineering that enhanced binding affinityCitation40 and recruitment of killer lymphocytes to the human Fcγ receptors (especially FcγRIIIa), to augment antibody-dependent cell-mediated cytotoxicity (ADCC), readied the antibody for clinical trials. In the pivotal clinical trials that applied trastuzumab on HER2-positive advanced breast cancer, the mAb conferred significant improvements in progression-free survival (PFS) and overall survival (OS), which led to the approval of a combination of trastuzumab and chemotherapy.Citation41 Unlike trastuzumab, pertuzumab is a mAb that inhibits heterodimerization of HER2 with other family members.Citation42 A large clinical trial that combined pertuzumab, trastuzumab and chemotherapyCitation43,44 demonstrated significant improvement in patient outcome, along with serious adverse events in 36% of patients who received pertuzumab, trastuzumab, and docetaxel. These results led to the approval of a combination of pertuzumab, trastuzumab and docetaxel for the treatment of patients with HER2-positive breast cancer, whose disease progressed during prior trastuzumab-based therapy.Citation45 Because pertuzumab inhibits recruitment of HER3 to HER2,Citation42 some side effects of the combination might be due to HER3 blockade. Another lesson from HER2 that might be applicable to anti-HER3 antibodies is Trastuzumab-DM1 (T-DM1). This is an antibody drug conjugate comprising trastuzumab and emtansine (DM1), a tubulin polymerization inhibitor. Following successful trials, which showed high efficacy and relatively mild side effects,Citation46,47 T-DM1 has been approved for the treatment of HER2-positive metastatic breast cancer patients, who previously received trastuzumab and chemotherapy.
HER3/ERBB3: How did the black sheep of the family become a prime target?
The third member of the EGFR family, HER3, has been discovered some 25 y ago (see ).Citation48,49 Initially, HER3, unlike EGFR and HER2, has been considered an unsuitable target for cancer treatments. This was due to an overall moderate expression levels in cancer cells, a catalytically impaired kinase domain,Citation50 a presumed lack of ability to form homodimeric HER3-HER3 complexes, and an initial inability to detect oncogenic mutations of HER3/ERBB3. Yet, it has been clear early on that HER3-containing heterodimers, especially with HER2, generate strong survival signals.Citation51,52 Moreover, ablation of HER3 uncovered one of its cellular roles, which is to couple active HER2 to the phosphatidylinositol 3-kinase/protein kinase B pathway (PI3K-AKT).Citation53 More recent studies were able to detect low but intrinsic tyrosine kinase activity of HER3, which prompted a model suggesting that transient HER3-HER2 hetero-interactions set the stage for signaling competent HER3 homodimers.Citation54 Another unique feature of HER3 relates to its constitutive (ligand-independent) endocytosis.Citation55,56 and its up-regulation when the AKT pathway is inhibited.Citation57 Unlike CBL-mediated downregulation of the majority of growth factor receptors, HER3 downregulation is mediated by a dedicated cascade involving a deubiquitinating enzyme, USP8, and 2 E3 ubiquitin ligases, NRDP1 and NEDD4.Citation58,59 In addition to regulation by means of ubiquitination, several microRNAs are reportedly able to modulate HER3 levels (see ).Citation60–63 As we describe below, 2 major lines of evidence motivated the current intensified interest in intercepting HER3 in human tumors: First, oncogenic mutant forms have recently been identified in approximately 10% of solid tumors and second, several studies indicated that HER3 plays pivotal roles in several compensatory processes that underlay emergence of resistance to certain cancer drugs.Citation64,65
Aberrant HER3 in human cancer
According to a recent report, somatic mutations exist in ∼11% of colon and gastric cancers ().Citation66 Moreover, when tested in vitro, the mutants were able to transform colonic and breast epithelial cells in a ligand-independent but HER2-dependent manner. In addition, co-expression of HER2 and HER3 is commonly detected in breast cancer.Citation67 In fact, a large fraction of human mammary tumors present an overexpressed HER3.Citation68 Although initially controversial, according to recent meta-analyses, relatively high expression levels of HER3 might associate with shorter survival of patients with breast, colorectal, melanoma, pancreatic, head and neck, and ovarian cancer.Citation69 Once again, co-expression of HER2 appears to strengthen this clinical association with poorer survival.
Roles for HER3 in drug resistance
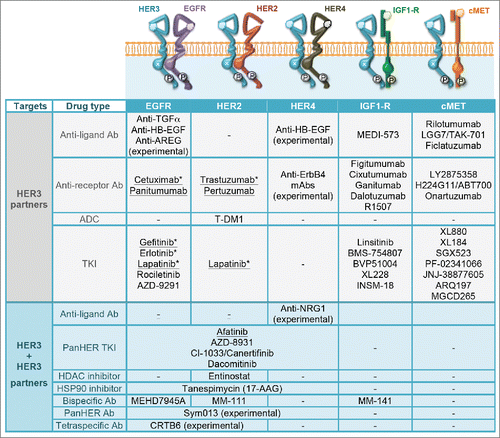
Unlike primary resistance, tolerance to HER/ERBB-intercepting drugs emerges in the majority of patients while under treatment with TKIs or with mAbs.Citation70,71 Mechanisms underlying resistance often involves compensatory pathways that pre-empt pharmacological intervention. Two features of HER3 make this receptor especially competent to launch such bypass loops, namely the ability to strongly stimulate the PI3K-AKT pathway and its propensity to form heterodimers not only with HER2 and EGFR but also with MET and IGF1-R. presents the major interaction partners of HER3, as well as drugs that target these partners, either alone or in combination with HER3. For example, HER3 expression is substantially increased after long-term exposure of breast cancer cells to trastuzumab.Citation72 Similarly, upregulation of HER3 accompanies inhibition of EGFR and HER2 using TKIs, such as gefitinib, erlotinib and lapatinib.Citation73,74 Yet another indirect effect underlies resistance of lung cancer to gefitinib; this involves focal amplification of MET, which confers resistance by driving HER3-dependent activation of PI3K.Citation75 HER3 has also been implicated in development of resistance to cetuximab: resistant NSCLC and HNSCC cancer cells manifested strong activation of HER2, HER3 and MET, along with coupling to PI3K-AKT.Citation76 Interestingly, IGF1-R inhibition in liver cancer cells evokes an analogous EGFR-dependent mechanism that involves HER3.Citation77 The roles of HER3 in evolving resistance extends to chemotherapy, such as resistance of HER2-overexpressing breast cancer cells to paclitaxelCitation65,78 and acquisition of resistance to tamoxifen by luminal B breast cancer.Citation79
Figure 4. Drugs targeting HER3s interaction partners. Listed are the main protein partners of HER3. Clinically approved drugs targeting HER3 partners are underlined. Other than afatinib, no drug targeting both HER3 and a direct partner (lower part of the table) has so far been approved. Asterisks indicate drugs and resistance mechanisms that might involve HER3.
Signature of HER3 activation and use as a biomarker
As aforementioned, HER3 is trans-activated by its dimerization partners. This activation leads to phosphorylation of some of the 14 tyrosine residues located at the C-terminal tail of HER3 ().Citation80 Several studies have identified phospho-sites able to dock SH2 (Src homology region 2) and PTB (phosphotyrosine-binding) domains of proteins involved in signaling pathways, such as SHC, GRB2 or the PI3K complex. Importantly, unlike EGFR and HER2, HER3 contains multiple phosphotyrosine binding sites for the regulatory subunit of PI3K complexes.Citation81 This unique feature of HER3 might explain HER2s action in breast cancer, such as inflammatory breast tumors overexpressing HER2.Citation82 In such patients, coexpression of pHER2 and pHER3 in tumors seems to predict for a favorable response to lapatinib, a HER2-specific TKI. Moreover, resistance of HER2-overexpressing breast cancer cells to lapatinib might be due to autocrine stimulation of HER3 by neuregulin and consequent transactivation of EGFR and the PI3K pathway.Citation83 In conclusion, the phosphorylated form of HER3, or a relevant phosphoprotein signature,Citation84,85 might serve as a biomarker enabling patient selection.
Indirect Strategies Targeting HER3/ERBB3
Several structural and functional features inherent to HER3 present a pharmacological challenge: attempts to intercept the protein must take into account its very low kinase activity.Citation50 In addition, HER3 seems to act as an auxiliary subunit of driver oncogenes, such as HER2/ERBB2 and EGFR, rather than a bona fide driver. Yet another feature, which is less understood, is the ability of HER3 to instigate compensatory feedback regulatory loops that adapt and compensate for inhibition of other receptors of the HER/ERBB family. Presumably, engagement of HER3 upon pathway inhibition involves multiple mechanisms, such as complex formation, de-phosphorylation, trans-phosphorylation and translocation to the plasma membrane, along with feedback regulation of the PI3K-AKT pathway, the major downstream effector of HER3.Citation74 Specific elements of the HER3 promoter might also be involved.Citation86 It is therefore conceivable that pharmacological elimination of HER3 would be accompanied by indirect side effects. Although immunological approaches have dominated the field of HER3 targeting, several additional strategies, which we briefly review below and in , might be effective by their own, or they might assist other strategies.
Figure 5. Direct and indirect inhibition of HER3. The last decade has witnessed the introduction of several experimental strategies able to target HER3/ERBB3. (1) The ligand trapping strategy consists of developing recombinant decoys (aka TRAP).Citation89 or anti-NRG antibodies,Citation88 thereby avoiding ligand-induced activation of HER3. (2) Since HER3 homodimers are weakly active compared to the heterodimers HER3-HER2 and HER3-EGFR, a recombinant bivalent-NRG has been developed that locks HER3 in the homodimeric conformation and restricts its ability to form heterodimers.Citation90 (3) Several monoclonal and multispecific antibodies have been developed to target HER3 and its dimerization partners, leading them to degradation or/and avoiding their phosphorylation.Citation172 Some of these antibodies are able to trigger CDC or ADCC. For example, the glycoengineered mAb RO5479599 causes enhanced ADCC, due to higher affinity to the human Fc-gamma receptor RIIIa expressed on the surface of immune effector cells. Antibodies competing with NRG and avoiding ligand-induced phosphorylation of HER3 have also been reported.Citation137,156 (4) Tyrosine kinase inhibitors (TKIs) have been widely used to inactivate the tyrosine kinase activity of HER3s partners, and several panHER TKIs (targeting all EGFR family members) have been developed, leading to inactive heterodimers.Citation94 (5) HDAC inhibitors (such as entinostat) can inhibit HER3 at the transcriptional level by inducing several miRNAs, such as miR125a, miR125b and miR205.Citation63 (6) Several other nucleotide-based drugs, such as the locked nucleic acid (LNA) called EZN-3920 and miR-450b-3p, might downregulate HER3. (7) Inhibiting the heat shock protein 90 (HSP90), and consequently HER3 maturation and refolding, is another way to reduce HER3 stabilization.Citation97 (8) HER3 can be targeted by small RNA aptamer molecules, which bind with the extracellular domain of HER3 and inhibit downstream signaling.Citation98 (9) Using a peptidomimetic molecule binding HER2 and mimicking HER2-HER3s dimerization site is another way to prevent HER3 activation.Citation99 (10) Recently, screening of approved drugs for their ability to selectively internalize and degrade HER3, identified an anti-anginal drug, perhexiline, as an agent that can target HER3.Citation100
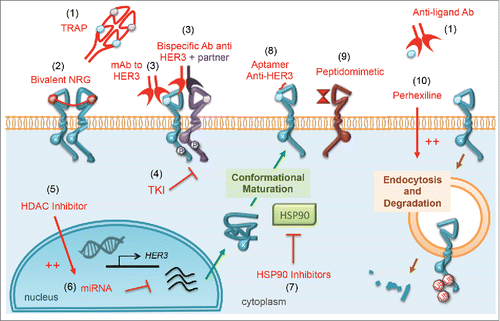
HER3 strategies involving ligand targeting
The potential of NRG targeting to inhibit tumorigenicity and metastasis of breast cancer has been first demonstrated by Tsai and colleagues, who applied an NRG-based antisense scenario.Citation87 Later approaches employed monoclonal antibodies to NRG. Some of the mAbs show synergy with chemotherapy on lung cancer cells.Citation88 Notably, because NRG1 binds both HER3/ERBB3 and HER4/ERBB4, and the latter receptor is expressed in cardiac and neural tissues, toxicity might become an issue when targeting NRGs. Another way to target the ligands is a decoy receptor strategy. This entails a recombinant fusion protein linking the Fc domain of human IgG1 to the truncated extracellular domains of EGFR and ERBB4/HER4.Citation89 This decoy molecule showed an ability to inhibit tumor growth and metastasis in several cancer models. Finally, an original strategy based on HER3 restricted ability to autophosphorylate was developed by Jay and colleagues, who made use of bivalent ligands.Citation90 In principle, the engineered ligands lock HER3/ERBB3 in a homodimeric conformation, thereby prevent HER3 from forming powerful heterodimers, such as HER2-HER3 or EGFR-HER3.
Tyrosine kinase inhibitors preventing HER3 auto- and trans-phosphorylation
An important approach to target HER3/ERBB3 entails inactivation of its own, very weak, kinase activity, as well as the catalytic activity of it dimerization partners, such as EGFR and HER2/ERBB2. As mentioned previously, tumors exhibiting hyper-activation of EGFR or HER2/ERBB2 often develop resistance following treatment with reversible TKIs, such as erlotinib, gefitinib or lapatinib, and this might involve up-regulation of HER3/ERBB3. PanHER inhibitors, which target all members of the HER/ERBB family, have been developed, as a default strategy that potentially overcomes this adaptive reaction. For example, afatinib (BIBW2992, Gilotrif), dacomitimib (PF299804), AZD-8931,Citation91 CI-1033Citation92,93 and several other PanHER TKIs are in different phases of clinical development.Citation94 Among these, dacomitinib and afatinib.Citation22 are particularly well advanced, and they possess novel features, such as irreversible binding to the target receptors and prevention of downstream signals. However, it has not been clearly documented in the clinic that panHER TKIs directly impact HER3 function. Specifically, IGF1-R, MET or other growth factor receptors might activate the HER3 signaling pathway and bypass inactivated EGFR or HER2.
Others indirect strategies
Entinostat (SNDX-275), a specific inhibitor of class I histone deacetylases (HDACs), appears capable of targeting HER3 at the transcriptional level. HDACs are involved in the de-acetylation of core nucleosomal histones, a process involved in aberrant cancer gene expression. Entinostat recently received BTD from FDA due to exciting data from Phase 2b data, showing a significant improvement of overall survival in patients with ER/PR positive breast cancer. Entinostat downregulates HER3 and HER2 via induction of specific microRNAs (miR-125a, miR125b, and miR205) in HER2-overexpressing breast cancer cells.Citation63 Likewise, several antisense oligonucleotides or microRNAs seems able to downregulate HER3, such as a locked nucleic acid (LNA)-based HER3 antisense oligonucleotide called EZN-3920,Citation95 which improves the anti-tumor activity of TKIs, and miR-450b-3p, which inhibits proliferation of breast cancer cells.Citation60 Targeting mRNAs with LNA EZN-3920 provided promising preclinical strategy but further development remains a challenge. Another indirect strategy is the use of HSP90 inhibitors, such as tanespimycin (17-AAG). The Heat Shock Protein 90 (HSP90) assists conformational maturation and refolding of various proteins, including the HER/ERBB family, IGF1-R and MET.Citation96 Inhibiting HSP90 reduces stability of these proteins and arrests subsequent signaling pathways. A phase 2 clinical trial showed efficacy of an HSP90 inhibitor when combined with trastuzumab in the treatment of HER2-overexpressing metastatic breast cancer.Citation97 Nevertheless, the HSP90 approach has been in development for over a decade in patients with advanced breast cancer without clear evidence of clinical efficacy. A30, an RNA aptamer specific to the extracellular domain of HER3, is able to inhibit NRG-induced signaling.Citation98 Likewise, a peptidomimetic molecule that binds HER2s domain IV and reduces HER2 dimerization with HER3 might inhibit HER2-HER3 signaling.Citation99 Lastly, Ren and associates recently re-purposed Perhexiline, an anti-anginal drug that inhibits mitochondrial carnitine palmitoyltransferase I (CPT1), as a promoter of HER3 internalization and degradation, as well as an inhibitor of breast cancer in an animal model.Citation100
Monoclonal Antibodies Targeting HER3
Monospecific antibodies to HER3
The first monoclonal antibody specific to human HER3 was generated in our lab in 1996,Citation101 but many more antibodies were generated later, following the understanding that HER3 acts as a partner of HER2/ERBB2 and serves as a node responsible for resistance to several cancer drugs. lists reagents specifically targeting HER3. By now, several antibodies have reached clinical trials (see a list in ). The first fully human antibody has been patritumab (U3–1287/AMG888).Citation102–104 Patritumab inhibits ligand-induced phosphorylation of HER3, as well as downstream signaling to AKT and ERK. In phase 1 trials patritumab was well tolerated, and showed some evidence of disease stabilization.Citation105 It is notable that clinical tests of patritumab and other anti-HER3 drugs in phase 1 and phase 2 studies has been conducted in unselected patient populations. Hence, lacklustre results are not surprising. When applied to lung and head and neck carcinoma, the antibody enhanced efficacy of radiation therapy.Citation106 It might also prevent cetuximab resistance in colorectal cancer.Citation107 This mAb is currently being tested in phase 2 trials on newly diagnosed HER2-positive metastatic breast cancer, in combination with trastuzumab and paclitaxel. Based on results of the HERALD trialCitation108 showing a statistically significant difference in PFS over placebo in NRG-high patient with advanced NSCLC, patritumab in combination with erlotinib is currently tested in trials that recruited patients with locally advanced and metastatic lung cancer.
Table 1. Experimental pharmacological agents specifically targeting HER3/ERBB3, along with their respective binding sites.
Table 2. Anti-HER3/ERBB3 antibodies currently in clinical trials.
MM-121(SAR256212, seribantumab) is a fully humanized IgG2 mAb that inhibits ligand –induced signaling by competing with NRG for binding to HER3. MM-121 decreases formation of HER2-HER3 dimers.Citation109,110 When singly applied, MM-121 decreased growth of pancreatic tumor cells (AsPC-1),Citation111 ovarian cancer cells (OVCAR8)Citation112 and also cisplatin-resistant cancer cells.Citation113 Multiple combinations of MM-121 with others drugs have been studied. Notably, the combination MM-121 and trastuzumab has been tested on a tumor xenograft model established from trastuzumab-resistant breast cancer cells, and dramatically inhibited tumor growth by activating apoptosis.Citation114 In combination with erlotinib, MM-121 inhibits growth of pancreatic ductal adenocarcinoma by abolishing AKT pathway activation.Citation112 Henry and colleagues examined the benefit of combining MM-121 and either a PanPI3K inhibitor (SAR245408) or a microtubule inhibitor (cabazitaxel), while treating lung (A549, NSCLC) and gastric cancer cells (N87, HER2-overexpressing), respectively. In both cases mAb-induced downregulation of HER3 enhanced the inhibitory activity of the other drug.Citation115 In the same vein, Curley and colleagues showed that MM-121 can re-sensitize estrogen receptor (ER) positive breast cancer to letrozole, an oral non-steroidal aromatase inhibitor.Citation116 Finally, the combination of MM-121 and cetuximab has been reported to inhibit head and neck tumors, in animals.Citation117 As reported in , several clinical trials currently examine safety and efficacy of these and additional combinations of anti-HER3 antibodies. Based on results reported in conferences, the safety of MM-121 combined with several chemotherapies (platinium or taxol)Citation118 or cetuximab and irinotecanCitation119 have been confirmed. The results from phase 2 trials in NSCLC (MM121 + Erlotinib),Citation120 or in ovarian cancer (MM121 + Paclitaxel),Citation121 showed the importance of patient selection, with improved PFS compared to TKI or chemotherapy alone, especially in a subgroup of patients who were NRG positive.
LJM716 is a fully human mAb that can lock HER3 in an inactive conformation by binding with an epitope localized in between domains II and IV of HER3. It has been reported to inhibit both ligand–dependent and ligand–independent HER3 activity in vitro, and prevent tumor growth in both NRG-dependent and HER2-driven cancer models. Importantly, this mAb does not inhibit NRG binding, and it acts synergistically with anti-EGFR (cetuximab) and anti-HER2 (trastuzumab) antibodies.Citation122 The result of a phase 1 clinical trial testing the safety LJM716 in combination with trastuzumab in HER2 positive patients (breast and gastric cancer), show mild to moderate adverse effects and some encouraging case of partial response (6%) and stable disease (36%).Citation123 In combination with BYL719 (a PI3K inhibitor), either alone or with trastuzumab, LJM716 showed promising results on HER2-positive breast cancer cells.Citation124 These data prompted additional clinical trials, which are currently ongoing (see ).
Another interesting mAb is AV-203, a humanized IgG1 directed to HER3.Citation125,126 AV-203 inhibits both NRG-induced and HER2-dependent activation of HER3, and it acts as a potent inhibitor of downstream signaling pathways by preventing HER2-HER3 dimerization. This mAb appears to be effective when tested in several cancer models, such as breast and pancreatic tumors; its safety has been successfully tested in a phase 1 clinical trial on patients with metastatic or advanced solid tumors.Citation127 TK-A3 and TK-A4 (aka, A3 and A4) are humanized IgG1 mAbs that inhibit NRG-dependent phosphorylation of HER3 and promote HER3 internalization and degradation. These mAbs showed an ability to decrease growth of several tumor types, especially melanoma, in animal models. TK-A3 recognizes the dimerization loop in domain II of HER3s extracellular domain.Citation128 According to recent reports, TK-A3 and TK-A4 can reduce melanoma resistance to BRAF/MEK inhibitors.Citation129
MP-RM-1 and its humanized form, EV-20, is a weak affinity anti-HER3 mAb, which is able to inhibit NRG-induced activation of HER3 and downstream pathways, by promoting HER3 degradation and inhibiting HER2-HER3 dimers. Notably, the antibody cannot displace HER3-bound NRG, yet able to decrease tumor growth in several animal models.Citation130,131 REGN1400 is a fully human IgG molecule directed to HER3 and able to inhibit NRG binding.Citation132 In combination with anti-EGFR antibodies, REGN1400 can synergistically promote regression of HNSCC (FaDu) and colorectal cancer (LIM1215) models in animals. The safety of REGN1400 in combination with erlotinib or cetuximab has been tested in a phase 1 trial, showing an acceptable safety profile. Stable disease cases have been reported when used in combination with cetuximab (23%) or with erlotinib (18%). An expansion cohort is planned in HNSCC and CRC.Citation133 SGP1 is another mAb that competes with NRG binding and cooperates with trastuzumab when tested in vitro on breast cancer cells (SKBR3 and MDA-MB-361).Citation134
Lazrek and colleagues generated mouse antibodies direc-ted to domain I, III and IV of HER3. Although some of their mAbs do not inhibit NRG binding, they are still able to diminish tumorigenic growth in nude mice xenografted with epidermoid (A431), pancreatic (BxPC3), or triple-negative breast cancer cells (MDA-MB-468). Mech-anistically, these mAbs arrested cells at the G1 phase of the cell cycle and induced apoptosis, while reducing HER2-HER3 dimers and AKT-induced phosphorylation of MDM-2, XIAP and FOXO1.Citation135 It was further reported that the combination of anti-HER3 mAbs and trastuzumab effectively inhibited growth of xenografts expressing relatively low HER2 levels (e.g., A431 and A549 cells). Similarly, a combination of pertuzumab and an antibody to HER3 (denoted 9F7-F11) enhanced pancreatic tumor inhibition in mice, in line with an added benefit of antibody mixtures containing mAbs to both HER3 and HER2.Citation136
Glycoengineered mAb to HER3
RG7116, also called RO5479599 (GE-huMab-HER3),Citation137 is a humanized glycoengineered IgG1 directed to domain I of HER3. This antibody prevents ligand binding and receptor heterodimerization, thereby blocks receptor phosphorylation and prevents downstream activation ok AKT.Citation138 Remarkably, the engineered glycosylation within the antibody's Fc region represents a novel feature allowing very high affinity recognition of the human Fc-gamma receptor RIIIa of immune effector cells. Hence, this mAb is expected to strongly trigger ADCC. Consistent with this attribute, RG7116/RO5479599 effectively inhibited NSCLC mouse models. RG7116s efficacy was tested in combination with an anti-EGFR (RG7160) or anti-HER2 (pertuzumab) mAbs in an animal model of HNSCC (FaDu), or in a subcutaneous patient-derived tumor xenograft model, respectively.Citation138 Several clinical trials are currently ongoing to test safety of RG7116/RO5479599, either alone or in combination with other drugs (). According to initial reports, RG7116 was well tolerated when used alone to treat patients with metastatic HER3 positive tumors.Citation139
GSK2849330Citation140 is an IgG1/IgG3 chimeric, glycoengineered humanized monoclonal antibody directed against domain III of HER3s extracellular domain and presenting enhanced ability to mediate ADCC and complement dependent cytoxicity (CDC) due to high binding affinity to human Fc-gamma receptor RIIIa and to human complement protein C1q, respectively. This mAb can block NRG binding, receptor dimerization and activation. It is currently being tested in phase 1 clinical studies.
In summary, despite encouraging preliminary results, glycoengineered mAbs against HER3 are still in an initial phase of clinical development. It is worthwhile, however, referring to a similar EGFR-targeting strategy, which yielded moderate efficacy and increased skin toxicity.Citation141 Hence, further clinical development has been discontinued.Citation142
Antibodies engaging 2 non-overlapping epitopes of HER3
Extensive animal studies observed synergistic anti-tumor effects of combining 2–3 anti-HER2 antibodies able to recognize distinct portions of HER2,Citation143-145 including a dimerization-inhibitory mAb.Citation146 In vitro, the more effective mAb mixture was also more effective than the respective single mAbs in inducing receptor degradationCitation147 and ADCC.Citation145 Synergistic anti-tumor effects were confirmed, as well as associated with receptor degradation, using another set of mAbs.Citation143 As aforementioned, a mixture of 2 mAbs to HER2, trastuzumab and pertuzumab, in combination with chemotherapy, significantly prolonged OS of breast cancer patients whose tumors overexpress HER2 compared to a mixture of placebo and trastuzumab combined with chemotherapy:Citation148,149 The median overall survival was 56.5 months in the group receiving the pertuzumab combination, as compared with 40.8 months in the group receiving the placebo combination. Surprisingly, clinical data did not support combination of T-DM1, a drug-conjugated analog of trastuzumab, and pertuzumab. Nevertheless, future mAb combinations might be identified by systematic selections of cooperating anti-HER2 antibodies that can improve efficacy relative to the trastuzumab/pertuzumab combination.Citation150 In analogy to anti-HER2 combinations, we noted that certain pairs of anti-EGFR antibodies could accelerate EGFR degradationCitation144 and they synergized in terms of inhibiting tumorigenic growth of triple negative breast cancer cells.Citation151 Sym004, a therapeutic antibody mixture comprising several mAbs targeting EGFR, was shown to elicit superior cancer cell inhibition and has already completed safety trials.Citation152 Using cellular models, it was shown that Sym004 might overcome acquired resistance to cetuximab.Citation153 In line with this, preclinicalCitation154 data supported tumor dependency on EGFR signaling. Moreover, recent clinical data provide evidence of clinical activity in patients with mCRC whose disease progressed while on anti-EGFR therapies.Citation155
Whether or not anti-HER3 antibodies can collaborate when administered in mixtures of 2 or more antibodies is still an open question. When studying in vitro mixtures of 2 mAbs to HER3/ERBB3, involving one mAb, which inhibits NRG binding, we observed clear benefit of combining 2 anti-HER3 mAbs in terms of inhibition of several cancer cells types. However, this cooperative effect was minimal in an animal model of pancreatic tumors.Citation156 In the same vein, D’Ouza and colleagues developed a bispecific molecule, called A5/F4, comprising 2 single chain variable fragments (ScFv) directed to HER3s domains I and III.Citation157 They later showed that the bispecific molecule, better than each mAb alone, inhibited cell proliferation in vitro. This result was extended to an in vivo model of gastric cancer, but no comparison to single mAbs was presented. In conclusion, combining 2 or more mAbs to EGFR and HER2 holds promise in terms of anti-tumor efficacy. Yet, not all antibodies targeting non-overlapping epitopes of EGFR or HER2 provide improved efficacy in preclinical studies, and the value of combining 2 or more anti-HER3 mAbs is still questionable.
Multispecific antibodies targeting HER3 and one of its direct partners
In line with the above-described antibody combinations, several bispecific and multispecific molecules have been developed in the past 5 y MEHD7945A is a 2-in-one human IgG1 molecule targeting both EGFR and HER3 with significantly different affinities to these receptors. By inhibiting EGFR, as well as signaling downstream to HER2-HER3 dimers, MEHD7945A strongly inhibited cancer cell growth in vitro and in vivo (cell lines NCI-H292, BxPC3, A431), especially in combination with chemotherapy (gemcitabine).Citation158 In later studies, MEHD7945A was shown to inhibit proliferation of cells that were resistant to anti-EGFR drugs, such as erlotinib and cetuximab,Citation159 and it could synergize with PI3K inhibitors in preclinical models of triple negative breast cancer.Citation160 Several clinical trials testing MEHD7945A alone, or in combination with chemotherapeutic agents, are currently in progress (see ). In phase 1 trials, MEHD7945A showed pharmacodynamic evidence supporting target inhibition, as well as anti-tumor activity in 25% of evaluable patients with head and neck squamous cell carcinoma (HNSCC; n = 3), mCRC (n = 6), and NSCLC (n = 3). However, these results were associated with grade 3 gastro-intestinal toxicities.Citation161 In addition, clinical data from randomized phase 2 studies failed to provide evidence of improved efficacy in patients with advanced HNSCCCitation162 or in patients with metastatic CRC (NCT01652482).Citation163
MM-111 is an engineered antibody fusion molecule directed to both HER2 and HER3. This bispecific antibody demonstrated an ability to decrease tumor growth in preclinical models of HER2-overexpressing cancer cells (cell lines BT474, breast cancer and N87, gastric carcinoma), especially when combined with lapatinib or with trastuzumab (see ).Citation164 The safety of MM-111 combined with several drugs, such as chemotherapy (taxol, platinium) or anti-HER2 therapies (TKI or mAbs), has been demonstrated in a phase 1 trial performed with patients with HER2-positive cancer.Citation165 Similarly, MM-141 is a tetravalent bispecific antibody harbouring 4 high-affinity binding sites, 2 are specific to the insulin-like growth factor 1 receptor (IGF1-R) and 2 to HER3.Citation166 Notably, MM-141 displaces both NRG and IGFs (I and II) from their respective receptors. This bispecific antibody could potentiate anti-tumoral effects of chemotherapy in animal models. Similarly, MM-141 potentiated the activity of everolimus (an mTOR inhibitor) in Caki-1 xenografted mice. Presumably, MM-141s efficacy is due to suppression of signaling downstream to both HER3 and IGF-1 receptors.Citation166
FL518 and CRTB6 are tetraspecific antibodies that recognize EGFR, HER2, HER3 and VEGF.Citation167 They were made out of 2 2-in-one antibodies, namely MEHD7945A, directed against EGFR and HER3 (as described above) and bH1–44, which binds both VEGF and HER2. Importantly, these tetraspecific molecules are able to strongly impact the crosstalk between HER proteins and the MET pathway. Accordingly, they were more effective, both in vitro and animals, than the corresponding bispecific antibodies in terms of inhibiting growth of drug-resistant cancer cells exhibiting elevated activation of MET. Similarly, Tab6 is a tetravalent antibody made out of trastuzumab and MM-121 (see above). Consequently, Tab6 binds both HER3 and HER2. So far, Tab6 was used in vitro, either alone or in combination with lapatinib, and showed an ability to decrease proliferation of breast cancer cells overexpressing HER2.Citation168
Sym013 (Pan-HER) is a mixture of 6 mAbs, comprising 3 pairs of synergistic mAbs, each targeting EGFR, HER2 and HER3.Citation169 The mixture has been reported to effectively inhibit growth of lung (NSCLC) and head and neck (HNSCC) cancer models in vitro and in vivo. Sym013 triggers degradation of EGFR, HER2 and HER3, prevents ligand binding to EGFR and HER3, and strongly inhibits subsequent activation of the AKT and MAPK/ERK pathways. Similarly, our team, recently demonstrated anti-cancer effects of a combination of 3 mouse mAbs directed to EGFR, HER2 and HER3.Citation170 The mixture was able to decrease tumorigenic growth of 2 lung cancer models expressing mutant forms of EGFR, which are resistant to erlotinib (PC9-ER and H1975 cell models). Importantly, when applied alone, anti-EGFR antibodies induced a feedback compensatory loop that up-regulated both HER2 and HER3, and resulted in robust activation of the ERK pathway. Importantly, the triple antibody mixture nullified compensatory activation of ERK and, accordingly, strongly inhibited tumorigenic growth of lung cancer models both in vitro and in animals.
Epilog and the power of drug combinations
While it is presently difficult predicting the true clinical potential of HER3 interceptors, experience gained in other domains of molecular targeted cancer therapy provide a glimpse of the future.Citation171 Thus, following the example of trastuzumab, which targets the major partner of HER3 and is commonly combined with paclitaxel for the treatment of patients with metastatic breast cancer overexpressing HER2,Citation41 it seems safe predicting that anti-HER3 agents, especially mAbs, will likely be combined with chemotherapy. It is also predictable that HER3 interceptors will induce growth arrest rather than blatant apoptosis or other types of cell death. The frequent involvement of HER3 in tumor recurrence following emergence of drug resistance raises another prediction: HER3 blockers might be especially effective in delaying onset of drug resistance in the context of genetically aberrant forms of HER/ERBB family members. Other than drug efficacy and emergence of resistance, application of the new drugs and especially their combinations, is expected to elicit mild or moderate adverse clinical effects. In similarity to other HER/ERBB-targeting agents, and in line with the phenotypes of HER3-ablated mice, side effects are expected to involve primarily skin, gastrointestinal tract, cardiac and neural tissues. In fact, serious gastrointestinal toxicities were reported when simultaneously targeting EGFR and HER3.Citation161 Nevertheless, the very large number of experimental drugs targeting EGFR and HER2, along with their manageable toxicities, promise that HER3 targeting will greatly expand the armamentarium available for therapy of patients with relatively hard to treat solid tumors.
Abbreviations
| ADCC | = | Antibody-Dependent Cell-mediated Cytotoxicity |
| ADC | = | Antibody Drug Conjugate |
| AKT | = | Protein kinase B |
| BTD | = | Breakthrough Therapy Designation |
| EGF | = | Epidermal Growth Factor |
| EGFR | = | Epidermal Growth Factor Receptor |
| FDA | = | Food and Drug Administration (USA) |
| MAPK | = | Mitogen-activated Protein Kinase |
| HDAC | = | Histone Deacetylase |
| HER | = | Human EGF Receptor |
| HGF | = | Hepatocyte Growth Factor |
| HNSCC | = | Head and Neck Squamous Cell Cancer |
| HSP90 | = | Heat Shock Protein 90 |
| IGF | = | Insulin-like Growth Factor |
| IGF1-R | = | Insulin-like Growth Factor 1 Receptor |
| IgG | = | Immunoglobuline G |
| mAb | = | Monoclonal Antibody |
| mCRC | = | Metastatic Colorectal Cancer |
| MET | = | Hepatocyte Growth Factor Receptor |
| NRG | = | Neuregulin |
| NSCLC | = | Non-Small Cell Lung Cancer |
| PFS | = | Progression-Free Survival |
| PI3K | = | Phosphatidylinositol 3-Kinase |
| RTK | = | Receptor Tyrosine Kinase |
| TKI | = | Tyrosine Kinase Inhibitor |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Maicol Mancini for insightful comments and Michael Sela for many long-term collaborations.Our laboratory is supported by the European Research Council, the Seventh Framework Program of the European Commission (LungTarget Consortium), the German-Israeli Project Cooperation (DIP), the Israel Cancer Research Fund and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. YY is the incumbent of the Harold and Zelda Goldenberg Professorial Chair.
References
- Stewart BW, Wild CP. World cancer report 2014. Lyon, France: IARC, WHO Press; 2014
- Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda) 2010; 25:85-101; PMID:20430953
- Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene 2000; 19:5548-57; PMID:11114734
- Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer 2012; 12:553-63; PMID:22785351
- Roskoski R, Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res 2014; 79:34-74; PMID:24269963
- Cohen S, Fava RA, Sawyer ST. Purification and characterization of epidermal growth factor receptor/protein kinase from normal mouse liver. Proc Natl Acad Sci USA 1982; 79:6237-41
- Lax I, Burgess WH, Bellot F, Ullrich A, Schlessinger J, Givol D. Localization of a major receptor-binding domain for epidermal growth factor by affinity labeling. Mol Cell Biol 1988; 8:1831-4; PMID:3260004
- Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, Lemmon MA, Sliwkowski MX, Ward CW, Yokoyama S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell 2003; 12:541-52; PMID:14527402
- Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol 1996; 16:5276-87; PMID:8816440
- Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors 2008; 26:263-74; PMID:18800267
- Clayton AH, Walker F, Orchard SG, Henderson C, Fuchs D, Rothacker J, Nice EC, Burgess AW. Ligand-induced dimer-tetramer transition during the activation of the cell surface epidermal growth factor receptor-A multidimensional microscopy analysis. J Biol Chem 2005; 280:30392-9; PMID:15994331
- Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Zhu HJ, Walker F, Frenkel MJ, Hoyne PA, et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor α. Cell 2002; 110:763-73; PMID:12297049
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001; 2:127-37; PMID:11252954
- Barros FF, Powe DG, Ellis IO, Green AR. Understanding the HER family in breast cancer: interaction with ligands, dimerization and treatments. Histopathol 2010; 56:560-72
- Levitzki A, Mishani E. Tyrphostins and other tyrosine kinase inhibitors. Annu Rev Biochem 2006; 75:93-109; PMID:16756486
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Eng J Med 2004; 350:2129-39
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004; 304:1497-500; PMID:15118125
- Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 2004; 101:13306-11; PMID:15329413; http://dx.doi.org/10.1073/pnas.0405220101
- Cohen MH, Johnson JR, Chen YF, Sridhara R, Pazdur R. FDA drug approval summary: erlotinib (Tarceva) tablets. Oncologist 2005; 10:461-6; PMID:16079312; http://dx.doi.org/10.1634/theoncologist.10-7-461
- Wood ER, Truesdale AT, McDonald OB, Yuan D, Hassell A, Dickerson SH, Ellis B, Pennisi C, Horne E, Lackey K, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res 2004; 64:6652-9; PMID:15374980; http://dx.doi.org/10.1158/0008-5472.CAN-04-1168
- Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol 2012; 9:16-32; http://dx.doi.org/10.1038/nrclinonc.2011.177
- Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, Kraemer O, Himmelsbach F, Haaksma E, Adolf GR. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Therapeutics 2012; 343:342-50; http://dx.doi.org/10.1124/jpet.112.197756
- Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31:3327-34; PMID:23816960; http://dx.doi.org/10.1200/JCO.2012.44.2806
- Janne PA, Ramalingam SS, Yang JC-H, Ahn MJ, Kim DW, Kim SW, Planchard D, Ohe Y, Felip E, Watkins C, et al. Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in patients (pts) with EGFR inhibitor-resistant non-small cell lung cancer (NSCLC). ASCO Meeting Abstracts 2014; 32:8009
- Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, Papadimitrakopoulou V, Solomon BJ, Oxnard GR, Dziadziuszko R, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Eng J med 2015; 372:1700-9; http://dx.doi.org/10.1056/NEJMoa1413654
- Kim DW, Lee DH, Kang JH, Park K, Han JY, Lee JS, Jang IJ, Kim HY, Son J, Kim JH. Clinical activity and safety of HM61713, an EGFR-mutant selective inhibitor, in advanced non-small cell lung cancer (NSCLC) patients (pts) with EGFR mutations who had received EGFR tyrosine kinase inhibitors (TKIs). ASCO Meeting Abstracts 2014; 32:8011
- Sato JD, Kawamoto T, Le AD, Mendelsohn J, Polikoff J, Sato GH. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med 1983; 1:511-29; PMID:6094961
- Aboud-Pirak E, Hurwitz E, Pirak ME, Bellot F, Schlessinger J, Sela M. Efficacy of antibodies to epidermal growth factor receptor against KB carcinoma in vitro and in nude mice. J Natl Cancer Inst 1988; 80:1605-11; PMID:3193478; http://dx.doi.org/10.1093/jnci/80.20.1605
- Prewett M, Rockwell P, Rockwell RF, Giorgio NA, Mendelsohn J, Scher HI, Goldstein NI. The biologic effects of C225, a chimeric monoclonal antibody to the EGFR, on human prostate carcinoma. J Immunother Emphasis Tumor Immunol 1996; 19:419-27; PMID:9041461; http://dx.doi.org/10.1097/00002371-199611000-00006
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Eng J Med 2004; 351:337-45; http://dx.doi.org/10.1056/NEJMoa033025
- Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, Salido M, Gallen M, Marsters S, Tsai SP, et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med 2012; 18:221-3; PMID:22270724; http://dx.doi.org/10.1038/nm.2609
- Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26:1626-34; PMID:18316791; http://dx.doi.org/10.1200/JCO.2007.14.7116
- Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006; 66:3992-5; PMID:16618717; http://dx.doi.org/10.1158/0008-5472.CAN-06-0191
- Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Eng J Med 2013; 369:1023-34; http://dx.doi.org/10.1056/NEJMoa1305275
- Bokemeyer C, Kohne CH, Ciardiello F, Lenz HJ, Heinemann V, Klinkhardt U, Beier F, Duecker K, van Krieken JH, Tejpar S. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer 2015; 51:1243-52; PMID:25937522; http://dx.doi.org/10.1016/j.ejca.2015.04.007
- Bertotti A, Sassi F. Molecular Pathways: Sensitivity and Resistance to Anti-EGFR Antibodies. Clin Cancer Res 2015; 21:3377-83; PMID:26071484; http://dx.doi.org/10.1158/1078-0432.CCR-14-0848
- Ciardiello F, Normanno N, Martinelli E, Troiani T, Cardone C, Nappi A, Rachiglio AM, Lambiase M, Pisconti S, Giuliani F, et al. LBA-09 • Cetuximab beyond progression in RAS wild type (WT) metastatic colorectal cancer (mCRC): the CAPRI-GOIM randomized phase II study of FOLFOX versus FOLFOX plus cetuximab. Ann Oncol 2015; 26:iv120-iv1; http://dx.doi.org/10.1093/annonc/mdv262.09
- Drebin JA, Link VC, Stern DF, Weinberg RA, Greene MI. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell 1985; 41:697-706; PMID:2860972; http://dx.doi.org/10.1016/S0092-8674(85)80050-7
- Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell biol 1989; 9:1165-72; PMID:2566907; http://dx.doi.org/10.1128/MCB.9.3.1165
- Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA 1992; 89:4285-9; PMID:1350088; http://dx.doi.org/10.1073/pnas.89.10.4285
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Eng J Med 2001; 344:783-92; http://dx.doi.org/10.1056/NEJM200103153441101
- Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer cell 2002; 2:127-37; PMID:12204533; http://dx.doi.org/10.1016/S1535-6108(02)00097-1
- Baselga J, Swain SM. CLEOPATRA: a phase III evaluation of pertuzumab and trastuzumab for HER2-positive metastatic breast cancer. Clin Breast Cancer 2010; 10:489-91; PMID:21147694; http://dx.doi.org/10.3816/CBC.2010.n.065
- Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2013; 14:461-71; PMID:23602601; http://dx.doi.org/10.1016/S1470-2045(13)70130-X
- Cortes J, Roche H. Docetaxel combined with targeted therapies in metastatic breast cancer. Cancer Treatment Rev 2012; 38:387-96; http://dx.doi.org/10.1016/j.ctrv.2011.08.001
- Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W, Girish S, Tibbitts J, Yi JH, Sliwkowski MX, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 2010; 28:2698-704; PMID:20421541; http://dx.doi.org/10.1200/JCO.2009.26.2071
- Krop IE, LoRusso P, Miller KD, Modi S, Yardley D, Rodriguez G, Guardino E, Lu M, Zheng M, Girish S, et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol 2012; 30:3234-41; PMID:22649126; http://dx.doi.org/10.1200/JCO.2011.40.5902
- Plowman GD, Whitney GS, Neubauer MG, Green JM, McDonald VL, Todaro GJ, Shoyab M. Molecular cloning and expression of an additional epidermal growth factor receptor-related gene. Proc Natl Acad Sci USA 1990; 87:4905-9; PMID:2164210; http://dx.doi.org/10.1073/pnas.87.13.4905
- Kraus MH, Issing W, Miki T, Popescu NC, Aaronson SA. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc Natl Acad Sci USA 1989; 86:9193-7; PMID:2687875; http://dx.doi.org/10.1073/pnas.86.23.9193
- Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA 2010; 107:7692-7; PMID:20351256; http://dx.doi.org/10.1073/pnas.1002753107
- Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J 1995; 14:4267-75; PMID:7556068
- Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J 1996; 15:2452-67; PMID:8665853
- Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA 2003; 100:8933-8; PMID:12853564; http://dx.doi.org/10.1073/pnas.1537685100
- Steinkamp MP, Low-Nam ST, Yang S, Lidke KA, Lidke DS, Wilson BS. erbB3 is an active tyrosine kinase capable of homo- and heterointeractions. Mol Cell Biol 2014; 34:965-77; PMID:24379439; http://dx.doi.org/10.1128/MCB.01605-13
- Waterman H, Sabanai I, Geiger B, Yarden Y. Alternative intracellular routing of ErbB receptors may determine signaling potency. J Biol Chem 1998; 273:13819-27; PMID:9593726; http://dx.doi.org/10.1074/jbc.273.22.13819
- Sak MM, Breen K, Ronning SB, Pedersen NM, Bertelsen V, Stang E, Madshus IH. The oncoprotein ErbB3 is endocytosed in the absence of added ligand in a clathrin-dependent manner. Carcinogenesis 2012; 33:1031-9; PMID:22436610; http://dx.doi.org/10.1093/carcin/bgs128
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 2011; 19:58-71; PMID:21215704; http://dx.doi.org/10.1016/j.ccr.2010.10.031
- Huang Z, Choi BK, Mujoo K, Fan X, Fa M, Mukherjee S, Owiti N, Zhang N, An Z. The E3 ubiquitin ligase NEDD4 negatively regulates HER3/ErbB3 level and signaling. Oncogene 2014; 34(9):1105-15; PMID:24662824; http://dx.doi.org/10.1038/onc.2014.56
- Cao Z, Wu X, Yen L, Sweeney C, Carraway KL, 3rd. Neuregulin-induced ErbB3 downregulation is mediated by a protein stability cascade involving the E3 ubiquitin ligase Nrdp1. Mol Cell Biol 2007; 27:2180-8; PMID:17210635; http://dx.doi.org/10.1128/MCB.01245-06
- Zhao Z, Li R, Sha S, Wang Q, Mao W, Liu T. Targeting HER3 with miR-450b-3p suppresses breast cancer cells proliferation. Cancer Biol Therapy 2014; 15:1404-12; http://dx.doi.org/10.4161/cbt.29923
- Yu J, Li Q, Xu Q, Liu L, Jiang B. MiR-148a inhibits angiogenesis by targeting ERBB3. J Biomed Res 2011; 25:170-7; PMID:23554686; http://dx.doi.org/10.1016/S1674-8301(11)60022-5
- Yan X, Chen X, Liang H, Deng T, Chen W, Zhang S, Liu M, Gao X, Liu Y, Zhao C, et al. miR-143 and miR-145 synergistically regulate ERBB3 to suppress cell proliferation and invasion in breast cancer. Mol Cancer 2014; 13:220; PMID:25248370; http://dx.doi.org/10.1186/1476-4598-13-220
- Wang S, Huang J, Lyu H, Lee CK, Tan J, Wang J, Liu B. Functional cooperation of miR-125a, miR-125b, and miR-205 in entinostat-induced downregulation of erbB2/erbB3 and apoptosis in breast cancer cells. Cell Death Dis 2013; 4:e556; PMID:23519125; http://dx.doi.org/10.1038/cddis.2013.79
- Mujoo K, Choi BK, Huang Z, Zhang N, An Z. Regulation of ERBB3/HER3 signaling in cancer. Oncotarget 2014; 5:10222-36; PMID:25400118; http://dx.doi.org/10.18632/oncotarget.2655
- Ma J, Lyu H, Huang J, Liu B. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol Cancer 2014; 13:105; PMID:24886126; http://dx.doi.org/10.1186/1476-4598-13-105
- Jaiswal BS, Kljavin NM, Stawiski EW, Chan E, Parikh C, Durinck S, Chaudhuri S, Pujara K, Guillory J, Edgar KA, et al. Oncogenic ERBB3 mutations in human cancers. Cancer Cell 2013; 23:603-17; PMID:23680147; http://dx.doi.org/10.1016/j.ccr.2013.04.012
- Bieche I, Onody P, Tozlu S, Driouch K, Vidaud M, Lidereau R. Prognostic value of ERBB family mRNA expression in breast carcinomas. Int J Cancer J Int Du Cancer 2003; 106:758-65; http://dx.doi.org/10.1002/ijc.11273
- Lemoine NR, Barnes DM, Hollywood DP, Hughes CM, Smith P, Dublin E, Prigent SA, Gullick WJ, Hurst HC. Expression of the ERBB3 gene product in breast cancer. Br J Cancer 1992; 66:1116-21; PMID:1333787; http://dx.doi.org/10.1038/bjc.1992.420
- Ocana A, Vera-Badillo F, Seruga B, Templeton A, Pandiella A, Amir E. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst 2013; 105:266-73; PMID:23221996; http://dx.doi.org/10.1093/jnci/djs501
- Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res 2008; 14:2895-9; PMID:18483355; http://dx.doi.org/10.1158/1078-0432.CCR-07-2248
- Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr Opin Genet Dev 2008; 18:73-9; PMID:18325754; http://dx.doi.org/10.1016/j.gde.2008.01.004
- Narayan M, Wilken JA, Harris LN, Baron AT, Kimbler KD, Maihle NJ. Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer Res 2009; 69:2191-4; PMID:19276389; http://dx.doi.org/10.1158/0008-5472.CAN-08-1056
- Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, Dave B, Cook RS, Pao W, McKinely E, et al. Transcriptional and posttranslational upregulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci USA 2011; 108:5021-6; PMID:21385943; http://dx.doi.org/10.1073/pnas.1016140108
- Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 2007; 445:437-41; PMID:17206155; http://dx.doi.org/10.1038/nature05474
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007; 316:1039-43; PMID:17463250; http://dx.doi.org/10.1126/science.1141478
- Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT, Harari PM. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene 2008; 27:3944-56; PMID:18297114; http://dx.doi.org/10.1038/onc.2008.19
- Desbois-Mouthon C, Baron A, Blivet-Van Eggelpoel MJ, Fartoux L, Venot C, Bladt F, Housset C, Rosmorduc O. Insulin-like growth factor-1 receptor inhibition induces a resistance mechanism via the epidermal growth factor receptor/HER3/AKT signaling pathway: rational basis for cotargeting insulin-like growth factor-1 receptor and epidermal growth factor receptor in hepatocellular carcinoma. Clin Cancer Res 2009; 15:5445-56; PMID:19706799; http://dx.doi.org/10.1158/1078-0432.CCR-08-2980
- Wang S, Huang X, Lee CK, Liu B. Elevated expression of erbB3 confers paclitaxel resistance in erbB2-overexpressing breast cancer cells via upregulation of Survivin. Oncogene 2010; 29:4225-36; PMID:20498641; http://dx.doi.org/10.1038/onc.2010.180
- Tovey S, Dunne B, Witton CJ, Forsyth A, Cooke TG, Bartlett JM. Can molecular markers predict when to implement treatment with aromatase inhibitors in invasive breast cancer? Clin Cancer Res 2005; 11:4835-42; PMID:16000581; http://dx.doi.org/10.1158/1078-0432.CCR-05-0196
- Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Sys Biol 2005; 1:2005 0008
- Prigent SA, Gullick WJ. Identification of c-erbB-3 binding sites for phosphatidylinositol 3'-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J 1994; 13:2831-41; PMID:8026468
- Johnston S, Trudeau M, Kaufman B, Boussen H, Blackwell K, LoRusso P, Lombardi DP, Ben Ahmed S, Citrin DL, DeSilvio ML, et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J Clin Oncol 2008; 26:1066-72; PMID:18212337; http://dx.doi.org/10.1200/JCO.2007.13.9949
- Xia W, Petricoin EF, 3rd, Zhao S, Liu L, Osada T, Cheng Q, Wulfkuhle JD, Gwin WR, Yang X, Gallagher RI, et al. An heregulin-EGFR-HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res 2013; 15:R85; PMID:24044505; http://dx.doi.org/10.1186/bcr3480
- Pierobon M, Wulfkuhle J, Liotta L, Petricoin E. Application of molecular technologies for phosphoproteomic analysis of clinical samples. Oncogene 2015; 34:805-14; PMID:24608425; http://dx.doi.org/10.1038/onc.2014.16
- Wulfkuhle JD, Berg D, Wolff C, Langer R, Tran K, Illi J, Espina V, Pierobon M, Deng J, DeMichele A, et al. Molecular analysis of HER2 signaling in human breast cancer by functional protein pathway activation mapping. Clin Cancer Res 2012; 18:6426-35; PMID:23045247; http://dx.doi.org/10.1158/1078-0432.CCR-12-0452
- Mancini M, Gaborit N, Lindzen M, Salame TM, Dall'Ora M, Sevilla-Sharon M, Abdul-Hai A, Downward J, Yarden Y. Combining three antibodies nullifies feedback-mediated resistance to erlotinib in lung cancer. Sci Signal 2015; 8:ra53; PMID:26038598
- Tsai MS, Shamon-Taylor LA, Mehmi I, Tang CK, Lupu R. Blockage of heregulin expression inhibits tumorigenicity and metastasis of breast cancer. Oncogene 2003; 22:761-8; PMID:12569369; http://dx.doi.org/10.1038/sj.onc.1206130
- Hegde GV, de la Cruz CC, Chiu C, Alag N, Schaefer G, Crocker L, Ross S, Goldenberg D, Merchant M, Tien J, et al. Blocking NRG1 and other ligand-mediated Her4 signaling enhances the magnitude and duration of the chemotherapeutic response of non-small cell lung cancer. Sci Trans Med 2013; 5:171ra18; http://dx.doi.org/10.1126/scitranslmed.3004438
- Lindzen M, Carvalho S, Starr A, Ben-Chetrit N, Pradeep CR, Kostler WJ, Rabinkov A, Lavi S, Bacus SS, Yarden Y. A recombinant decoy comprising EGFR and ErbB-4 inhibits tumor growth and metastasis. Oncogene 2012; 31:3505-15; PMID:22105361; http://dx.doi.org/10.1038/onc.2011.518
- Jay SM, Kurtagic E, Alvarez LM, de Picciotto S, Sanchez E, Hawkins JF, Prince RN, Guerrero Y, Treasure CL, Lee RT, et al. Engineered bivalent ligands to bias ErbB receptor-mediated signaling and phenotypes. J Biol Chem 2011; 286:27729-40; PMID:21622572; http://dx.doi.org/10.1074/jbc.M111.221093
- Hickinson DM, Klinowska T, Speake G, Vincent J, Trigwell C, Anderton J, Beck S, Marshall G, Davenport S, Callis R, et al. AZD8931, an equipotent, reversible inhibitor of signaling by epidermal growth factor receptor, ERBB2 (HER2), and ERBB3: a unique agent for simultaneous ERBB receptor blockade in cancer. Clin Cancer Res 2010; 16:1159-69; PMID:20145185; http://dx.doi.org/10.1158/1078-0432.CCR-09-2353
- Slichenmyer WJ, Elliott WL, Fry DW. CI-1033, a pan-erbB tyrosine kinase inhibitor. Seminars Oncol 2001; 28:80-5; http://dx.doi.org/10.1016/S0093-7754(01)90285-4
- Rixe O, Franco SX, Yardley DA, Johnston SR, Martin M, Arun BK, Letrent SP, Rugo HS. A randomized, phase II, dose-finding study of the pan-ErbB receptor tyrosine-kinase inhibitor CI-1033 in patients with pretreated metastatic breast cancer. Cancer Chemother Pharmacol 2009; 64:1139-48; PMID:19294387; http://dx.doi.org/10.1007/s00280-009-0975-z
- Wang X, Batty KM, Crowe PJ, Goldstein D, Yang JL. The Potential of panHER Inhibition in Cancer. Front Oncol 2015; 5:2; PMID:25674538; http://dx.doi.org/10.3389/fonc.2015.00002
- Wu Y, Zhang Y, Wang M, Li Q, Qu Z, Shi V, Kraft P, Kim S, Gao Y, Pak J, et al. Downregulation of HER3 by a novel antisense oligonucleotide, EZN-3920, improves the antitumor activity of EGFR and HER2 tyrosine kinase inhibitors in animal models. Mol Cancer Therapeutics 2013; 12:427-37; http://dx.doi.org/10.1158/1535-7163.MCT-12-0838
- Citri A, Gan J, Mosesson Y, Vereb G, Szollosi J, Yarden Y. Hsp90 restrains ErbB-2/HER2 signalling by limiting heterodimer formation. EMBO Rep 2004; 5:1165-70; PMID:15568014; http://dx.doi.org/10.1038/sj.embor.7400300
- Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, D'Andrea G, Dickler M, Moynahan ME, Sugarman S, et al. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res 2011; 17:5132-9; PMID:21558407; http://dx.doi.org/10.1158/1078-0432.CCR-11-0072
- Chen CH, Chernis GA, Hoang VQ, Landgraf R. Inhibition of heregulin signaling by an aptamer that preferentially binds to the oligomeric form of human epidermal growth factor receptor-3. Proc Natl Acad Sci USA 2003; 100:9226-31; PMID:12874383; http://dx.doi.org/10.1073/pnas.1332660100
- Kanthala S, Banappagari S, Gokhale A, Liu YY, Xin G, Zhao Y, Jois S. Novel Peptidomimetics for Inhibition of HER2:HER3 Heterodimerization in HER2-Positive Breast Cancer. Chem Biol Drug Design 2014; 85(6):702-14; http://dx.doi.org/10.1111/cbdd.12453
- Ren XR, Wang J, Osada T, Mook RA, Jr., Morse MA, Barak LS, Lyerly HK, Chen W. Perhexiline promotes HER3 ablation through receptor internalization and inhibits tumor growth. Breast Cancer Res 2015; 17:528; http://dx.doi.org/10.1186/s13058-015-0528-9
- Chen X, Levkowitz G, Tzahar E, Karunagaran D, Lavi S, Ben-Baruch N, Leitner O, Ratzkin BJ, Bacus SS, Yarden Y. An immunological approach reveals biological differences between the two NDF/heregulin receptors, ErbB-3 and ErbB-4. J Biol Chem 1996; 271:7620-9; PMID:8631797; http://dx.doi.org/10.1074/jbc.271.13.7620
- Freeman D, Ogbagabriel S, Rothe M, Radinsky R, Treder M. Abstract LB-21: Fully human Anti-HER3 monoclonal antibodies (mAbs) have unique in vitro and in vivo functional and antitumor activities versus other HER family inhibitors. Proc 99th AACR Annual Meeting. Cancer Res 2008; 68:LB-21
- Treder M, Hartmann S, Ogbagabriel S, Borges E, Green L, Kang J, Radinsky R, Rothe M, Freeman D. Abstract LB-20: Fully human Anti-HER3 monoclonal antibodies (mAbs) inhibit oncogenic signaling and tumor cell growth in vitro and in vivo. Proceedings of the 99th AACR Annual Meeting. Cancer Res 2008; 68:LB-20
- Freeman DJ, Ogbagabriel S, Bready J, Sun J-R, Radinsky R, Hettmann T. Abstract A182: U3–1287 (AMG 888), a fully human anti-HER3 mAb, demonstrates in vitro and in vivo efficacy in the FaDu model of human squamous cell carcinoma of the head and neck (SCCHN). AACR-NCI-EORTC Int Conf Mol Targets Cancer Therapeutics. Mol Cancer Ther 2011; 10:A182; http://dx.doi.org/10.1158/1535-7163.TARG-11-A182
- LoRusso P, Janne PA, Oliveira M, Rizvi N, Malburg L, Keedy V, Yee L, Copigneaux C, Hettmann T, Wu CY, et al. Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 2013; 19:3078-87; PMID:23591447; http://dx.doi.org/10.1158/1078-0432.CCR-12-3051
- Li C, Brand TM, Iida M, Huang S, Armstrong EA, van der Kogel A, Wheeler DL. Human epidermal growth factor receptor 3 (HER3) blockade with U3-1287/AMG888 enhances the efficacy of radiation therapy in lung and head and neck carcinoma. Discov Med 2013; 16:79-92; PMID:23998444
- Kawakami H, Okamoto I, Yonesaka K, Okamoto K, Shibata K, Shinkai Y, Sakamoto H, Kitano M, Tamura T, Nishio K, et al. The anti-HER3 antibody patritumab abrogates cetuximab resistance mediated by heregulin in colorectal cancer cells. Oncotarget 2014; 5:11847-56; PMID:25474137; http://dx.doi.org/10.18632/oncotarget.2663
- Von Pawel J, Tseng J, Dediu M, Schumann C, Moritz B, Mendell-Harary J, Jin X, Feng W, Copigneaux C, Beckman RA. Abstract 8045: Phase 2 HERALD study of patritumab (P) with erlotinib (E) in advanced NSCLC subjects (SBJs). J Clin Oncol - 2014 ASCO Annual Meeting 2014; 32:5s
- Schoeberl B, Faber AC, Li D, Liang MC, Crosby K, Onsum M, Burenkova O, Pace E, Walton Z, Nie L, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res 2010; 70:2485-94; PMID:20215504; http://dx.doi.org/10.1158/0008-5472.CAN-09-3145
- Schoeberl B, Pace EA, Fitzgerald JB, Harms BD, Xu L, Nie L, Linggi B, Kalra A, Paragas V, Bukhalid R, et al. Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci Signal 2009; 2:ra31; PMID:19567914; http://dx.doi.org/10.1126/scisignal.2000352
- Sheng Q, Liu X, Fleming E, Yuan K, Piao H, Chen J, Moustafa Z, Thomas RK, Greulich H, Schinzel A, et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer cell 2010; 17:298-310; PMID:20227043; http://dx.doi.org/10.1016/j.ccr.2009.12.047
- Liles JS, Arnoletti JP, Kossenkov AV, Mikhaylina A, Frost AR, Kulesza P, Heslin MJ, Frolov A. Targeting ErbB3-mediated stromal-epithelial interactions in pancreatic ductal adenocarcinoma. Br J Cancer 2011; 105:523-33; PMID:21792199; http://dx.doi.org/10.1038/bjc.2011.263
- Curley M, Kalra A, Fulgham A, Xiao D, Allen J, Wainszelbaum M, Garcia G, Kubasek W, MacBeath G. Abstract 141: MM-121, an Anti-ErbB3 antibody, Inhibits PI3K/AKT Signaling and Viability in platinum-resistant Ovarian Cells and in Primary Ascites Derived From Chemo-resistant Ovarian Cancer Patients. Proc 24 EORTC – NCI – AACR Symposium Mol Targets Cancer Therapeutics 2012; 48 (6 suppl):43-4
- Huang J, Wang S, Lyu H, Cai B, Yang X, Wang J, Liu B. The anti-erbB3 antibody MM-121/SAR256212 in combination with trastuzumab exerts potent antitumor activity against trastuzumab-resistant breast cancer cells. Mol Cancer 2013; 12:134; PMID:24215614; http://dx.doi.org/10.1186/1476-4598-12-134
- Henry C, Geslin C, Blanchet AM, Nicolazzi C, Garcia G, Vincent L, Blanc V, Tabah-Fisch I, Chiron M. Abstract 325: MM-121 (SAR256212), an Anti-ErbB3 Monoclonal Antibody, Shows Synergistic Tumor Growth Inhibition in Combination with a Pan-PI3K Inhibitor or a Microtubule Inhibitor Through ErbB3 Expression Modulation. Proc 24 EORTC – NCI – AACR Symposium Mol Targets Cancer Therapeutics 2012; 48:99
- Curley MD, Sabnis GJ, Wille L, Adiwijaya BS, Garcia G, Moyo V, Kazi AA, Brodie A, MacBeath G. Seribantumab, an anti-ERBB3 antibody, delays the onset of resistance and restores sensitivity to letrozole in an estrogen receptor-positive breast cancer model. Mol Cancer Therapeutics 2015
- Jiang N, Wang D, Hu Z, Shin HJ, Qian G, Rahman MA, Zhang H, Amin AR, Nannapaneni S, Wang X, et al. Combination of anti-HER3 antibody MM-121/SAR256212 and cetuximab inhibits tumor growth in preclinical models of head and neck squamous cell carcinoma. Mol Cancer Therapeutics 2014; 13:1826-36; http://dx.doi.org/10.1158/1535-7163.MCT-13-1093
- Arnedos M, Shereen Denlinger C, Harb WA, Rixe O, Charles Morris J, Dy GK, Adjei AA, Pearlberg J, Follows S, Gabor Czibere A, et al. Abstract 2609: A phase I study of MM-121 in combination with multiple anticancer therapies in patients with advanced solid tumors. J Clin Oncol ASCO Annual Meeting. J Clin Oncol 2013; 31(suppl; abstr 2609)
- Cleary JM, Jackson McRee A, O'Neil BH, Sharma S, Pearlberg J, Manoli S, Lloyd Kubasek W, Korn WM. Abstract 3076: A phase 1 study of MM-121 (a fully human monoclonal antibody targeting the epidermal growth factor receptor family member ErbB3) in combination with cetuximab and irinotecan in patients with advanced cancers. J Clin Oncol ASCO Annual Meeting 2014; 32:5s
- Sequist LV, Lopez-Chavez A, Doebele RC, Gray JE, Harb WA, Modiano MR, Jackman DM, Baggstrom M, Atmaca A, Felip E, et al. Abstract 8051: A randomized phase 2 trial of MM-121, a fully human monoclonal antibody targeting ErbB3, in combination with erlotinib in EGFR wild-type NSCLC patients. J Clin Oncol ASCO Annual Meeting 2014; 32:5s
- Liu J, Ray-Coquard IA, Selle F, Poveda A, Cibula D, Hirte HW, Raspagliesi F, Gladieff L, Harter P, Schiavetto I, et al. Abstract 5519: A phase II randomized open-label study of MM-121, a fully human monoclonal antibody targeting ErbB3, in combination with weekly paclitaxel versus weekly paclitaxel in patients with platinum-resistant/refractory ovarian cancers. J Clin Oncol ASCO Annual Meeting 2014; 32:5s
- Garner AP, Bialucha CU, Sprague ER, Garrett JT, Sheng Q, Li S, Sineshchekova O, Saxena P, Sutton CR, Chen D, et al. An antibody that locks HER3 in the inactive conformation inhibits tumor growth driven by HER2 or neuregulin. Cancer Res 2013; 73:6024-35; PMID:23928993; http://dx.doi.org/10.1158/0008-5472.CAN-13-1198
- Im S-A, Juric D, Baselga J, Kong A, Martin P, Lin CC, Dees EC, Schellens JHM, De Braud FG, Delgado L, et al. Abstract 2519: A phase 1 dose-escalation study of anti-HER3 monoclonal antibody LJM716 in combination with trastuzumab in patients with HER2-overexpressing metastatic breast or gastric cancer. J Clin Oncol ASCO Annual Meeting 2014; 32:5s
- Garrett JT, Sutton CR, Kurupi R, Bialucha CU, Ettenberg SA, Collins SD, Sheng Q, Wallweber J, Defazio-Eli L, Arteaga CL. Combination of antibody that inhibits ligand-independent HER3 dimerization and a p110alpha inhibitor potently blocks PI3K signaling and growth of HER2+ breast cancers. Cancer Res 2013; 73:6013-23; PMID:23918797; http://dx.doi.org/10.1158/0008-5472.CAN-13-1191
- Vincent S, Fleet C, Bottega S, McIntosh D, Winston W, Chen T, Tyler S, Meetze K, Weiler S, Gyuris J. Abstract 2509: AV-203, a humanized ERBB3 inhibitory antibody inhibits ligand-dependent and ligand-independent ERBB3 signaling in vitro and in vivo. Proc AACR 103rd Annual Meeting. Cancer Res 2012; 72:2509; http://dx.doi.org/10.1158/1538-7445.AM2012-2509
- Meetze K, Vincent S, Tyler S, Mazsa EK, Delpero AR, Bottega S, McIntosh D, Nicoletti R, Winston WM, Weiler S, et al. Neuregulin 1 Expression Is a Predictive Biomarker for Response to AV-203, an ERBB3 Inhibitory Antibody, in Human Tumor Models. Clin Cancer Res 2015; 21:1106-14; PMID:25542901; http://dx.doi.org/10.1158/1078-0432.CCR-14-2407
- Sarantopoulos J, Gordon MS, Harvey RD, Sankhala KK, Malik L, Mahalingam D, Owonikoko TK, Lewis CM, Payumo F, Miller J, et al. First-in-human phase 1 dose-escalation study of AV-203, a monoclonal antibody against ERBB3, in patients with metastatic or advanced solid tumors. ASCO Meeting Abstracts 2014; 32:11113
- Aurisicchio L, Marra E, Luberto L, Carlomosti F, De Vitis C, Noto A, Gunes Z, Roscilli G, Mesiti G, Mancini R, et al. Novel anti-ErbB3 monoclonal antibodies show therapeutic efficacy in xenografted and spontaneous mouse tumors. J Cell Physiol 2012; 227:3381-8; PMID:22213458; http://dx.doi.org/10.1002/jcp.24037
- Fattore L, Malpicci D, Marra E, Camerlingo R, Roscilli G, Belleudi F, Ribas A, Mancini R, Torrisi MR, Aurisicchio L, et al. ErbB3 plays a key role in the early phase of establishment of resistance to BRAF and/or MEK inhibitors. J Transl Med 2015; 13:2041; http://dx.doi.org/10.1186/1479-5876-13-S1-K3
- Sala G, Traini S, D'Egidio M, Vianale G, Rossi C, Piccolo E, Lattanzio R, Piantelli M, Tinari N, Natali PG, et al. An ErbB-3 antibody, MP-RM-1, inhibits tumor growth by blocking ligand-dependent and independent activation of ErbB-3/Akt signaling. Oncogene 2012; 31:1275-86; PMID:21822299; http://dx.doi.org/10.1038/onc.2011.322
- Sala G, Rapposelli IG, Ghasemi R, Piccolo E, Traini S, Capone E, Rossi C, Pelliccia A, Di Risio A, D'Egidio M, et al. EV20, a Novel Anti-ErbB-3 Humanized Antibody, Promotes ErbB-3 Down-Regulation and Inhibits Tumor Growth In Vivo. Transl Oncol 2013; 6:676-84; PMID:24466370; http://dx.doi.org/10.1593/tlo.13475
- Zhang L, Castanaro C, Luan B, Yang K, Fan L, Fairhurst JL, Rafique A, Potocky TB, Shan J, Delfino FJ, et al. ERBB3/HER2 signaling promotes resistance to EGFR blockade in head and neck and colorectal cancer models. Mol Cancer Therapeutics 2014; 13:1345-55; http://dx.doi.org/10.1158/1535-7163.MCT-13-1033
- Papadopoulos KP, Adjei AA, Rasco DW, Liu L, Kao RJ, Brownstein CM, DiCioccio AT, Lowy I, Trail P, Wang D. Phase 1 study of REGN1400 (anti-ErbB3) combined with erlotinib or cetuximab in patients (pts) with advanced non-small cell lung cancer (NSCLC), colorectal cancer (CRC), or head and neck cancer (SCCHN). ASCO Meeting Abstracts 2014; 32:2516
- Blackburn E, Zona S, Murphy ML, Brown IR, Chan SK, Gullick WJ. A monoclonal antibody to the human HER3 receptor inhibits Neuregulin 1-β binding and co-operates with Herceptin in inhibiting the growth of breast cancer derived cell lines. Breast Cancer Res Treatment 2012; 134:53-9; http://dx.doi.org/10.1007/s10549-011-1908-1
- Lazrek Y, Dubreuil O, Garambois V, Gaborit N, Larbouret C, Le Clorennec C, Thomas G, Leconet W, Jarlier M, Pugniere M, et al. Anti-HER3 domain 1 and 3 antibodies reduce tumor growth by hindering HER2/HER3 dimerization and AKT-induced MDM2, XIAP, and FoxO1 phosphorylation. Neoplasia 2013; 15:335-47; PMID:23479511; http://dx.doi.org/10.1593/neo.121960
- Thomas G, Chardès T, Gaborit N, Mollevi C, Leconet W, Robert B, Radosevic-Robin N, Penault-Llorca F, Gongora C, Colombo P-E, et al. HER3 as biomarker and therapeutic target in pancreatic cancer: new insights in pertuzumab therapy in preclinical models. Oncotarget 2014; 5:7138-48; PMID:25216528; http://dx.doi.org/10.18632/oncotarget.2231
- Bossenmaier B, Friess T, Gerdes CA, Kolm I, Dimoudis N, Lifke V, Reiff U, Moessner E, Hoelzlwimmer G, von Hirschheydt T, et al. Abstract 2508: GE-huMab-HER3, a novel humanized, glycoengineered HER3 antibody with enhanced ADCC and superior preclinical in vitro and in vivo efficacy. Proc AACR 103rd Annual Meeting 2012. Cancer Res 2012; 72:2508; http://dx.doi.org/10.1158/1538-7445.AM2012-2508
- Mirschberger C, Schiller CB, Schraml M, Dimoudis N, Friess T, Gerdes CA, Reiff U, Lifke V, Hoelzlwimmer G, Kolm I, et al. RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation. Cancer Res 2013; 73:5183-94; PMID:23780344; http://dx.doi.org/10.1158/0008-5472.CAN-13-0099
- Meulendijks D, Lolkema MPJK, Voest EE, De Jonge MJ, Sleijfer S, Schellens JH, Fleitas T, Cervantes-Ruiperez A, Martinez-Garcia M, Taus A, et al. A first-in-human trial of RG7116, a glycoengineered monoclonal antibody targeting HER3, in patients with advanced/metastatic tumors of epithelial cell origin expressing HER3 protein. ASCO Meeting Abstracts 2013; 31:2522
- Clarke N, Hopson C, Hahn A, Sully K, Germaschewski F, Yates J, Akinseye C, Mangatt B, Jonak Z, Matheny C. Abstract 300: Preclinical pharmacologic characterization of GSK2849330, a monoclonal AccretaMab® antibody with optimized ADCC and CDC activity directed against HER3. Proc 26th EORTC – NCI – AACR Symposium Mol Targets Cancer Therapeutics 2014; 50(6 Suppl):98-9
- Gerdes CA, Nicolini VG, Herter S, van Puijenbroek E, Lang S, Roemmele M, Moessner E, Freytag O, Friess T, Ries CH, et al. GA201 (RG7160): a novel, humanized, glycoengineered anti-EGFR antibody with enhanced ADCC and superior in vivo efficacy compared with cetuximab. Clin Cancer Res 2013; 19:1126-38; PMID:23209031; http://dx.doi.org/10.1158/1078-0432.CCR-12-0989
- Cervantes-Ruiperez A, Markman B, Siena S, Pericay C, Aprile G, Bridgewater JA, Cubillo A, Waterston AM, Garcia-Carbonero R, Kozloff M, et al. The GAIN-C study (BP25438): Randomized phase II trial of RG7160 (GA201) plus FOLFIRI, compared to cetuximab plus FOLFIRI or FOLFIRI alone in second-line KRAS wild type (WT) or mutant metastatic colorectal cancer (mCRC). ASCO Meeting Abstracts 2012; 30:TPS3637
- Ben-Kasus T, Schechter B, Lavi S, Yarden Y, Sela M. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: relevance of receptor endocytosis. Proc Natl Acad Sci USA 2009; 106:3294-9; PMID:19218427; http://dx.doi.org/10.1073/pnas.0812059106
- Friedman LM, Rinon A, Schechter B, Lyass L, Lavi S, Bacus SS, Sela M, Yarden Y. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc Natl Acad Sci USA 2005; 102:1915-20; PMID:15684082; http://dx.doi.org/10.1073/pnas.0409610102
- Spiridon CI, Ghetie MA, Uhr J, Marches R, Li JL, Shen GL, Vitetta ES. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res 2002; 8:1720-30; PMID:12060609
- Klapper LN, Vaisman N, Hurwitz E, Pinkas-Kramarski R, Yarden Y, Sela M. A subclass of tumor-inhibitory monoclonal antibodies to ErbB-2/HER2 blocks crosstalk with growth factor receptors. Oncogene 1997; 14:2099-109; PMID:9160890; http://dx.doi.org/10.1038/sj.onc.1201029
- Kasprzyk PG, Song SU, Di Fiore PP, King CR. Therapy of an animal model of human gastric cancer using a combination of anti-erbB-2 monoclonal antibodies. Cancer Res 1992; 52:2771-6; PMID:1349849
- Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015; 372:724-34; PMID:25693012; http://dx.doi.org/10.1056/NEJMoa1413513
- Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Eng J Med 2012; 366:109-19; http://dx.doi.org/10.1056/NEJMoa1113216
- Pedersen MW, Jacobsen HJ, Koefoed K, Dahlman A, Kjaer I, Poulsen TT, Meijer PJ, Nielsen LS, Horak ID, Lantto J, et al. Targeting Three Distinct HER2 Domains with a Recombinant Antibody Mixture Overcomes Trastuzumab Resistance. Mol Cancer Therapeutics 2015; 14:669-80; http://dx.doi.org/10.1158/1535-7163.MCT-14-0697
- Ferraro DA, Gaborit N, Maron R, Cohen-Dvashi H, Porat Z, Pareja F, Lavi S, Lindzen M, Ben-Chetrit N, Sela M, et al. Inhibition of triple-negative breast cancer models by combinations of antibodies to EGFR. Proc Natl Acad Sci USA 2013; 110:1815-20; PMID:23319610; http://dx.doi.org/10.1073/pnas.1220763110
- Skartved NJ, Jacobsen HJ, Pedersen MW, Jensen PF, Sen JW, Jorgensen TK, Hey A, Kragh M. Preclinical pharmacokinetics and safety of Sym004: a synergistic antibody mixture directed against epidermal growth factor receptor. Clin Cancer Res 2011; 17:5962-72; PMID:21825041; http://dx.doi.org/10.1158/1078-0432.CCR-11-1209
- Iida M, Brand TM, Starr MM, Li C, Huppert EJ, Luthar N, Pedersen MW, Horak ID, Kragh M, Wheeler DL. Sym004, a novel EGFR antibody mixture, can overcome acquired resistance to cetuximab. Neoplasia 2013; 15:1196-206; PMID:24204198; http://dx.doi.org/10.1593/neo.131584
- Pedersen MW, Jacobsen HJ, Koefoed K, Hey A, Pyke C, Haurum JS, Kragh M. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res 2010; 70:588-97; PMID:20068188; http://dx.doi.org/10.1158/0008-5472.CAN-09-1417
- Dienstmann R, Patnaik A, Garcia-Carbonero R, Cervantes A, Benavent M, Rosello S, Tops BB, van der Post RS, Argiles G, Skartved NJ, et al. Safety and Activity of the First-in-Class Sym004 Anti-EGFR Antibody Mixture in Patients with Refractory Colorectal Cancer. Cancer Discov 2015; 5:598-609; PMID:25962717; http://dx.doi.org/10.1158/2159-8290.CD-14-1432
- Gaborit N, Abdul-Hai A, Mancini M, Lindzen M, Lavi S, Leitner O, Mounier L, Chentouf M, Dunoyer S, Ghosh M, et al. Examination of HER3 targeting in cancer using monoclonal antibodies. Proc Natl Acad Sci USA 2015; 112:839-44; PMID:25564668; http://dx.doi.org/10.1073/pnas.1423645112
- D'Souza JW, Reddy S, Goldsmith LE, Shchaveleva I, Marks JD, Litwin S, Robinson MK. Combining anti-ERBB3 antibodies specific for domain I and domain III enhances the anti-tumor activity over the individual monoclonal antibodies. PloS One 2014; 9:e112376; PMID:25386657; http://dx.doi.org/10.1371/journal.pone.0112376
- Schaefer G, Haber L, Crocker LM, Shia S, Shao L, Dowbenko D, Totpal K, Wong A, Lee CV, Stawicki S, et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 2011; 20:472-86; PMID:22014573; http://dx.doi.org/10.1016/j.ccr.2011.09.003
- Huang S, Li C, Armstrong EA, Peet CR, Saker J, Amler LC, Sliwkowski MX, Harari PM. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res 2013; 73:824-33; PMID:23172311; http://dx.doi.org/10.1158/0008-5472.CAN-12-1611
- Tao JJ, Castel P, Radosevic-Robin N, Elkabets M, Auricchio N, Aceto N, Weitsman G, Barber P, Vojnovic B, Ellis H, et al. Antagonism of EGFR and HER3 enhances the response to inhibitors of the PI3K-Akt pathway in triple-negative breast cancer. Sci Signal 2014; 7:ra29; PMID:24667376
- Juric D, Dienstmann R, Cervantes A, Hidalgo M, Messersmith W, Blumenschein GR, Jr., Tabernero J, Roda D, Calles A, Jimeno A, et al. Safety and Pharmacokinetics/Pharmacodynamics of the First-in-Class Dual Action HER3/EGFR Antibody MEHD7945A in Locally Advanced or Metastatic Epithelial Tumors. Clin Cancer Res 2015; 21:2462-70; PMID:26034219; http://dx.doi.org/10.1158/1078-0432.CCR-14-2412
- Fayette J, Wirth LJ, Oprean C, Hitt R, Udrea A, Jimeno A, Rischin D, Nutting C, Harari PM, Csőszi T, et al. Randomized phase II study of MEHD7945A (MEHD) vs cetuximab (Cet) in >= 2nd-line recurrent/metastatic squamous cell Carcinoma of the head & neck Annals of Oncology. ESMO 2014; 25:iv340-iv56
- Hill AG, Findlay M, Burge M, Jackson C, Garcia Alfonso P, Samuel L, Ganju V, Karthaus M, Amatu A, Jeffery M, et al. CT110 - Randomized phase II study of duligotuzumab + FOLFIRI versus cetuximab + FOLFIRI in 2nd-line patients with KRAS wild-type (wt) metastatic colorectal cancer (mCRC). In: AACR, ed. 106th Annual Meeting of the American Association for Cancer Research. Philadelphia, PA, 2015
- McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, Zhang B, Luus L, Overland R, Nguyen S, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Therapeutics 2012; 11:582-93; http://dx.doi.org/10.1158/1535-7163.MCT-11-0820
- Richards DA, Braiteh FS, Garcia AA, Denlinger CS, Conkling PR, Edenfield WJ, Anthony SP, Hellerstedt BA, Raju RN, Becerra C, et al. A phase 1 study of MM-111, a bispecific HER2/HER3 antibody fusion protein, combined with multiple treatment regimens in patients with advanced HER2-positive solid tumors. ASCO Meeting Abstracts 2014; 32:651
- Fitzgerald JB, Johnson BW, Baum J, Adams S, Iadevaia S, Tang J, Rimkunas V, Xu L, Kohli N, Rennard R, et al. MM-141, an IGF-IR- and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol Cancer Therapeutics 2014; 13:410-25; http://dx.doi.org/10.1158/1535-7163.MCT-13-0255
- Hu S, Fu W, Xu W, Yang Y, Cruz M, Berezov SD, Jorissen D, Takeda H, Zhu W. Four-in-one antibodies have superior cancer inhibitory activity against EGFR, HER2, HER3, and VEGF through disruption of HER/MET crosstalk. Cancer Res 2015; 75:159-70; PMID:25371409; http://dx.doi.org/10.1158/0008-5472.CAN-14-1670
- Kang JC, Poovassery JS, Bansal P, You S, Manjarres IM, Ober RJ, Ward ES. Engineering multivalent antibodies to target heregulin-induced HER3 signaling in breast cancer cells. Mabs 2014; 6:340-53; PMID:24492289; http://dx.doi.org/10.4161/mabs.27658
- Jacobsen HJ, Poulsen TT, Dahlman A, Kjaer I, Koefoed K, Sen JW, Weilguny D, Bjerregaard B, Andersen CR, Horak ID, et al. Pan-HER, an Antibody Mixture Simultaneously Targeting EGFR, HER2 and HER3 Effectively Overcomes Tumor Heterogeneity and Plasticity. Clin Cancer Res 2015; 21(18):4110-22; PMID:25908781; http://dx.doi.org/10.1158/1078-0432.CCR-14-3312
- Mancini M, Gaborit N, Lindzen M, Meir Salame T, Dall’Ora M, Sevilla-Sharon M, Abdul-Hai A, Downward J, Yarden Y. Combining Three Antibodies Nullifies Feedback and Inhibits Erlotinib Resistant Lung Cancer. Sci Signal 2015; 8(379):ra53 in press; PMID:26038598; http://dx.doi.org/10.1126/scisignal.aaa0725
- Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biozztechnol 2012; 30:679-92; http://dx.doi.org/10.1038/nbt.2284
- Aurisicchio L, Marra E, Roscilli G, Mancini R, Ciliberto G. The promise of anti-ErbB3 monoclonals as new cancer therapeutics. Oncotarget 2012; 3:744-58; PMID:22889873; http://dx.doi.org/10.18632/oncotarget.550
