ABSTRACT
A great number of cystic fibrosis (CF) pathogens such as Pseudomonas aeruginosa, the Burkholderia cepacia and the Mycobacterium abscessus complex raised difficult therapeutic problems due to their intrinsic multi-resistance to numerous antibiotics. Vaccine strategies represent one of the key weapons against these multi-resistant bacteria in a number of clinical settings like CF. Different strategies are considered in order to develop such vaccines, linked either to priming the host response, or by exploiting genomic data derived from the bacterium. Interestingly, virulence factors synthesized by various pathogens might serve as targets for vaccine development and have been, for example, evaluated in the context of CF.
Cystic fibrosis and microbial lung infections
Cystic fibrosis (CF) is a disease which arises from a Mendelian defect due to a series of mutations in the cftr gene encoding the Cl− channel.Citation1 The resulting flaw in this protein is responsible for increasing the viscosity of the mucus, which promotes the accumulation and the attachment of bacteria to mucins. Chronic inflammationCitation2 and early bacterial infection maintain a vicious circle and are each responsible for the lung damage which ensues. Lung infections in CF patients represent the most frequent but also the more serious manifestations since they are responsible for more than 90% of CF patient deaths.Citation3 The microorganisms that may infect the respiratory system are bacteria, fungi and viruses. Bacterial colonization occurs very early in the natural history of the disease.Citation4 The first causative organisms are Haemophilus influenzae and Staphylococcus aureus. S. aureus is usually the first detectedCitation5 and its prevalence is rising.Citation6 Affinity of S. aureus for CF mucus contributes to persistent colonization and progressive pulmonary damage increasing the potential for further infections to set in, for example Pseudomonas spp.Citation5 Pseudomonas aeruginosa colonization arises several months to several years after. Finally, several bacterial complexes are found responsible for severe infections in CF, in addition to be the most difficult to treat: the Burkholderia cepacia complex (Bcc) and the Mycobacterium abscessus complex, which has emerged recently as a threat in CF patients, and may present with Mycobacterium avium, the major non-tuberculous mycobacterium (NTM) present in CF lungs with a significant prevalence.Citation7,8
Opportunistic pathogens becoming untreatable weapons in CF patients
P. aeruginosa is the environmental opportunistic pathogen in CF patients. It is the most commonly isolated bacterium that infects individuals with CF, with colonization and chronic infections that may affect up to 80% of adult CF patients.Citation9 P. aeruginosa establishes a chronic endobronchial infection which impacts on morbidity and mortality of CF patients. P. aeruginosa is also notable for its resistance to antibiotics, making it therefore a difficult to treat pathogen, which, once acquired, is rarely, if ever, eradicated. In addition, P. aeruginosa frequently colonized CF lungs as a biofilm, which reduces the patient's immune response and access by antibiotics.Citation10 A second opportunistic pathogen, represented as a complex is the Burkholderia cepacia complex (Bcc). It is composed of 18 species that are able to cause opportunistic and lethal infections CF patients.Citation11 The two most clinically relevant species are Burkholderia cenocepacia and Burkholderia multivorans.Citation12 These environmental, intracellular and biofilm-forming bacteria are extremely antibiotic resistant organism.Citation12 Bcc infections are rarely cleared from CF patients once they are colonized, as observed in P. aeruginosa infections. The third antibiotic-resistant bacterium found in CF patients with frequency between 3 to 7%Citation7,13 is Mycobacterium abscessus. It is a rapidly growing mycobacterium also existing as a complex: the Mycobacterium abscessus complex,Citation14 with 2 subspecies M. abscessus abscessus and M. abscessus bolletii respectively. M. abscessus is, within the group of rapid-growers, responsible for a broad spectrum of diseases in humans. Lung infections are frequent, with CF patients particularly susceptible,Citation7,13,Citation15 in addition to muco-cutaneous infections often of nosocomial origin.Citation16 Recent reports of human-to-human transmission in the context of CF care have been described.Citation17,18 M. abscessus raises very challenging therapeutic issues because of its natural resistance to most available antibiotics.Citation19,20 Severe, even fatal, infections in CF patients have been described due to therapeutic deadlock.Citation21 M. abscessus infection might represent a contraindication for lung transplantation in several countries,Citation22 leaving CF patients without therapeutic options.
As such, antibiotic treatment exemplifies a clear challenge now faced with these opportunistic pathogens. We demonstrated for example a significant link between previous intravenous antibiotic courses and the isolation of M. abscessus in CF patient lungs, underlining the role of broad-spectrum antimicrobial therapy in the emergence of M. abscessus disease.Citation23 And this is true for the continuous emergence of resistant P. aeruginosa or Bcc due to the repeated antibiotic therapeutic regimens given to CF patients.Citation24 Emergence of multi-resistant bacteria leads to therapeutic impasses with severe and fatal infections.Citation24
Vaccine approaches
Pathogens can be divided into 2 groups according to whether vaccines exist against them or not. However, no human vaccine has been developed so far against the antibiotic-resistant pathogens described above. As such, the development of prophylactic or therapeutic vaccines is of extreme importance when confronted with bacteria of this high resistance.
When defining an appropriate target, making the correct choice and how such tools can be developed is a long and tedious process. Development of vaccine targets can profit from genome sequence comparisons,Citation25 and, using a process known as reverse vaccinology, might introduce a novel strategy to identify target antigens that might serve as potential vaccines.Citation25 We chose this strategy for the development of a first vaccine against M. abscessusCitation26 (as described in the following paragraph). Indeed, the presence of specific genes in opportunistic pathogen genomes that are absent from saprophytic bacterial genomes belonging to the same genus represents the key to unravel virulence factors that can then be targeted using a vaccine approach. A better understanding of the "virulence" genes contributes greatly to the development of new control strategies against these microorganisms. In addition, the choice of a virulence factor as a vaccine target has been shown to be relevant in the scientific literature.Citation25
Vaccination can then be performed using recombinant proteins when expression and purification are possible; or using the expression of plasmid DNA with modified eukaryotic gene sequences, as has been performed with M. abscessus.Citation26 In fact, in the different vaccine strategies, DNA vaccines exemplify one of these new strategies and has been used successfully in the context of infectious diseases.Citation27 Over the last 15 years, DNA vaccines have proved effective in animal models including candidates against HIV, malaria and influenza.Citation28 DNA vaccines have been extensively evaluated in humans with a recent review identifying 72 Phase I, 20 Phase II and 2 Phase III human trials.Citation29 Mycobacteria, such as Mycobacterium tuberculosis, have received particular attention in this respect.Citation30,31 Finally, some common antigens might be present in a variety of different CF pathogens, and the development of a vaccine might have the potential for conferring cross-protection against several CF pathogens (see below).
Vaccines against opportunistic CF pathogens
Some infectionsCitation32,33 frequently associated with impaired respiratory function in patients with cystic fibrosis are the subject of vaccine development: for example infections with P. aeruginosa.
The development of a vaccine against P. aeruginosa has so far mobilized many research teams, even though no human trials have been conducted yet. Numerous vaccine attempts have been made against this pathogen that is generally considered to be the most targeted among pathogens infecting cystic fibrosis patients, and in order to obtain a state of the art overview in this domain, you would do well to explore the following recent reviews.Citation34-37 Major target antigens include the O-glycosylated lipopolysaccharide, cell-surface alginate, flagella, components of the Type III secretion system and outer membrane proteins.Citation38 The FliC flagellin protein widely considered as a virulence factor has pro-inflammatory activity on respiratory epithelial cells. Flagellin has been one of the major targets for vaccines and therapeutic development especially for CF patients. For example, as early as 1995, P. aeruginosa flagella were developed as a vaccine against P. aeruginosa and a Phase I study demonstrated that intramuscular immunization in healthy human adults results in high and long-lasting serum IgG flagella antibody titers and IgG, IgA and secretory IgA isotypes in the secretory immune system.Citation39 Then, in a phase III study, an immunization with a bivalent vaccine for flagella subtypes significantly lowered the risk for initial P. aeruginosa infection in CF patients.Citation40 Other proteins have been used in human trials: OprF and OprI that are outer membrane proteins are able to induce a specific antibody response in the lung after nasal and oral vaccinations, and are as such promising candidates for the development of anti-pseudomonas immunization.Citation41 Furthermore, a fusion protein of the P. aeruginosa OprF fragment, OprI, and FliC promoted the clearance of P. aeruginosa in a pulmonary challenge model.Citation42 Another study which also tested these 2 outer membrane proteins as mucosal vaccines, leads to the development of airway immunogenicity against the pathogen with superior efficacy compared to systemic vaccination.Citation43 We can add to this, as more recently, among the latest antigens and strategies tested, an assay using the conserved surface exopolysaccharide alginate, a virulence factor produced by mucoid strains, has been tried in miceCitation44 and conferred protection after intranasal challenge. It was also efficacious as a therapeutic vaccine. Previously, an assay was performed in humans with O-polysaccharide conjugated to toxin A and vaccinated children developed less chronic Pseudomonas lung infections than non-vaccinated children.Citation45,46 An element of alginate (polymannuronic acid) has also been conjugated to flagellin leading to protective efficacy in a mouse lung infection model.Citation47 Some P. aeruginosa antigens conjugated to bovine serum albumin have also been tested in mice.Citation48 However, despite more than 50 y of research efforts, a licensed vaccine against P. aeruginosa is still a long way off from being available for CF patients.
With reference to the Burkholderia cepacia complex (Bcc), several virulence factors associated with human infection were tested for their potential as vaccine candidates.Citation49 Such an approach was undertaken by a group that sought immunoreactive proteins expressed by both Burkholderia cenocepacia and Burkholderia multivorans.Citation50 A recent review summarizes in detail all vaccination experiments that have been undertaken against Bcc.Citation51 As a recent example, the flagellar protein FliC from Burkholderia pseudomallei and considered as a virulence factor has been shown to confer protection in mice.Citation52 This study shows that the epitopes of interest in B. pseudomallei FliC cross-react with orthologous B. multivorans and B. cenocepacia FliC sequences suggesting protection can be conferred against members of the Bcc. Other proteins like OMPs from different Burkolderia species are also able to generate protection in mice.Citation53,54
Vaccination in the context of mycobacteria
The most widely used global vaccine is the "Bacille Calmette et Guérin" (BCG) strain of Mycobacterium bovis used in the fight against tuberculosis, a disease caused by Mycobacterium tuberculosis, which presently kills 1.3 million individuals around the world every year.Citation55 This technique harnessed the historically defined strategy developed by Jenner,Citation56 by using the antigenic repertoire of a non-pathogenic strain for human, in order to confer protection against the human pathogen. Despite its widespread use, BCG is still a controversial vaccine, and its deficiencies have lead to the development of new research axes, using either purified compounds or a DNA vaccines in a quest to improve anti-mycobacterial vaccines. Several mycobacterial proteins (Ag85A/B, 65-kDa heat shock protein, hsp65, 36-kDa proline-rich antigen, MPB83, MPB70, CFP-10 and ESAT-6) have been evaluated as DNA vaccines in experimental models.Citation57-63 By virtue of its strong capacity to induce CD4+-mediated Th1 and CD8+-mediated cytotoxic T-lymphocyte responses,Citation25 DNA vaccine approaches are particularly attractive for their preventive and therapeutic activity against intracellular pathogens such as pathogenic mycobacteria.Citation61 The majority of these studies were conducted in the fields of humanCitation30,31,Citation57,60,Citation61 and bovine tuberculosisCitation58,59; and even in leprosy (immuno-dominant 35-kDa proteinCitation64). Few DNA vaccines have been developed which are related to pathogenic or opportunistic NTM, for example M. avium (35-kDa proteinCitation65; p85A-EGFP, p65K-EGFPCitation66); Mycobacterium ulceransCitation67 or Mycobacterium marinum.Citation62,63 None were developed in the context of tackling infections in inherited diseases such as CF.
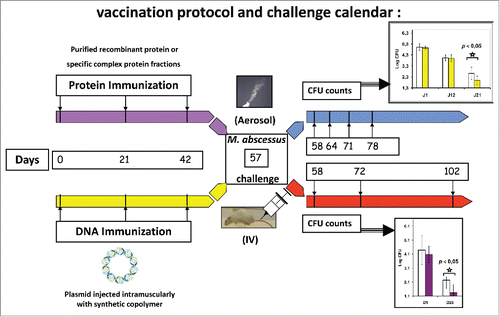
While the presence of NTM is demonstrated in 7 to 13% of CF patients,Citation8 infections by M. tuberculosis are rare in CF patients.Citation68,69 BCG remains a currently recommended vaccine in children at risk of exposure to TB; and as a consequence of partial efficacy against NTM, continues to be recommended in CF children. The two mycobacteria that together are responsible for the majority of infections in cystic fibrosis patients are M. avium and M. abscessus, a slow and a rapid growing mycobacteria (SGM and RGM), respectively, and to our knowledge, no vaccine approach against these bacteria has been considered in the context of CF. As mentioned above, development of vaccine targets can benefit from knowledge derived from genome sequence comparison.Citation25 Genome sequence comparison between the M. abscessus genome and Mycobacterium chelonae, Mycobacterium smegmatis genomes (2 rapid growing NTM, which are less or non-pathogenic respectively), allowed the unraveling of several key virulence factors.Citation19 As described in each genome sequences, several of these virulence factors were acquired by horizontal gene transfer, from non-fermenting Gram negative bacteria such as those found in CF patient lungs: P. aeruginosa and Bcc.Citation19 Among others we characterized MAB_0555, a phospholipase C (PLC) with the highest homology with PLC-N from P. aeruginosa.Citation70 We have demonstrated the impact of MAB_0555 PLC in the virulence of M. abscessus in mice,Citation70 when pre-cultivated on amoeba. We have also shown that the recombinant protein or the plasmid encoding PLC conferred protection, after an aerosol or IV challenge (), in Δ508 or CF miceCitation71 only.Citation26 The two formulations () gave quite similar results, namely a diminution of bacterial load in lungs 3 weeks after a M. abscessus aerosol challenge.Citation26 PLC are also present in other CF pathogens like P. aeruginosa and the immune cross reactivity between M. abscessus PLC and P. aeruginosa PLC could lead to a vaccine protective against both mycobacterial and Gram negative infection in CF patients, as we were able to show the recognition of the MAB_0555 PLC by sera from CF patients only infected by P. aeruginosa. This approach is currently underway in our laboratory with other target designs recently unraveled.Citation72
Figure 1. Vaccination protocol and challenge calendar. Mice are immunized three times following a prime/boost scheme (days 0, 21 and 42) either with recombinant proteins or protein complexes, either with a plasmid coding for a protein of interest in the case of DNA immunization. Two weeks after the third immunization, mice are challenged (day 57) with M. abscessus, either by an aerosol way, either intravenously (IV). Lungs are collected, homogenized by dislocation and CFUs are counted at various times after bacterial challenge according to the type of challenge practised: day 1, 7, 14 and 21 for aerosol challenge or day 1, 15 and 45 for IV challenge.
Conclusion
Vaccination is effective in preventing infections and we advocate their use in patients with CF, specifically for the prevention of respiratory infections. In addition to the traditional vaccination schedules,Citation73 evaluation studies to demonstrate the immunological and clinical efficacy of novel vaccines against multi-resistant bacteria remain necessary in this particular patient population.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989; 245:1066-73; PMID:2475911; http://dx.doi.org/10.1126/science.2475911.
- Ratjen FA. Cystic fibrosis: pathogenesis and future treatment strategies. Resp Care 2009; 54:595-602; PMID:19393104; http://dx.doi.org/10.4187/aarc0427.
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005; 352:1992-2001; PMID:15888700; http://dx.doi.org/10.1056/NEJMra043184.
- May JR, Herrick NC, Thompson D. Bacterial Infection in cystic fibrosis. Arch Dis Child 1972; 47:908-13; PMID: 4630478; http://dx.doi.org/10.1136/adc.47.256.908.
- Marks MI. Clinical significance of Staphylococcus aureus in cystic fibrosis. Infection 1990; 18:53-6; PMID: 2107147; http://dx.doi.org/10.1007/BF01644186.
- LiPuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev 2010; 23:299-323; PMID:20375354; http://dx.doi.org/10.1128/CMR.00068-09.
- Roux AL, Catherinot E, Ripoll F, Soismier N, Macheras E, Ravilly S, Bellis G, Vibet MA, Le Roux E, Lemonnier L, et al. Multicenter study of prevalence of nontuberculous mycobacteria in patients withcystic fibrosis in France. J Clin Microbiol 2009; 47:4124-8; PMID:19846643; http://dx.doi.org/10.1128/JCM.01257-09.
- Olivier KN, Weber DJ, Lee JH, Handker A, Tudor G, Molina PL, Tomashefski J, Knowles MR; Nontuberculous Mycobacteria in Cystic Fibrosis Study Group. Nontuberculous mycobacteria. Am J Respir Crit Care Med 2003; 167:835-40; PMID:12433669; http://dx.doi.org/10.1164/rccm.200207-679OC.
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003; 168:918-51; PMID:14555458; http://dx.doi.org/10.1164/rccm.200304-505SO.
- Drenkard E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microb Infect 2003; 5:1213-1219; PMID:14623017; http://dx.doi.org/10.1016/j.micinf.2003.08.009.
- Lipuma J. Update on the Burkholderia cepacia complex. Curr Opin Pulm Med 2005; 11:528-33; PMID:16217180; http://dx.doi.org/10.1097/01.mcp.0000181475.85187.ed.
- Caraher E, Duff C, Mullen T, Mc Keon S, Murphy P, Callaghan M, McClean S. Invasion and biofilm formation of Burkholderia dolosa is comparable with Burkholderia cenocepacia and Burkholderia multivorans. J Cyst Fibros 2007; 6:49-56; PMID:16781896; http://dx.doi.org/10.1016/j.jcf.2006.05.007.
- Olivier KN, Weber DJ, Wallace RJ Jr, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, et al. Nontubercu-lous mycobacteria I: multicenter prevalence study in cystic fibrosis. Am J RespirCrit Care Med 2003; 167:828-34; PMID:12433668; http://dx.doi.org/10.1164/rccm.200207-678OC.
- Leao SC, Tortoli E, Euzeby JP, Garcia MJ. Proposal that Mycobacterium massiliense and Mycobacterium bolletii be united and reclassified as Mycobacterium abscessus subsp. bolletii comb. nov., designation of Mycobacterium abscessus subsp.abscessus subsp. nov. and emended description of Mycobacterium abscessus. IntJ Syst Evol Microbiol 2011; 61:2311-3; PMID:21037035; http://dx.doi.org/10.1099/ijs.0.023770-0.
- Pierre-Audigier C, Ferroni A, Sermet-Gaudelus I, Le Bourgeois M, Offredo C, Vu-Thien H, Fauroux B, Mariani P, Munck A, Bingen E, et al. Age-related prevalence and distribution of nontuberculousmycobacterial species among patients with cystic fibrosis. J Clin Microbiol 2005; 43:3467-70; PMID:16000480; http://dx.doi.org/10.1128/JCM.43.7.3467-3470.2005.
- Wallace RJ Jr, Brown BA, Griffith DE. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol 1998; 52:453-90; PMID:9891805; http://dx.doi.org/10.1146/annurev.micro.52.1.453.
- Aitken ML, Limaye A, Pottinger P, Whimbey E, Goss CH, Tonelli MR, Cangelosi GA, Dirac MA, Olivier KN, Brown-Elliott BA, et al. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am J Respir Crit Care Med 2012; 185:231-2; PMID:22246710; http://dx.doi.org/10.1164/ajrccm.185.2.231.
- Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, et al. Whole-genome sequencing to identify transmission of Mycobacterium absces-sus between patients with cystic fibrosis: a retrospective cohort study. Lancet 2013; 381:1551-60; PMID:23541540; http://dx.doi.org/10.1016/S0140-6736(13)60632-7.
- Ripoll F, Pasek S, Schenowitz C, Dossat C, Barbe V, Rottman M, Macheras E, Heym B, Herrmann JL, Daffé M, et al. Nonmycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PLoS ONE 2009; 4:e5660; PMID:19543527; http://dx.doi.org/10.1371/journal.pone.0005660.
- Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 2009; 53:1367-76; PMID: 19171799; http://dx.doi.org/10.1128/AAC.01275-08.
- Sanguinetti M, Ardito F, Fiscarelli E, La Sorda M, D'Argenio P, Ricciotti G, Fadda G. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J Clin Microbiol 2001;39:816-9; PMID:11158161; http://dx.doi.org/10.1128/JCM.39.2.816-819.2001.
- Taylor JL, Palmer SM. Mycobacterium abscessus chest wall and pulmonary infection in a cystic fibrosis lung transplant recipient. J Heart Lung Transplant 2006; 25:985-8; PMID:16890122; http://dx.doi.org/10.1016/j.healun.2006.04.003.
- Catherinot E, Roux AL, Vibet MA, Bellis G, Ravilly S, Lemonnier L, Le Roux E, Bernède-Bauduin C, Le Bourgeois M, Herrmann JL, et al. Mycobacterium avium and Mycobacterium abscessus complex target distinct cysticfibrosis patient subpopulations. J Cyst Fibros 2013;12:74-80; PMID:22857820; http://dx.doi.org/10.1016/j.jcf.2012.06.009.
- Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med 1998; 4:1241-3; PMID:9809543; http://dx.doi.org/10.1038/3218.
- Hoft DF, Brusic V, Sakala IG. Optimizing vaccine development. Cell Microbiol 2011 13:934-942; PMID:21631691; http://dx.doi.org/10.1111/j.1462-5822.2011.01609.x.
- Le Moigne V, Rottman M, Goulard C, Barteau B, Poncin I, Soismier N, Canaan S, Pitard B, Gaillard JL, Herrmann JL. Bacterial phospholipases C as vaccine candidate antigens against cystic fibrosis respiratory pathogens: the Mycobacterium abscessus model. Vaccine 2015; 33:2118-24; PMID: 25804706; http://dx.doi.org/10.1016/j.vaccine.2015.03.030.
- Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today 1998; 19:89-97; PMID:9509764; http://dx.doi.org/10.1016/S0167-5699(97)01201-2.
- Klinman DM, Klaschik S, Tross D, Shirota H, Steinhagen F. FDA guidance on prophylactic DNA vaccines: analysis and recommendations. Vaccine 2010; 28:2801-05; PMID:19941989; http://dx.doi.org/10.1016/j.vaccine.2009.11.025.
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet 2008; 9:776-88; PMID:18781156; http://dx.doi.org/10.1038/nrg2432.
- Tascon RE, Colston MJ, Ragno S, Stavropoulos E, Gregory D, Lowrie DB. Vaccination against tuberculosis by DNA injection. Nat Med 1996; 2:888-92; PMID:8705858; http://dx.doi.org/10.1038/nm0896-888.
- Huygen K, Content J, Denis O, Montgomery DL, Yawman AM, Deck RR, DeWitt CM, Orme IM, Baldwin S, D'Souza C, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med 1996; 2:893-8; PMID:8705859; http://dx.doi.org/10.1038/nm0896-893.
- Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, Massie J, Hall GL, Sly P, Stick S, et al. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med 2011; 184:75-81; PMID:21493738; http://dx.doi.org/10.1164/rccm.201011-1892OC.
- Sagel SD, Gibson RL, Emerson J, McNamara S, Burns JL, Wagener JS, Ramsey BW; Inhaled Tobramycin in Young Children Study Group; Cystic Fibrosis Foundation Therapeutics Development Network. Impact of pseudomonas and staphylococcal infection on inflammation and clinical status in young children with cystic fibrosis. J Pediatr 2009; 154:183-8; PMID:18822427; http://dx.doi.org/10.1016/j.jpeds.2008.08.001.
- Grimwood K, Kyd JM, Owen SJ, Massa HM, Cripps AW. Vaccination against respiratory Pseudomonas aeruginosa infection. Hum Vaccin Immunother 2015; 11:14-20; PMID: 25483510; http://dx.doi.org/10.4161/hv.34296.
- Savoia D. New perspectives in the management of Pseudomonas aeruginosa infections. Future Microbiol 2014; 9:917-28; PMID:25156380; http://dx.doi.org/10.2217/fmb.14.42.
- Johansen HK, Gøtzsche PC. Vaccines for preventing infection with Pseudomonas aeruginosa in cystic fibrosis. Cochrane Database Syst Rev 2013; 6:CD001399; PMID: 23771707.
- Sharma A, Krause A, Worgall S. Recent developments for Pseudomonas vaccines. Hum Vaccin 2011; 7:999-1011; PMID:21941090; http://dx.doi.org/10.4161/hv.7.10.16369.
- Pier G. Application of vaccine technology to prevention of Pseudomonas aeruginosa infections. Expert Rev Vaccines 2005; 4:645-56; PMID:16221066; http://dx.doi.org/10.1586/14760584.4.5.645.
- Döring G, Pfeiffer C, Weber U, Mohr-Pennert A, Dorner F. Parenteral application of a Pseudomonas aeruginosa flagella vaccine elicits specific anti-flagella antibodies in the airways of healthy individuals. Am J Respir Crit Care Med 1995; 151, 983-985; PMID:7697276.
- Döring G, Meisner C, Stern M; Flagella Vaccine Trial Study Group. Flagella vaccine trial study, a double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc Natl Acad Sci USA 2007; 104:11020-5; PMID:17585011; http://dx.doi.org/10.1073/pnas.0702403104.
- Bumann D, Behre C, Behre K, Herz S, Gewecke B, Gessner JE, von Specht BU, Baumann U. Systemic, nasal and oral live vaccines against Pseudomonas aeruginosa: a clinical trial of immunogenicity in lower airways of human volunteers. Vaccine 2010; 28:707-13; PMID:19887136; http://dx.doi.org/10.1016/j.vaccine.2009.10.080.
- Weimer ET, Lu H, Kock ND, Wozniak DJ, Mizel SB. A fusion protein vaccine containing OprF epitope 8, OprI, and type A and B flagellins promotes enhanced clearance of nonmucoid Pseudomonas aeruginosa. Infect Immun 2009; 77:2356-66; PMID:19349426; http://dx.doi.org/10.1128/IAI.00054-09.
- Sorichter S, Baumann U, Baumgart A, Walterspacher S, von Specht BU. Immune responses in the airways by nasal vaccination with systemic boosting against Pseudomonas aeruginosa in chronic lung disease. Vaccine 2009; 27:2755-9; PMID:19366571; http://dx.doi.org/10.1016/j.vaccine.2009.03.010.
- Farjah A, Owlia P, Siadat SD, Mousavi SF, Ardestani MS, Mohammadpour HK. Immunological evaluation of an alginate-based conjugate as a vaccine candidate against Pseudomonas aeruginosa. APMIS 2015; 123:175-83; PMID:25470757; http://dx.doi.org/10.1111/apm.12337.
- Zuercher AW, Horn MP, Que JU, Ruedeberg A, Schoeni MH, Schaad UB, Marcus P, Lang AB. Antibody responses induced by long-term vaccination with an octovalent conjugate Pseudomonas aeruginosa vaccine in children with cystic fibrosis. FEMS Immunol Med Microbiol 2006; 47:302-8; PMID:16831219; http://dx.doi.org/10.1111/j.1574-695X.2006.00103.x.
- Zuercher AW, Imboden MA, Jampen S, Bosse D, Ulrich M, Chtioui H, Lauterburg BH, Lang AB. Cellular immunity in healthy volunteers treated with an octavalent conjugate Pseudomonas aeruginosa vaccine. Clin Exp Immunol 2006; 143:132-8; PMID:16367944; http://dx.doi.org/10.1111/j.1365-2249.2005.02964.x.
- Campodónico VL, Llosa NJ, Bentancor LV, Maira-Litran T, Pier GB. Efficacy of a conjugate vaccine containing polymannuronic acid and flagellin against experimental Pseudomonas aeruginosa lung infection in mice. Infect Immun 2011; 79:3455-64; PMID: 21628521; http://dx.doi.org/10.1128/IAI.00157-11.
- Rodrigues NF, van Tilburg Bernardes E, Rocha RP, da Costa LC, Coutinho AC, dos Santos Muniz M, Pereira AA, da Silva PH, Malaquias LC, Coelho LF. Bovine serum albumin nanoparticle vaccine reduces lung pathology induced by live Pseudomonas aeruginosa infection in mice. Vaccine 2013; 31:5062-6; PMID: 24021308; http://dx.doi.org/10.1016/j.vaccine.2013.08.078.
- Casey WT, McClean S. Exploiting molecular virulence determinants in Burkholderia to develop vaccine antigens. Curr Med Chem 2015; 22:1719-33; PMID:25850766; http://dx.doi.org/10.2174/0929867322666150408111304.
- Shinoy M, Dennehy R, Coleman L, Carberry S, Schaffer K, Callaghan M, Doyle S, McClean S. Immunoproteomic analysis of proteins expressed by two related pathogens, Burkholderia multivorans and Burkholderia cenocepacia, during human infection. PLoS One 2013; 8:e80796; PMID: 24260482; http://dx.doi.org/10.1371/journal.pone.0080796.
- Choh LC, Ong GH, Vellasamy KM, Kalaiselvam K, Kang WT, Al-Maleki AR, Mariappan V, Vadivelu J. Burkholderia vaccines: are we moving forward? Front Cell Infect Microbiol 2013; 5;3:5; PMID:23386999.
- Musson JA, Reynolds CJ, Rinchai D, Nithichanon A, Khaenam P, Favry E, Spink N, Chu KK, De Soyza A, Bancroft GJ, et al. CD4 + T cell epitopes of FliC conserved between strains of Burkholderia: implications for vaccines against melioidosis and cepacia complex in cystic fibrosis. J Immunol 2014; 193:6041-9; PMID:25392525; http://dx.doi.org/10.4049/jimmunol.1402273.
- Makidon PE, Knowlton J, Groom JV 2nd, Blanco LP, LiPuma JJ, Bielinska AU, Baker JR Jr. Induction of immune response to the 17 kDa OMPA Burkholderia cenocepacia polypeptide and protection against pulmonary infection in mice after nasal vaccination with an OMP nanoemulsion-based vaccine. Med Microbiol Immunol 2010; 199:81-92; PMID: 19967396; http://dx.doi.org/10.1007/s00430-009-0137-2.
- Bertot GM, Restelli MA, Galanternik L, Aranibar Urey RC, Valvano MA, Grinstein S. Nasal immunization with Burkholderia multivorans outer membrane proteins and the mucosal adjuvant adamantylamide dipeptide confers efficient protection against experimental lung infections with B. multivorans and B. cenocepacia. Infect Immun 2007; 75:2740-52; PMID: 17296759; http://dx.doi.org/10.1128/IAI.01668-06.
- World Health Organization. Global tuberculosis report 2013. Available at www.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf
- Jenner E. The Three Original Publications on Vaccination Against Smallpox. 1798, 1799, 1800. New York: P.F. Collier & Son Company, 1909–14. Harvard Classics, Vol. 38, Part 4.
- Montgomery DL, Huygen K, Yawman AM, Deck RR, Dewitt CM, Content J, Liu MA, Ulmer JB. Induction of humoral and cellular immune responses by vaccination with M. tuberculosis antigen 85 DNA. Cell Mol Biol (Noisy-le-grand) 1997; 43:285-92; PMID:9193782.
- Chambers MA, Vordermeier H, Whelan A, Commander N, Tascon R, Lowrie D, Hewinson RG. Vaccination of mice and cattle with plasmid DNA encoding the Mycobacterium bovis antigen MPB83. Clin Infect Dis 2000; Suppl 3:S283-7. PMID:10875801; http://dx.doi.org/10.1086/313875.
- Vordermeier HM, Whelan A, Cockle PJ, Farrant L, Palmer N, Hewinson RG. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin Diagn Lab Immunol 2001; 8:571-8; PMID:11329460.
- Huygen K. On the use of DNA vaccines for the prophylaxis of mycobacterial diseases. Infect Immun 2003; 71:1613-21; PMID:12654772; http://dx.doi.org/10.1128/IAI.71.4.1613-1621.2003.
- Romano M, Huygen K. DNA vaccines against mycobacterial diseases. Expert Rev Vaccines 2009; 8:1237-50; PMID:19722896; http://dx.doi.org/10.1586/erv.09.87.
- Oksanen KE, Halfpenny NJ, Sherwood E, Harjula SK, Hammarén MM, Ahava MJ, Pajula ET, Lahtinen MJ, Parikka M, Rämet M. An adult zebrafish model for preclinical tuberculosis vaccine development. Vaccine 2013; 31:5202-9; PMID:24055305; http://dx.doi.org/10.1016/j.vaccine.2013.08.093.
- Feng G, Jiang Q, Xia M, Lu Y, Qiu W, Zhao D, Lu L, Peng G, Wang Y. Enhanced immune response and protective effects of nano-chitosan-based DNA vaccine encoding T cell epitopes of Esat-6 and FL against Mycobacterium tuberculosis infection. PLoS One 2013; 8:e61135; PMID:23637790; http://dx.doi.org/10.1371/journal.pone.0061135.
- Martin E, Roche PW, Triccas JA, Britton WJ. DNA encoding a single mycobacterial antigen protects against leprosy infection. Vaccine 2001; 19:1391-6. PMID:11163661; http://dx.doi.org/10.1016/S0264-410X(00)00374-1.
- Martin E, Kamath AT, Triccas JA, Britton WJ. Protection against virulent Mycobacterium avium infection following DNA vaccination with the 35-kgdalton antigen is accompanied by induction of gamma interferon-secreting CD4(+) T cells. Infect Immun 2000; 68:3090-6; PMID:10816448; http://dx.doi.org/10.1128/IAI.68.6.3090-3096.2000.
- Velaz-Faircloth M, Cobb AJ, Horstman AL, Henry SC, Frothingham R. Protection against Mycobacterium avium by DNA vaccines expressing mycobacterial antigens as fusion proteins with green fluorescent protein. Infect Immun 1999; 67:4243-50; PMID:10417198.
- Tanghe A, Content J, Van Vooren JP, Portaels F, Huygen K. Protective efficacy of a DNA vaccine encoding antigen 85A from Mycobacterium bovis BCG against Buruli ulcer. Infect Immun 2001; 69:5403-11; PMID:11500410; http://dx.doi.org/10.1128/IAI.69.9.5403-5411.2001.
- Morand PC, Burgel PR, Carlotti A, Desmazes-Dufeu N, Farhi D, Martin C, Kanaan R, Mangialavori L, Palangié E, Dusser D, et al. Mediastinal tuberculosis in an adult patient with cystic fibrosis. J Clin Microbiol 2011; 49:750-1; PMID:21106788; http://dx.doi.org/10.1128/JCM.01313-10.
- Asherova IK, Feigelson J, Vasilyeva LA, Gabitov VJ. Cystic fibrosis complicated by multiresistant tuberculosis. Acta Paediatr 2006; 95:1513-4; PMID:17062491; http://dx.doi.org/10.1080/08035250600724515.
- Bakala N'Goma JC, Le Moigne V, Soismier N, Laencina L, Le Chevalier F, Roux AL, Poncin I, Serveau-Avesque C, Rottman M, Gaillard JL, et al. Mycobacterium abscessus Phospholipase C expression is induced during coculture within amoebae and enhances M. abscessus virulence in mice. Infect Immun 2015; 83:780-91; PMID:25486995; http://dx.doi.org/10.1128/IAI.02032-14.
- van Doorninck JH, French PJ, Verbeek E, Peters RH, Morreau H, Bijman J, Scholte BJ. A mouse model for the cystic fibrosis delta F508 mutation. EMBO J 1995; 14:4403-11; PMID:7556083.
- Roux AL, Ray A, Pawlik A, Medjahed H, Etienne G, Rottman M, Catherinot E, Coppée JY, Chaoui K, Monsarrat B, et al. Overexpression of proinflammatory TLR-2-signalling lipoproteins in hypervirulent mycobacterial variants. Cell Microbiol 2011; 13:692-704; PMID:21143571; http://dx.doi.org/10.1111/j.1462-5822.2010.01565.x.
- Malfroot A, Adam G, Ciofu O, Döring G, Knoop C, Lang AB, Van Damme P, Dab I, Bush A; European Cystic Fibrosis Society (ECFS) Vaccination Group. Immunisation in the current management of cystic fibrosis patients. J Cyst Fibros 2005; 4:77-87; PMID:15978534; http://dx.doi.org/10.1016/j.jcf.2004.10.003.
