ABSTRACT
Dendritic cells (DCs) are known to be a set of morphology, structure and function of heterogeneous professional antigen presenting cells (APCs), as well as the strongest functional antigen presenting cells, which can absorb, process and present antigens. As the key regulators of innate and adaptive immune responses, DCs are at the center of the immune system and capable of interacting with both B cells and T cells, thereby manipulating the humoral and cellular immune responses. DCs provide an essential link between the innate and adaptive immunity, and the strong immune activation function of DCs and their properties of natural adjuvants, make them a valuable target for antigen delivery. Targeting antigens to DC-specific endocytic receptors in combination with the relevant antibodies or ligands along with immunostimulatory adjuvants has been recently recognized as a promising strategy for designing an effective vaccine that elicits a strong and durable T cell response against intracellular pathogens and cancer. This opinion article provides a brief summary of the rationales, superiorities and challenges of existing DC-targeting approaches.
Introduction
DCs, derived from pluripotent hematopoietic stem cells, belong to the antigen presenting cells (APCs) families together with B cells and macrophages. They were originally discovered in 1973 by a Canada researcher named Ralph Steinman as a previously undefined cell type in the mouse spleen,Citation1 subsequently they were named because of the characteristics of extending many dendritic or pseudopodia-like protrusions in maturation, and are now recognized as a group of related cell populations that elicit and regulate adaptive immune responses. DCs occupy a small population, which is only about 1% of the mononuclear cell components in human bodies. However, DCs were found to distribute to all of the organs except for the brain, mostly located in the inner layer of skin or mucosa parts consisting of epidermi, nasal cavity, lung, stomach and intestine that contact with the outside.
DCs possess intrinsic specialized features, which made them particularly efficient to capture, process and present antigens. Current studies demonstrated that DCs can positively and negatively regulate immune responses.Citation2 This unique immunoregulation function of DCs provides mechanism for the immune stabilization. In pathological states, however, aforementioned characteristics of DCs along with their own disorders would become the dynamic factors of inducing inflammatory diseases as well as escaping immune surveillance of organism for pathogens and tumors.Citation3 Consequently, as the important regulatory factor of the humoral and cellular immune response, DCs determine the different immune reaction by recognizing self or foreign antigens, maintaining the immune balance ultimately.
Most of the DCs in human bodies are present in immature state, they are poor at antigen presentation because of suboptimal levels of major histocompatibility complex (MHC) class II and low levels of co-stimulator molecules as well as adhesion molecules, which mediated interactions between cells such as stimulating the maturation of T lymphocyte cells.Citation4 Whereas the immature DCs possess a strong ability of capturing and phagocytosing antigen, and they can capture antigens in several methods as follows: Firstly, immature DCs can take up exogenous antigens by phagocytosis.Citation5-8 Secondly, they can take advantage of macropinocytosis to form large pinocytic vesicles.Citation9 And thirdly, they can mediate adsorptive endocytosis by expressing C-type lectin receptors such as DEC-205,Citation10 as well as Fcg and Fce receptors.Citation11 Once the immature DCs encounter with antigens or stimulus signals, they will be activated and differentiated into mature DCs, which are equipped with the levels of MHC class I/II–antigen complexes and co-stimulator molecules as well as adhesion molecules. Subsequently DCs migrate from the peripheral tissue into the secondary lymphoid organs, producing an appropriate immune response by interacting with both B cells and T cells. In this review, we will discuss the roles of DCs in immunity by interacting with B lymphocytes and T lymphocytes, and then discuss recent progress and challenges about DCs targeted vaccines.
DCs and B lymphocytes
DCs and B cell activation
DCs, famous for their function of stimulating T cells, were also known to regulate B-cell growth and immunoglobulin secretion. Both B cells and DCs are APCs and essential for antibody responses. As the professional APCs, as we all know, DCs phagocytose and process the exogenous antigens, which subsequently combine with MHC-II molecules of secretory vesicles into complexes, exhibiting on the DCs surface to be recognized by CD4 T cells, while B cell receptor (BCR) can combine with the dissociative antigens. Depending on different antigen types, B cell activation processes are divided into thymus-dependent and thymus-independent antigens cell activation processes. In the thymus-dependent antigens cell activation process, 2 kinds of basic stimulus signals are acquired for B cells activation. First, BCRs recognize and interact with antigens,Citation12 which produces the first stimulus signal. And the second stimulus signal was produced in the process of CD40L molecule on the Th cell membrane combining with CD40 molecule on the B cell membrane.Citation13 After production of the first stimulus signal, B cells upregulate the expression of MHC-II molecules and B7 costimulatory molecules to become APCs, which can present processed antigens through MHC-II molecules in the way of forming MHC-II-antigen peptide complexes. Helper T(Th) cells recognize antigens presented by MHC-II molecules through T cell receptors (TCRs), simultaneously combine with B7 molecules by costimulatory molecules CD28 expressed on the surface of Th cells membrane, all of which ultimately activate the Th cells. In turn, the activated Th cells upregulate the expression of CD40L, which combine with CD40 (a kind of B cell membrane receptor) to produce the second stimulus signal, making B cells to be plasmocytes that mediate humoral immune response by secreting antibodiesCitation14 (). In addition to providing co-stimulatory signals by CD40L combing with CD40, the activated Th cells can also secrete corresponding cytokines depending on the nature of the antigens to modulate immune responses. IL-4, for instance, promotes Th2 immune response, which may be inclined to the humoral immunity. On the contrary, IL-12 promotes Th1 immune response, which may tend toward the cellular immunity.Citation15
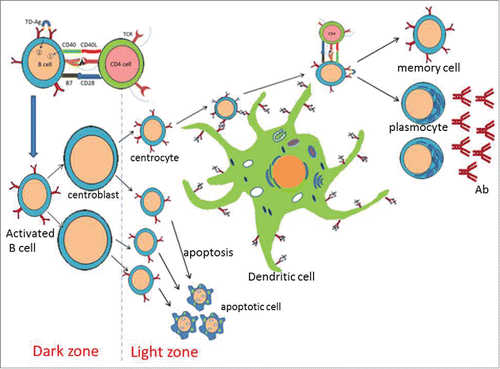
Figure 1. B cell activation and antibody affinity maturation with DC. B cell activation needs 2 essential stimulus signals. First, BCRs recognize and combine with antigens, which produce the first stimulus signal. And the second stimulus signal is produced in the process of CD40L molecule on the Th cell membrane combining with CD40 molecule on the B cell membrane. Antibody affinity of B cells can be gradually improved by the mature process of B cells. In the germinal center of lymph nodes or spleen, centrocytes with different affinities are produced through the cell division and proliferation, then migrate to the light zone. The combination of antigens and antibodies is a dynamic equilibrium (Ag+Ab== Ag-Ab), as a consequence, centrocytes with higher affinity can combine with Ag competitively from antigen-antibody complex displayed on the surface of the DCs. With the help of Th cells, centrocytes with higher affinity can be activated to be memory cells and plasmocytes, which are able to produce antibodies of higher affinity. Whereas centrocytes with lower affinity cannot compete to the antigens, resulting in apoptosis subsequently and eliminating by macrophages.
DCs and antibody affinity maturation
In lymphoid tissues, antigens are captured by B cells or DCs, and then presented by MHC-II to activate Th cells. With the help of Th cells, B cells activate, proliferate, and further differentiate into plasmocytes produced antibodies with a certain affinity.Citation16 In the early immune responses, plasmocytes generally produce antibodies of low affinity, which may be not sufficient to eliminate antigens. But high affinity antibodies are produced gradually during antibody affinity maturation, where DCs mediated and played an essential role in this process.Citation17 Antibodies combine with antigens to form the antigen-antibody complex, exhibiting on the surface of the DCs by binding the Fc fragment of antibodies. The B cells that lied in proliferating phase are known as centroblasts aggregated in the dark zone of germinal center, where centroblasts are characterized by large in size, hyperplasia of the cytoplasm and the lack of mIg. In the germinal center of lymph nodes or spleen, centrocytes with different affinities are produced through the cell division and proliferation, and then migrate to the light zone where centrocytes become smaller, as well as display mIg on the surface of membrane. The combination of antigen (Ag) and antibody (Ab) is a dynamic equilibrium (Ag+Ab==Ag-Ab), as a consequence, centrocytes with higher affinity competitively combine with Ag competitively from antigen-antibody complex displayed on the surface of the DCs. With the help of Th cells, centrocytes with higher affinity are activated to become memory cells and plasmocytes,Citation18 which produce antibodies of higher affinity. Whereas centrocytes with lower affinity cannot compete to the antigens, underwent apoptosis subsequently and eliminated by macrophages ().
DCs and T lymphocytes
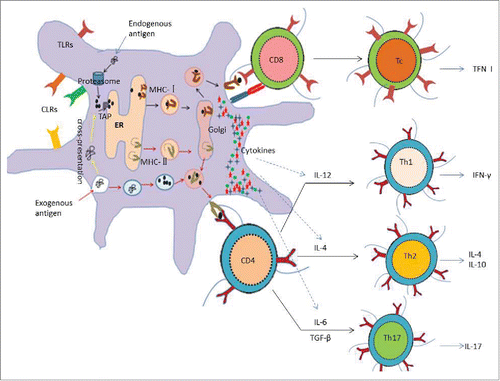
Unlike BCRs combine with dissociative antigens, TCRs only recognize antigenic peptides presented by MHC molecules, which determine T cells cannot neutralize antigens directly in the bodies, but recognize cells showed specific antigenic peptides on their surfaces. As the professional antigen presenting cells, DCs express both MHC-I molecules and MHC-II molecules. T cells that express CD4 only recognize antigens presented by MHC-II molecules, consequently initiate MHC-II molecules restrictive CD4+ Th reaction, while those T cells who express CD8 only recognize antigens presented by MHC-I molecules, as a consequence initiating MHC-Imolecules restrictive cytotoxic T lymphocyte (CTL) responses.Citation19 CTL has strong ability to kill target cells through medium such as perforin, granzyme, tumor necrosis factor (TNF), interferon-γ(IFN-γ) and so on. Additionally, DCs also provide the second signal for T cells activation through its high expression of co-stimulus molecules (CD80, CD86 / B7 / B7–1–2, CD40, etc.), ultimately initiating the immune responses. And also, the activated Th cells directly regulate immune responses by secreting a variety of cytokines, which regulate the direction of the immune responses. For example, both IL–4 and IL–12 are the important cytokines. IL-4 promotes Th2 immune response, which may be inclined to the humoral immunity. On the contrary, IL-12 is oriented toward the cellular immunity. Th1 cells promote the secretion of cytokines such as IL-2 and IFN-γ, which promote inflammation, activation of T cells and macrophage. While Th2 cells promote the secretion of cytokines such as IL-4 and IL-10, which activate B cells to secret the antibodies. What's more, the combination of DCs and T cells may also promote the secretion of IL-12 and IL-18, which induce the production of CTL, killing the specific target cells ().
Figure 2. Antigen processing and presentation of DCs and the activation of T cells As the professional antigen-presenting cells, DCs are able to capture, process and present both exogenous antigens and endogenous antigens. Exogenous antigens are degraded to be oligopeptides in the endocyst, and then presented by MHC-II molecules to the surface of DCs for recognization of CD4 T cells; while endogenous antigens are degraded to be oligopeptides in the cytoplasm, subsequently presented by MHC- Imolecules to the surface of DCs for recognization of CD8 T cells. CD8 T cells are activated to be CTL, which have strong ability to kill target cells through medium such as TNF, IFN-γetc. However, CD4 T cells are activated to be Th cells, which can be regulated by a variety of cytokines secreted by DCs. For example, IL-12, the main cytokine, promotes Th1 immune response, which may be inclined to the humoral immunity and secret IFN-γ; On the contrary, IL-4 promotes Th2 immune response, which may tend to the humoral immunity by secreting IL-4, IL-5, IL-10 and IL-13. In addition, IL-6 promotes Th17 immune response, which may activate neutrophils and boost the local inflammatory responses by secreting cytokines such as IL–17 and IL–22. What's more, DCs surface exists varieties of receptors such as TLRs and CLRs, which may be convenient for DC targeting.
Most traditional vaccine researches were mainly focusing on humoral immunities during the past decades, which were based on the induction of long-lived Ab responses. For example, vaccinia virus—the earliest successful live attenuated pathogens vaccines—have been used to induce protective immunity. Subsequently, many kinds of vaccines consist of inactivated viruses, subunit vaccines and protein–polysaccharide conjugate vaccines were developed to protect against different viral and bacterial infections by inducing antibody-mediated immunities.Citation20 However, with the deepening of the research, researchers found that the cellular immunity plays a more significant role, because there are still many pathogens that no efficient vaccines are available, including HIV, Hepatitis C virus, Mycobacteria, Chlamydia, and Plasmodium, a parasite causing malaria. Most of these pathogens cause chronic diseases where strong cellular immunity, in particular CTL response, is critical for the clearance of the pathogens by inducing Ag-specific killing of pathogen-infected cells or tumor cells. Therefore, inducing both humoral immune responses and cellular immune responses simultaneously are of significance against pathogens infections.
DC-based vaccines
DCs, the important regulatory factors of the humoral and cellular immune responses, are characterized by immunologic activator and natural adjuvants, which making DCs valuable in improving vaccine immunogenicity, enhancing the efficiency of the vaccines and the clinical immunotherapy. And nowadays using DCs to improve the immunogenicity of the vaccines has been one of the important strategies in the field of vaccine development.Citation21 In recent years, the most extensively studied approach to induce both humoral immune responses and cellular immune responses is designing DC targeted vaccines,Citation22,23 which induce the clonal expansion of T cells,Citation24 consequently inducing durable and effective CTL immune response. Additionally, DC-targeted vaccines are also used for the development of immunotherapies against cancer, autoimmunity, and infectious diseases, all of which require T cell immunity. Therefore, the design and application of vaccines developed from the original preventive vaccines to the present therapeutic vaccines. Currently, DC-based vaccines are divided into 2 types, namely ex vivo antigen-loaded DC based vaccines and in vivo DC targeted vaccines,Citation25 below is the detailed summary of these 2 DC-based vaccines.
Ex vivo antigen-loaded DC-based vaccines
The first attempt utilizing DCs to induce protective immune responses in humans involved the adoptive transfer of in vitro-cultured DCs loaded with antigens, which was mainly used as immunotherapy against cancer.Citation26 CD14 + cells, obtained from bone marrow-derived CD34+ progenitor cells and peripheral blood mononuclear cells (PBMC) through density gradient centrifugation and magnetic-activated cell sorting, were cultured to be immature DCs in vitro by adding cytokines such as granulocyte-macrophage colony stimulating factors(GM-CSF) and IL-4.Citation27 Subsequently, immature DCs can be activated by stimulus signals to be mature DCs, which loaded with tumor antigens or tumor antigen alternatives by several methods such as actively absorbing, electroporation and adenovirus mediation.Citation28 Self-DCs, loaded with tumor cells or tumor antigens, re-injected into patients' bodies. According to the function of migration, DCs migrate into T cell areas of the lymph organs, then activate T cells, stimulate the antitumor immune responses.Citation29
At present, DC-based vaccines have made huge progress in basic and clinical researches, which indicate that in vitro tumor antigen loaded DCs are efficient for tumor suppression as well as optimistic application prospect in the treatment of prostate cancer and malignant glioma tumor.Citation30,31 Besides, more than 10 years of clinical trials indicated that ex vivo antigen-loaded DC-based vaccines are safe and effective in inducing tumor-specific CD4+ T cells and CTLs in humans, therefore, DC-based vaccines used as the tumor biological treatment scheme have been approved to enter III phase of clinical trials by the United States Food and Drug Administration. However, the approach of ex vivo antigen-loaded DC-based vaccines is expensive, labor-intensive and operation process-complex. In addition, ex vivo-cultured DCs loaded with antigens need to be operated individually for each patient, which limited the clinical application on a large scale. What's more, because of the fact that ex vivo antigen-loaded DCs are re-injected into the bodies of patients after culture in vitro and artificial modification, induction of immune response may exist some uncertainty and uncontrollability. As a result, generation of DC-based vaccines that more simple and suitable for clinical operation will be the next phase for DC vaccine development.
In vivo DC targeted vaccines
As the important regulators of the humoral and cellular immune responses, DCs can maintain the immune balance by judging self or foreign antigens. Recent studies indicated that DCs are the initiator of immune responses, which play an important role in the resistance of pathogen infection and the generation of anti-tumor immunity.Citation32 In recent years, using DCs to improve the immunogenicity of the vaccines has been one of the important strategies in the fields of vaccine designation.Citation21 The DC targeted vaccine, as the name implies, is targeting the antigens, DNA molecules or drug molecules to DCs by identifying the specific receptors expressed on the cell surface, then stimulate the corresponding humoral and cellular immune responses. At present, several ways of DC targeting mainly adopted are detailed next.
Ligands based DC targeted vaccines
DCs are able to induce the clonal expansion and the activation of T cells simultaneously undergo innate immune activation.Citation33,34 Therefore targeting DC in the development of vaccines to induce effective and durable protective T cell immunity will be the main strategy nowadays. The surface of DCs exists many pattern recognition receptors (PRRs), which combine specifically with the corresponding natural ligands. As a consequence, linking proteins to pattern recognition receptor ligands (PRRLs) is reasonable to generate antigen–PRR-ligand conjugate vaccines, which ensures that antigen process and stimulation occur simultaneously in the same DCs.
When design the antigen–PRR-ligand conjugate vaccines, a variety of PRRs are candidates to be selected according to the desired immune responses such as Th1 cells, Th2 cells, Th17 cells and Tc cells reaction. PRRs also known as the pattern recognition molecules (PRMs), are the special molecules of host cells that identified infectious pathogens. Therefore, the host cells initiate the immune responses by recognizing pathogen associated molecular patterns (PAMPs), which are conserved among microbial species. The type and intensity of the immune responses are closely related to the recognition between PRRs and PAMPs. Nowadays four PRRs families have been found, namely Toll-like receptors (TLRs), Nucleotide-binding oligomerization domain-like receptors (NLRs), Retinoic acid-inducible gene I-like helicases receptors (RLRs) and C-type lectin receptors (CLRs), while the PRRs that participate in antigen–PRR–ligand conjugate vaccines are TLRs and CLRs 2 main PRRs families expressed on DC membranes.
TLRs are a kind of pattern recognition receptors that belong to the membrane binding proteins. They are distributed on the cell membrane surface and phage membrane, making them feasible to identify pathogens including bacteria, viruses, fungi and protozoa. TLR9, the most extensively studied PRR in antigen–PRR-ligand conjugate vaccines, combine specifically with CpG, which is a TLR9 ligand, as well as the adjuvant of stimulating DCs. Model antigen OVA conjugate with murine CpG by using method of chemical crosslinking to design the antigens–TLR–ligand conjugate vaccines, which produce a stronger adaptive immune response, and induce the more potent Th1 cells and IgG2a antibody responses as well as cross-primed CTLs at lower dose compared with free antigen and ligand.Citation34-36 Besides, as the natural ligand, CpG can also conjugate with the HIV gp120 protein by taking advantages of chemical crosslinking reagents. The immunization of injected mice showed that conjugate vaccines were able to elicit apparent antibody responses, CD4+ and CD8+ T cell responses.Citation37 Based on these results, antigens–TLR–ligand conjugate vaccines are promising for the development of an effective preventive and therapeutic HIV vaccine. What's more, James W employed a recombinant fusion protein strategy, which was using flagellin- the TLR5 ligand -fused to specific antigens to generate protective immune responses, and the immunization of mice with the recombinant-flagellin-OVA fusion protein STF2.OVA resulted in efficacious antigen specific T and B cell responses that were equal to or even better than the responses induced by OVA emulsified in Complete Freund's adjuvant.Citation38 A summary of part selected studies about Ligands based DC targeting is provided in .
Table 1. Ligands based DC targeted vaccines.
CLRs are another kind of PRRs, which are key receptors for the induction of intracellular signaling cascades. As is known to all, most CLRs function as antigen uptake receptors with a large number of expression on the DCs. CLRs contain carbohydrate recognition domains (CRDs) on their extracellular components, which recognize glycosyl ligands of pathogenic microorganism selectively, subsequently resulting in the internalization of antigens by MHC class I/II molecules, then initiating the immunity against pathogens.Citation39-41 DC specific ICAM grabbing nonintegrin (DC-SIGN), is one of CLRs discovered in recent years, simultaneously proved to be a highly efficient antigen receptor, which can take in both particulate antigens and soluble antigens, then presented by MHC - I molecules and MHC - II molecules in a restrictive manner. As an antigen receptor, DC-SIGN combine specifically with high-affinity ligands-Lewis X oligosaccharides, which are utilized to design the antigens–TLR–ligand conjugate vaccines. Hongjie Chen demonstrated that modifing DCs with Lewis X oligosaccharides–heparanase complex could enhance the specific IFN-c production and cytotoxic T cell response.Citation37 Furthermore, the modified DCs also significantly suppress the established tumor growth and prolong the life span of tumor-bearing mice. In addition, there are still many ligands that can be used for DCs targeting such as heat shock protin (HSP), belongs to the molecular chaperone family, which plays an important role in repairing of the thermal denaturation proteins. Researchers have found that CD91 molecules expressed on the DCs surface are the specific receptors of HSP,Citation42 therefore, antigens-HSP complex obtained by several methods such as chemical conjugation, in vitro mixting and separation from lysates of expressed interest proteins can be presented specifically by DCs to promote the immune responses. Some studies about Ligands based DC targeting are summarized in .
Antibody based DC targeted vaccines
With the exploration of acceptor molecules expressed on DCs surface and the production of monoclonal antibodies, targeting antigens to DCs in combination with antibodies nowadays has been the most extensively studied methods for activating T cells, making antibodies a favorable delivery vehicles for DC targeting. Many researches verified that antigens in combination with specific monoclonal antibodies of the DC acceptor molecules by many ways consist of chemical conjugation and genetic recombination can be targeted to particular DC subsets. The success of producing humanized monoclonal antibodies making the antibody based DC targeting has great utility value in the transition of clinic treatment. Recently antibody based DC targeting researches have mainly concentrated on the DC acceptor molecules such as CLRs, integrin and Fc receptors etc. (see ).
CLRs are targeted for the induction of antigen-specific immune responses because of the reason that CLRs are able to internalize antigens and deliver them to endocytic compartments for the antigen processing and presentation, which subsequently stimulate the specific immune response. Along with the deepening of researches, more and more CLRs have been identified nowadays with a variety of distributions. For example, DC-SIGN and Dectin-1 expressed in many DC subsets such as epidermal DCs and the myeloid dendritic cells(MDC) in the blood. On the contrary, the expression of certain CLRs is relatively limited to specific DC subsets, for instance, langerin/CD207 is mainly expressed in a certain subset of langerhans cells(LC) and dermal DCs, while BDCA-2(CLEC4C)is mainly expressed in plasmacytoid dendritic cells(pDC). Different DC subsets have their respective patterns of antigen capture, processing and presentation, therefore, targeting different CLRs makes it feasible for antigens to combine with the specific DC subsets, consequently manipulate and regulate the vaccine immunization.
Currently the antibody based DC targeting studies have been mainly focusing on DEC205, DNGR1 (Clec9A) and DC-SIGN, etc. For example, DEC205 is highly expressed in CD8+ DCs, but is also found in Langerhans cells, dermal DCs and B cells. Targeting HIV, malaria, Leishmania spp or tumor-associated antigens to DEC205 in combination with its specific antibodies along with adjuvants has a significant superiority in the proliferation of antigen-specific T cells and the production of IFN-γ compared with the non-targeted antigen,Citation57,58 while the absence of adjuvants results in immunosuppression.Citation59 DNGR1, however, was found to be expressed in mice at high levels by CD8+ DCs and at low levels by plasmacytoid DCs but not by other hematopoietic cells. Therefore, antigens targeted to DNGR1 can be transported to CD8+ DC, which stimulate and activate the antigen-specific CD8+ T cells, then elicit the CTL responses.Citation60 Sancho David demonstrated that antigens in combination with an antibody specific for mouse DNGR-1 were selectively cross-presented by CD8 α + DCs in vivo and elicited potent CTL responses with the help of adjuvants.Citation61
In addition, there are still many other acceptor molecules that can be used for antibody based DC targeting. For example, CD11c, considered as the main iconic molecule as well as an effective immune receptor in mouse, is expressed in all DC subsets at high levels including CD8+ DC and CD8− DC, which can be targeted to induce the production of specific antibodies and the generation of cellular immune responses.Citation62-64 Wang H et found that antigens combined with an antibody specific for mouse CD11c were targeted to DCs and, when immunized only once, could generate antigen-specific humoral immune responses.Citation65 Fc receptor, another DC targeting acceptor molecule, is capable of interacting with the constant region of immunoglobulin. DC surface exists many acceptor molecules of immunoglobulins consist of IgG, IgA and IgE, such as FcγR, FcαR and FcεR,Citation66 from which, FcγR participates in many biological processes including cytophagy, antigen presentation, immune complex-mediated DC maturation and the activation of natural effector cells, etc.Citation67 Wallace et targeted prostate-specific antigen(PSA) conjugated with the specific monoclonal antibodies to FcγR of the humanized THP-1 cells, and found that PSA could be internalized and cross-presented by MHC-I molecules, which ultimately stimulated the antigen-specific CTL responses.Citation68
Delivery system based DC targeted vaccines
As discussed above, the most widely studied approach to activate T cells is targeting DC-specific endocytic receptors by linking the relevant antigens to antibodies or ligands along with the immunostimulatory adjuvants, ultimately eliciting strongly CTL responses. However, the requirement for systemic administration of adjuvants and the consequent untoward systemic effects should be taken into consideration, because we cannot guarantee that the DCs exposed to antigens are the ones activated by adjuvants. Consequently, developing a delivery system for vaccine design is of great necessity, which ensures that antigens and adjuvants can be co-delivered within the same compartment. Therefore this approach will eliminate such disadvantages, and will be a promising strategy.
Nanoparticles (NP) are rapidly emerging and have received much attention as potential vehicles for delivering vaccines because of several aspectsCitation78: Firstly, the development of the nanotechnology in recent years making NP available to delivery vaccine antigens as well as drugs; secondly, the size and particular structure of NP are similar to natural pathogens, which permits DC taking up immune complex efficiently. Besides, NPs possess many characteristics such as large payloads, high signal intensity/stability and the high surface area to volume ratio, which allow delivery of high dose of immunogenic cargo, all within the same vector. What's more, NPs can stabilize vaccine antigens by preventing immune complex from degradation rapidly upon injection. In addition, as a kind of adjuvant, NPs can delivery antigens in a slow and sustained manner to maximize exposure to the immune system, which inducing the long-lasting immune responses. Lastly, the release properties of NPs can be easily controlled by modulating their physic-chemical properties, subsequently inducing protective Th1-type immune responses to intracellular pathogens.
A wide variety of NPs delivery systems have been studied nowadays such as polymeric particles, liposomes, live-vectors, nanocrystals, immune stimulating complexes (ISCOMs) and non-degradable nanospheres, etc. Among some of the first studied NPs delivery systems are polymeric NPs made of FDA-approved biocompatible poly-lactic-co-glycolic acid (PLGA), which have been most extensively studied as competent vaccine delivery devices because of their biodegradable properties and the exceptional ability to improve vaccine-induced immune responses compared to soluble antigens. Polymeric NPs can be decorated on their surface with antibodies or carbohydrate ligands through physical adsorptions or chemical conjugation, resulting in binding specifically to DC receptors such as CLRs and TLRs. Several studies have demonstrated significant induction of DC maturation, simultaneous activation of CD4+ and CD8+ T–cell responses following co-delivery of antigens along with immunostimulatory adjuvants (such as TLR ligands). For instance, Paul J et al. encapsulated TLR ligands (TLRL) for intracellular TLRs within biodegradable PLGA-NPs coated with DC-SIGN-specific humanized Abs targeting to human DC-specific receptor.Citation79 In addition, he also encapsulated TLRL and NPs loaded with Ag targeting to the DC-specific CLR DEC-205 of mice in vivo, because DC-SIGN is differentially expressed in mice and in human, while DEC-205 has been harnessed for DC-targeted vaccination strategies in numerous studies in mice. Both of the examinations demonstrated that targeted PLGA-NPs were able to improve the antigen presentation and the generation of CTL responses. The similar conclusion was also proved by Samar Hamdy et al., who demonstrated that mannose receptor (MR)-decorated antigen loaded PLGA-NPs simultaneously enhanced antigen-specific CD4+ and CD8+ T–cell responses in mouse in vivo and in vitro system compared to non-decorated NPs.Citation80 What's more, using PLGA-NPs presented melanoma-associated antigens to target to specific human DC subsets BDCA3+ MDCs through CLEC9A has also been evaluated, which demonstrated that antigens delivered with antibodies to CLEC9A are presented by CD8+ T cells to both CD4+ and CD8+ T cells and induce antitumor immunity in a melanoma model.Citation81
In addition to polymeric NPs, other NP delivery systems such as liposomes and live-vectors are also effective at inducing cytotoxic response and protection against tumor ().Citation82 In regard to live-vectors, lentiviral and adenoviral vector are the most extensive studied live-vectors. For instance, Lili Yang et al. enveloped the lentivector with a viral glycoprotein from Sindbis virus engineered to be DC-SIGN–specific, after injection of a targeted lentiviral vector encoding an ovalbumin (OVA) transgene into naïve mice, they observed a high frequency (up to 12%) of OVA-specific CD8+ T cells and a significant antibody response.Citation83 Co-delivery of antigens and adjuvants by suitable vaccine carriers improve antigen processing and presentation as well as the maturation of DCs, which enhanced the efficient CD8+ T cells priming.
Table 2. Antibody based DC targeted vaccines.
Table 3. Delivery system based DC targeted vaccines.
Based on the aforementioned discussion, we noticed that targeting of antigens to specific DC subsets in combination with co-delivery strategies reduces the lower dose of antigens and adjuvants substantially and therefore be proved an attractive model for the priming of strong T-cell responses. And co-delivery of antigens and adjuvants avoids potential adverse effects, such as induction of tolerance, autoimmunity and cytokine release syndrome. What's more, co-delivery of antigens and adjuvants into the same compartment of DCs promotes cross-priming. More importantly, we can tailor immune responses to what we expected such as Th1, Th2 and Th17 responses by fine-tuning vaccine formulation. Despite of extensive researches, however, there is no consensus on preferred targeting molecules and cell types. Additionally, researchers have to face the technical challenges of co-delivery antigens and adjuvant to particular DC subsets, which may also be labor-intensive and cost-higher.
Conclusions and future prospects
The past decades witnessed the rapid progresses made in the knowledge of DC biology along with the mature and perfect technology of isolating and culturing DCs from blood and bone marrow, which opened the avenues for development of DC -targeting vaccines. Vaccination is by far the greatest success within the field of immunology to date, and the most efficient vaccines might actually be those who are able to generate the protective and therapeutic immunities including Tfh cells which dictate the quality of humoral immunity and CD8+ T cells that give rise to CTLs able to eliminate infected/transformed cells. Nowadays DC immunotherapy has been extensively used in the clinic, and has been demonstrated to be feasible, nontoxic and effective for translating these promising results in experimental mouse models to human application.
In spite of aforementioned advancements, there are still many challenges and difficulties that scientists and clinicians to be solved. For example, many characteristics of DCs need to be identified in more detail to facilitate successful modulation of the immune system. Besides, although active targeting of DCs in situ is emerging as an attractive approach to generate strong protective cellular immunity against chronic infectious diseases and cancer, there is no consensus on preferred targeting molecules and cell types. Therefore, a feasible strategy is the combinatorial targeting of multiple DC subsets, which was verified to significantly enhance the efficacy of DC targeting compared to targeting of only one DC subset. What's more, further studies need to be conducted to compare whether targeting of multiple DC subsets is more efficient than currently well-formulated protein-based vaccines in eliciting the strong and durable protective immunities against intracellular pathogens and cancer.
Abbreviations
| DCs | = | Dendritic cells |
| APCs | = | antigen presenting cells |
| MHC | = | major histocompatibility complex |
| BCR | = | B cell receptor |
| Th | = | Helper T |
| TCR | = | T cell receptor |
| IL | = | interleukin |
| Ag | = | antigen |
| Ab | = | antibody |
| CTL | = | Cytotoxic T lymphocyte |
| TNF | = | tumor necrosis factor |
| IFN | = | interferon |
| PBMC | = | peripheral blood mononuclear cells |
| GM-CSF | = | granulocyte-macrophage colony stimulating factor |
| PRRs | = | pattern recognition receptors |
| PRMs | = | pattern recognition moleculars |
| PAMPs | = | pathogen associated molecular patterns |
| TLRs | = | Toll-like receptors |
| NLRs | = | Nucleotide-binding oligomerization domain-like receptors |
| RLRs | = | Retinoic acid-inducible gene-like helicases receptors |
| CLRs | = | C-type lectin receptors |
| DC-SIGN | = | DC specific ICAM grabbing non-integrin |
| CRDs | = | carbohydrate recognition domains |
| HSP | = | heat shock protin |
| OVA | = | ovalbumin |
| NPs | = | Nanoparticles |
| ISCOMs | = | immune stimulating complexes |
| PLGA | = | poly-lactic-co-glycolic acid |
| MR | = | mannose receptor |
Disclosure of potential conflicts of interest
The authors declare they have no competing interests.
Funding
This study was financially supported by the Chinese “863” National Programs for High Technology Research and Development (Grant No.: 2011AA10A211), the National Pig Industrial System (CARS-36–06B), and the Special Fund for Agro-scientific Research in the Public Interest (201203039).
References
- Ueno H, Klechevsky E, Schmitt N, Ni L, Flamar AL, Zurawski S, Zurawski G, Palucka K, Banchereau J, Oh S. Targeting human dendritic cell subsets for improved vaccines. Semin Immunol 2011; 23:21-7; PMID:21277223; http://dx.doi.org/10.1016/j.smim.2011.01.004
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YT, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18:767; PMID:10837075; http://dx.doi.org/10.1146/annurev.immunol.18.1.767
- Garcia-Vallejo JJ, van Kooyk Y. The physiological role of DC-SIGN: A tale of mice and men. Trends Immunol 2013; 34:482-6; PMID:23608151; http://dx.doi.org/10.1016/j.it.2013.03.001
- Caminschi I, Shortman K. Boosting antibody responses by targeting antigens to dendritic cells. Trends Immunol 2012; 33:71-7; PMID:22153931; http://dx.doi.org/10.1016/j.it.2011.10.007
- Inaba K, Inaba M, Naito M, Steinman RM. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. The Journal of experimental medicine 1993; 178:479-88; PMID:7688024; http://dx.doi.org/10.1084/jem.178.2.479
- Moll H, Fuchs H, Blank C, Rollinghoff M. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. European journal of immunology 1993; 23:1595-601; PMID:8325337; http://dx.doi.org/10.1002/eji.1830230730
- Reis e Sousa C, Stahl PD, Austyn JM. Phagocytosis of antigens by Langerhans cells in vitro. The Journal of experimental medicine 1993; 178:509-19; PMID:8393477; http://dx.doi.org/10.1084/jem.178.2.509
- Svensson M, Stockinger B, Wick MJ. Bone marrow-derived dendritic cells can process bacteria for MHC-I and MHC-II presentation to T cells. J Immunol 1997; 158:4229-36; PMID:9126984
- Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic Cells Use Macropinocytosis and the Mannose Receptor to Concentrate Macromolecules in the Major Histocompatibility Complex Class-Ii Compartment - down-Regulation by Cytokines and Bacterial Products. Journal of Experimental Medicine 1995; 182:389-400; PMID:7629501; http://dx.doi.org/10.1084/jem.182.2.389
- Jiang WP, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The Receptor Dec-205 Expressed by Dendritic Cells and Thymic Epithelial-Cells Is Involved in Antigen-Processing. Nature 1995; 375:151-5; PMID:7753172; http://dx.doi.org/10.1038/375151a0
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. The Journal of experimental medicine 1994; 179:1109-18; PMID:8145033; http://dx.doi.org/10.1084/jem.179.4.1109
- Yuseff MI, Pierobon P, Reversat A, Lennon-Dumenil AM. How B cells capture, process and present antigens: a crucial role for cell polarity. Nat Rev Immunol 2013; 13:475-86; PMID:23797063; http://dx.doi.org/10.1038/nri3469
- Khan WN, Wright JA, Kleiman E, Boucher JC, Castro I, Clark ES, et al. B-lymphocyte tolerance and effector function in immunity and autoimmunity. Immunol Res 2013; 57:335-53; PMID:24293007; http://dx.doi.org/10.1007/s12026-013-8466-z
- Lanzavecchia A. Antigen-specific interaction between T and B cells. J Immunol 2007; 179:7206-8; PMID:18025160
- Mukhopadhyay S, George A, Bal V, Ravindran A, Rath S. Bruton's tyrosine kinase deficiency in macrophages inhibits nitric oxide generation leading to enhancement of IL-12 induction. J Immunol 1999; 163:1786-92; PMID:10438910
- Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol 2001; 13(2):13195-201
- de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. Germinal centers without T cells. J Exp Med 2000; 191:485-93; PMID:10662794; http://dx.doi.org/10.1084/jem.191.3.485
- van Zelm MC, van der Burg M, van Dongen JJM. Homeostatic and maturation-associated proliferation in the peripheral B-cell compartment. Cell Cycle 2007; 6:2890-5; PMID:18156800; http://dx.doi.org/10.4161/cc.6.23.4952
- Unger WWJ, van Beelen AJ, Bruijns SC, Joshi M, Fehres CM, van Bloois L, Verstege MI, Ambrosini M, Kalay H, Nazmi K, et al. Glycan-modified liposomes boost CD4(+) and CD8(+) T-cell responses by targeting DC-SIGN on dendritic cells. J Control Release 2012; 160:88-95; PMID:22366522; http://dx.doi.org/10.1016/j.jconrel.2012.02.007
- Kastenmuller W, Kastenmuller K, Kurts C, Seder RA. Dendritic cell-targeted vaccines - hope or hype? Nat Rev Immunol 2014; 14:705-11; PMID:25190285; http://dx.doi.org/10.1038/nri3727
- Shortman K, Lahoud MH, Caminschi I. Improving vaccines by targeting antigens to dendritic cells. Exp Mol Med 2009; 41:61-6; PMID:19287186; http://dx.doi.org/10.3858/emm.2009.41.2.008
- De Souza JB. Protective immunity against malaria after vaccination. Parasite Immunol 2014;36:131-9; PMID:24188045; http://dx.doi.org/10.1111/pim.12086
- Andersen P, Woodworth JS; Tuberculosis vaccines - rethinking the current paradigm. Trends Immunol 2014; 35:387-95; PMID:24875637; http://dx.doi.org/10.1016/j.it.2014.04.006
- Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol 2002; 20:621-67; PMID:11861614; http://dx.doi.org/10.1146/annurev.immunol.20.100301.064828
- Palucka K, Ueno H, Fay J, Banchereau J. Harnessing Dendritic Cells to Generate Cancer Vaccines. Ann Ny Acad Sci 2009; 1174:88-98; PMID:19769741; http://dx.doi.org/10.1111/j.1749-6632.2009.05000.x
- Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol 2003; 15:138-47; PMID:12633662; http://dx.doi.org/10.1016/S0952-7915(03)00015-3
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265-77; PMID:22437871; http://dx.doi.org/10.1038/nrc3258
- Ueno H, Schmitt N, Klechevsky E, Pedroza-Gonzalez A, Matsui T, Zurawski G, Oh S, Fay J, Pascual V, Banchereau J, et al. Harnessing human dendritic cell subsets for medicine. Immunol Rev 2010; 234:199-212; PMID:20193020; http://dx.doi.org/10.1111/j.0105-2896.2009.00884.x
- Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 2007; 26:503-17; PMID:17398124; http://dx.doi.org/10.1016/j.immuni.2007.03.006
- Draube A, Klein-Gonzalez N, Mattheus S, Brillant C, Hellmich M, Engert A, von Bergwelt-Baildon M. Dendritic Cell Based Tumor Vaccination in Prostate and Renal Cell Cancer: A Systematic Review and Meta-Analysis. Plos One 2011; 6:e18801; PMID:21533099; http://dx.doi.org/10.1371/journal.pone.0018801
- Lesterhuis WJ, de Vries IJM, Schreibelt G, Lambeck AJA, Aarntzen EHJG, Jacobs JFM, Scharenborg NM, van de Rakt MW, de Boer AJ, Croockewit S, et al. Route of Administration Modulates the Induction of Dendritic Cell Vaccine-Induced Antigen-Specific T Cells in Advanced Melanoma Patients. Clin Cancer Res 2011; 17:5725-35; PMID:21771874; http://dx.doi.org/10.1158/1078-0432.CCR-11-1261
- Den Dunnen J, Gringhuis SI, Geijtenbeek TBH. Innate signaling by the C-type lectin DC-SIGN dictates immune responses. Cancer Immunol Immun 2009; 58:1149-57; http://dx.doi.org/10.1007/s00262-008-0615-1
- Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 2006; 440:808-12; PMID:16489357; http://dx.doi.org/10.1038/nature04596
- Sporri R, Sousa CRE. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4(+) T cell populations lacking helper function. Nature Immunology 2005; 6:163-70; PMID:15654341; http://dx.doi.org/10.1038/ni1162
- Tighe H, Takabayashi K, Schwartz D, Marsden R, Beck L, Corbeil J, Richman DD, Eiden JJ Jr, Spiegelberg HL, Raz E. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur J Immunol 2000; 30:1939-47; PMID:10940883; http://dx.doi.org/10.1002/1521-4141(200007)30:7%3c1939::AID-IMMU1939%3e3.0.CO;2-
- Tighe H, Takabayashi K, Schwartz D, Van Nest G, Tuck S, Eiden JJ, Kagey-Sobotka A, Creticos PS, Lichtenstein LM, Spiegelberg HL, et al. Conjugation of immunostimulatory DNA to the short ragweed allergen Amb a 1 enhances its immunogenicity and reduces its allergenicity. J Allergy Clin Immun 2000; 106:124-34; PMID:10887315; http://dx.doi.org/10.1067/mai.2000.107927
- Horner AA, Datta SK, Takabayashi K, Belyakov IM, Hayashi T, Cinman N, Nguyen MD, Van Uden JH, Berzofsky JA, Richman DD, et al. Immunostimulatory DNA-based vaccines elicit multifaceted immune responses against HIV at systemic and mucosal sites. J Immunol 2001; 167:1584-91; PMID:11466380; http://dx.doi.org/10.4049/jimmunol.167.3.1584
- Huleatt JW, Jacobs AR, Tang J, Desai P, Kopp EB, Huang Y, Song L, Nakaar V, Powell TJ. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine 2007; 25:763-75; PMID:16968658; http://dx.doi.org/10.1016/j.vaccine.2006.08.013
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8(+) T cell tolerance. J Exp Med 2002; 196:1627-38; PMID:12486105; http://dx.doi.org/10.1084/jem.20021598
- Moris A, Nobile C, Buseyne F, Porrot F, Abastado JP, Schwartz O, et al. DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood 2004; 103:2648-54; PMID:14576049; http://dx.doi.org/10.1182/blood-2003-07-2532
- Tacken PJ, de Vries IJM, Gijzen K, Joosten B, Wu DY, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ, et al. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood 2005; 106:1278-85; PMID:15878980; http://dx.doi.org/10.1182/blood-2005-01-0318
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001; 14:303-13; PMID:11290339; http://dx.doi.org/10.1016/S1074-7613(01)00111-X
- Heit A, Gebhardt F, Lahl K, Neuenhahn M, Schmitz F, Anderl F, Wagner H, Sparwasser T, Busch DH, Kastenmüller K. Circumvention of regulatory CD4(+) T cell activity during cross-priming strongly enhances T cell-mediated immunity. Eur J Immunol 2008; 38:1585-97; PMID:18465771; http://dx.doi.org/10.1002/eji.200737966
- Cho HJ, Takabayashi K, Cheng PM, Nguyen MD, Corr M, Tuck S, Raz E. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nat Biotechnol 2000; 18:509-14; PMID:10802617; http://dx.doi.org/10.1038/75365
- Kastenmuller K, Wille-Reece U, Lindsay RWB, Trager LR, Darrah PA, Flynn BJ, Becker MR, Udey MC, Clausen BE, Igyarto BZ, et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J Clin Invest 2011; 121:1782-96; PMID:21540549; http://dx.doi.org/10.1172/JCI45416
- Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. HIV Gag protein conjugated to a toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8(+) T cell responses in nonhuman primates. Proc Natl Acad Sci USA 2005; 102:15190-4; PMID:16219698; http://dx.doi.org/10.1073/pnas.0507484102
- Wille-Reece U, Wu CY, Flynn BJ, Kedl RM, Seder RA. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8(+) T cell responses. J Immunol 2005; 174:7676-83; PMID:15944268; http://dx.doi.org/10.4049/jimmunol.174.12.7676
- Szeles L, Meissner F, Dunand-Sauthier I, Thelemann C, Hersch M, Singovski S, Haller S, Gobet F, Fuertes Marraco SA, Mann M, et al. TLR3-Mediated CD8+ Dendritic Cell Activation Is Coupled with Establishment of a Cell-Intrinsic Antiviral State. J Immunol 2015; 195(3):1025-33; PMID:26101320
- Jackson DC, Lau YF, Le T, Suhrbier A, Deliyannis G, Cheers C, Smith C, Zeng W, Brown LE. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci USA 2004; 101:15440-5; PMID:15489266; http://dx.doi.org/10.1073/pnas.0406740101
- Wang BL, Henao-Tamayo M, Harton M, Ordway D, Shanley C, Basaraba RJ, Orme IM. A toll-like receptor-2-directed fusion protein vaccine against tuberculosis. Clin Vaccine Immunol 2007; 14:902-6; PMID:17616633; http://dx.doi.org/10.1128/CDLI.00077-07
- Singh SK, Stephani J, Schaefer M, Kalay H, Garcia-Vallejo JJ, den Haan J, Saeland E, Sparwasser T, van Kooyk Y. Targeting glycan modified OVA to murine DC-SIGN transgenic dendritic cells enhances MHC class I and II presentation. Mol Immunol 2009; 47:164-74; PMID:19818504; http://dx.doi.org/10.1016/j.molimm.2009.09.026
- Chen HJ, Yuan BQ, Zheng ZC, Liu Z, Wang SS. Lewis X oligosaccharides-heparanase complex targeting to DCs enhance antitumor response in mice. Cell Immunol 2011; 269:144-8; PMID:21570677; http://dx.doi.org/10.1016/j.cellimm.2011.03.021
- Garcia-Vallejo JJ, Ambrosini M, Overbeek A, van Riel WE, Bloem K, Unger WWJ, Chiodo F, Bolscher JG, Nazmi K, Kalay H, et al. Multivalent glycopeptide dendrimers for the targeted delivery of antigens to dendritic cells. Mol Immunol 2013; 53:387-97; PMID:23103377; http://dx.doi.org/10.1016/j.molimm.2012.09.012
- Cruz LJ, Tacken PJ, Pots JM, Torensma R, Buschow SI, Figdor CG. Comparison of antibodies and carbohydrates to target vaccines to human dendritic cells via DC-SIGN. Biomaterials 2012; 33:4229-39; PMID:22410170; http://dx.doi.org/10.1016/j.biomaterials.2012.02.036
- Singh SK, Streng-Ouwehand I, Litjens M, Kalay H, Burgdorf S, Saeland E, Kurts C, Unger WW, van Kooyk Y. Design of neo-glycoconjugates that target the mannose receptor and enhance TLR-independent cross-presentation and Th1 polarization. Eur J Immunol 2011; 41:916-25; PMID:21400496; http://dx.doi.org/10.1002/eji.201040762
- Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8(+) T cells. Nat Immunol 2005; 6:593-9; PMID:15864309; http://dx.doi.org/10.1038/ni1201
- Trumpfheller C, Finke JS, Lopez CB, Moran TM, Moltedo B, Soares H, Huang Y, Schlesinger SJ, Park CG, Nussenzweig MC, et al. Intensified and protective CD4(+) T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med 2006; 203:607-17; PMID:16505141; http://dx.doi.org/10.1084/jem.20052005
- Boscardin SB, Hafalla JCR, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U, Morrot A, Zavala F, Steinman RM, Nussenzweig RS, et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med 2006; 203:599-606; PMID:16505139; http://dx.doi.org/10.1084/jem.20051639
- Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee CN, Phipson B, Shi W, Smyth GK, Lew AM, et al. Targeting Antigen to Mouse Dendritic Cells via Clec9A Induces Potent CD4 T Cell Responses Biased toward a Follicular Helper Phenotype. J Immunol 2011; 187:842-50; PMID:21677141; http://dx.doi.org/10.4049/jimmunol.1101176
- Corbett AJ, Caminschi I, McKenzie BS, Brady JL, Wright MD, Mottram PL, Hogarth PM, Hodder AN, Zhan Y, Tarlinton DM, et al. Antigen delivery via two molecules on the CD8(−) dendritic cell subset induces humoral immunity in the absence of conventional “danger”. Eur J Immunol 2005; 35:2815-25; PMID:16143986; http://dx.doi.org/10.1002/eji.200526100
- Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 2009; 458:899-903; PMID:19219027; http://dx.doi.org/10.1038/nature07750
- Caminschi I, Ahmet F, Heger K, Brady J, Nutt SL, Vremec D, Pietersz S, Lahoud MH, Schofield L, Hansen DS, et al. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J Exp Med 2007; 204:2579-90; PMID:17923506; http://dx.doi.org/10.1084/jem.20071351
- Beyer M, Wang HW, Peters N, Doths S, Koerner-Rettberg C, Openshaw PJ, Schwarze J. The beta2 integrin CDIIc distinguishes a subset of cytotoxic pulmonary T cells with potent antiviral effects in vitro and in vivo. Resp Res 2005; 6:70; http://dx.doi.org/10.1186/1465-9921-6-70
- Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins MF, Lanier LL, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med 2006; 12:207-13; PMID:16444266; http://dx.doi.org/10.1038/nm1352
- Wang H, Griffiths MN, Burton DR, Ghazal P. Rapid antibody responses by low-dose, single-step, dendritic cell-targeted immunization. Proc Natl Acad Sci USA 2000; 97:847-52; PMID:10639168; http://dx.doi.org/10.1073/pnas.97.2.847
- Tacken PJ, Torensma R, Figdor CG. Targeting antigens to dendritic cells in vivo. Immunobiology 2006; 211:599-608; PMID:16920498; http://dx.doi.org/10.1016/j.imbio.2006.05.021
- Bolland S, Ravetch JV. Inhibitory pathways triggered by ITIM-containing receptors. Adv Immunol 1999; 72:149-77; PMID:10361574; http://dx.doi.org/10.1016/S0065-2776(08)60019-X
- Wallace PK, Tsang KY, Goldstein J, Correale P, Jarry TM, Schlom J, Guyre PM, Ernstoff MS, Fanger MW. Exogenous antigen targeted to Fc gamma RI on myeloid cells is presented in association with MHC class I. J Immunol Methods 2001; 248:183-94; PMID:11223078; http://dx.doi.org/10.1016/S0022-1759(00)00351-3
- Flacher V, Tripp CH, Haid B, Kissenpfennig A, Malissen B, Stoitzner P, Idoyaga J, Romani N. Skin Langerin(+) Dendritic Cells Transport Intradermally Injected Anti-DEC-205 Antibodies but Are Not Essential for Subsequent Cytotoxic CD8(+) T Cell Responses. J Immunol 2012; 188:2146-55; PMID:22291181; http://dx.doi.org/10.4049/jimmunol.1004120
- Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M, Nussenzweig MC, Piperno AG, Steinman RM. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8(+) T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci USA 2007; 104:1289-94; PMID:17229838; http://dx.doi.org/10.1073/pnas.0610383104
- Njongmeta LM, Bray J, Davies CJ, Davis WC, Howard CJ, Hope JC, Palmer GH, Brown WC, Mwangi W. CD205 antigen targeting combined with dendritic cell recruitment factors and antigen-linked CD40L activation primes and expands significant antigen-specific antibody and CD4(+) T cell responses following DNA vaccination of outbred animals. Vaccine 2012; 30:1624-35; PMID:22240344; http://dx.doi.org/10.1016/j.vaccine.2011.12.110
- Ramakrishna V, Treml JF, Vitale L, Connolly JE, O'Neill T, Smith PA, Jones CL, He LZ, Goldstein J, Wallace PK, et al. Mannose receptor targeting of tumor antigen pmel17 to human dendritic cells directs anti-melanoma T cell responses via multiple HLA molecules. J Immunol 2004; 172:2845-52; PMID:14978085; http://dx.doi.org/10.4049/jimmunol.172.5.2845
- Caminschi I, Vremec D, Ahmet F, Lahoud MH, Villadangos JA, Murphy KM, Heath WR, Shortman K. Antibody responses initiated by Clec9A-bearing dendritic cells in normal and Batf3(−/−) mice. Mol Immunol 2012; 50:9-17; PMID:22209163; http://dx.doi.org/10.1016/j.molimm.2011.11.008
- Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest 2008; 118:2098-110; PMID:18497879; http://dx.doi.org/10.1172/JCI34584
- Castro FVV, Tutt AL, White AL, Teeling JL, James S, French RR, Glennie MJ. CD11c provides an effective immunotarget for the generation of both CD4 and CD8 T cell responses. Eur J Immunol 2008; 38:2263-73; PMID:18651710; http://dx.doi.org/10.1002/eji.200838302
- Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, et al. Fc gamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med 1999; 189:371-80; PMID:9892619; http://dx.doi.org/10.1084/jem.189.2.371
- Flinsenberg TWH, Compeer EB, Koning D, Klein M, Amelung FJ, van Baarle D, Boelens JJ, Boes M. Fc gamma receptor antigen targeting potentiates cross-presentation by human blood and lymphoid tissue BDCA-3(+) dendritic cells. Blood 2012; 120:5163-72; PMID:23093620; http://dx.doi.org/10.1182/blood-2012-06-434498
- Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Mi 2013; 3:13
- Tacken PJ, Zeelenberg IS, Cruz LJ, van Hout-Kuijer MA, van de Glind G, Fokkink RG, Lambeck AJ, Figdor CG. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood 2011; 118:6836-44; PMID:21967977; http://dx.doi.org/10.1182/blood-2011-07-367615
- Hamdy S, Haddadi A, Shayeganpour A, Samuel J, Lavasanifar A. Activation of Antigen-Specific T Cell-Responses by Mannan-Decorated PLGA Nanoparticles. Pharm Res-Dordr 2011; 28:2288-01; http://dx.doi.org/10.1007/s11095-011-0459-9
- Schreibelt G, Klinkenberg LJJ, Cruz LJ, Tacken PJ, Tel J, Kreutz M, Adema GJ, Brown GD, Figdor CG, de Vries IJ. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3(+) myeloid dendritic cells. Blood 2012; 119:2284-92; PMID:22234694; http://dx.doi.org/10.1182/blood-2011-08-373944
- Thomann JS, Heurtault B, Weidner S, Braye M, Beyrath J, Fournel S, Schuber F, Frisch B. Antitumor activity of liposomal ErbB2/HER2 epitope peptide-based vaccine constructs incorporating TLR agonists and mannose receptor targeting. Biomaterials 2011; 32:4574-83; PMID:21474175; http://dx.doi.org/10.1016/j.biomaterials.2011.03.015
- Yang L, Yang H, Rideout K, Cho T, Il Joo K, Ziegler L, Elliot A, Walls A, Yu D, Baltimore D, et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol 2008; 26:326-34; PMID:18297056; http://dx.doi.org/10.1038/nbt1390
- Cruz LJ, Tacken PJ, Fokkink R, Joosten B, Stuart MC, Albericio F, Torensma R, Figdor CG. Targeted PLGA nano- but not microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. J Control Release 2010; 144:118-26; PMID:20156497; http://dx.doi.org/10.1016/j.jconrel.2010.02.013
- Arigita C, Bevaart L, Everse LA, Koning GA, Hennink WE, Crommelin DJA, van de Winkel JG, van Vugt MJ, Kersten GF, Jiskoot W. Liposomal meningococcal B vaccination: Role of dendritic cell targeting in the development of a protective immune response. Infect Immun 2003; 71:5210-8; PMID:12933866; http://dx.doi.org/10.1128/IAI.71.9.5210-5218.2003
- Cruz LJ, Rueda F, Cordobilla B, Simon L, Hosta L, Albericio F, Domingo JC. Targeting Nanosystems to Human DCs via Fc Receptor as an Effective Strategy to Deliver Antigen for Immunotherapy. Mol Pharmaceut 2011; 8:104-16; http://dx.doi.org/10.1021/mp100178k
- Hangalapura BN, Oosterhoff D, de Groot J, Boon L, Tuting T, van den Eertwegh AJ, Gerritsen WR, van Beusechem VW, Pereboev A, Curiel DT, et al. Potent Antitumor Immunity Generated by a CD40-Targeted Adenoviral Vaccine. Cancer Res 2011; 71:5827-37; PMID:21747119; http://dx.doi.org/10.1158/0008-5472.CAN-11-0804
- Brandao JG, Scheper RJ, Lougheed SM, Curiel DT, Tillman BW, Gerritsen WR, van den Eertwegh AJ, Pinedo HM, Haisma HJ, de Gruijl TD. CD40-targeted adenoviral gene transfer to dendritic cells through the use of a novel bispecific single-chain Fv antibody enhances cytotoxic T cell activation. Vaccine 2003; 21:2268-72; PMID:12744857; http://dx.doi.org/10.1016/S0264-410X(03)00050-1
