ABSTRACT
Influenza vaccination is recommended for healthcare workers (HCWs), but coverage is often low. We reviewed studies evaluating interventions to increase seasonal influenza vaccination coverage in HCWs, including a meta-regression analysis to quantify the effect of each component. Fourty-six eligible studies were identified. Domains conferring a high risk of bias were identified in most studies. Mandatory vaccination was the most effective intervention component (Risk Ratio of being unvaccinated [RRunvacc] = 0.18, 95% CI: 0.08–0.45), followed by “soft” mandates such as declination statements (RRunvacc = 0.64, 95% CI: 0.45–0.92), increased awareness (RRunvacc = 0.83, 95% CI: 0.71–0.97) and increased access (RRunvacc = 0.88, 95% CI: 0.78–1.00). For incentives the difference was not significant, while for education no effect was observed. Heterogeneity was substantial (τ2 = 0.083). These results indicate that effective alternatives to mandatory HCWs influenza vaccination do exist, and need to be further explored in future studies.
Introduction
Healthcare workers (HCWs) are at increased risk for influenza,Citation1 and may transmit the virus to their patientsCitation2; therefore annual influenza vaccination has been recommended for HCWs by public health authorities worldwide for over 2 decades.Citation3-5 Despite the vaccine's variable year-on-year efficacy,Citation6 there is evidence that influenza vaccination of HCWs can reduce patient morbidity and mortality in long term care and in hospital settings,Citation7-9 as well as prevent sickness absence among healthcare staff.Citation10,11 As a result, influenza vaccination is regarded both as an occupational health measure and as an important element in infection control, commensurate with HCWs' duty to protect patients.Citation12
Despite long-standing recommendations, influenza vaccine uptake remains low in most healthcare settings and in most countries,Citation5,13 falling far short of specified targets that usually reach 80% or 90%. As a result, various interventions to increase coverage of HCWs have been explored,Citation14,15 with a particular focus on mandatory influenza vaccination, whose merits and ethics remain strongly debated.Citation16-21 To a large extent this debate is driven by the fact that the evidence on influenza vaccination of HCWs is not unequivocally conclusiveCitation22,23; the benefit, in terms of both personal protection and protection of patients, is smaller and much less clearCitation6 than in the case of diseases for which vaccination is universally accepted and/or mandated, such as measlesCitation24 and Hepatitis BCitation25.
Setting aside the “ethics” part of the question, for which there are arguments on both sides, we tried to address the “merits” part and find out how effective mandatory vaccination is, compared to other, less controversial alternatives. To that end, we undertook a systematic review of epidemiologic studies evaluating interventions to increase influenza vaccination coverage in HCWs, including a meta-regression analysis to quantify the benefit of each intervention component. We tried to include the widest possible range of studies in our review, but focused only in seasonal (i.e. not pandemic) influenza vaccination campaigns, in order to maximize generalizability of the results.
Results
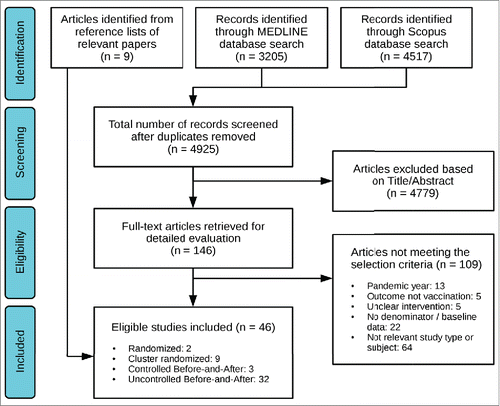
Our literature search yielded 4,925 unique (non-duplicate) citations. After screening the title and abstract, we selected 146 full-text articles for retrieval. Thirty-seven studies, plus another 9 identified from reference lists of relevant articles, met our eligibility criteria and were included in the analysis ().Citation26-71 Publication years ranged from 1992 to 2015. Most studies were BnA without a control group,Citation27-33,38-40,42-46,48,50,52,54-57,59-63,65-67,69,70 and there were also 3 BnA studies with a control group,Citation36,68,71 9 cRCTsCitation26,34,37,41,47,49,51,58,64 and 2 RCTs.Citation35,53 Two uncontrolled BnA studies compared the intervention year (2005 in both cases) with the second-to-last preceding year (2003), because of a reported vaccine shortage during the immediately preceding one (2004).Citation43,56
Five studiesCitation35,41,54,58,71 evaluated separately more than one intervention on the same population or using the same controls, and 2 studiesCitation27,28 were also performed on the same population. In two studies,Citation34,42 the same intervention was evaluated in independent populations or settings. As a result, the 46 studies contributed a total of 53 comparisons, nested within 47 clusters ().
Table 1. Characteristics of included studies.
Most comparisons were performed in a hospital or nursing home setting, 32 and 11 respectively. Seven studies were limited to particular types of HCWs (). The majority of comparisons examined an intervention with multiple simultaneous components; the exception was “hard” mandates, which were assessed in 8 comparisons (7 studies, all uncontrolled BnACitation28,29,42,54,56,66,67) with no other simultaneous component. In one study the mandatory policy included termination of employment for unvaccinated workers, but due to reactions this was ultimately put in abeyanceCitation42; we considered this intervention as a “hard” mandate nonetheless, in an “intention-to-treat” manner. Regarding the remaining intervention components, increased access (mostly involving mobile carts) was assessed in 23 comparisons, increased awareness in 27, education in 18, incentives in 11, and “soft” mandates in 7. Of the 7 comparisons assessing “soft” mandates, all but oneCitation61 involved the use of a declination form, i.e., a mandatory written statement that the HCW refuses vaccination and provides the reasons for doing so.
Regarding the risk of bias, out of the 11 RCTs or cRCTs, in 7 the method of randomization was unclear,Citation26,34,35,51,53,58,64 and one study employed a factorial design with partial randomization.Citation41 Allocation concealment in the 2 RCTs was unclear,Citation35,53 5 studies did not report vaccination coverage at baseline for the intervention and control groups,Citation34,35,37,47,53 and 9 studies (including the 3 controlled BnA studies) did not sufficiently report on potential baseline imbalances.Citation34-36,47,51,53,58,68,71 Completeness of outcome data was not clarified in all but 5 studies,Citation26,41,47,49,58 and in 3 of them the response rate was low enough to potentially bias the results.Citation41,47,58 Most studies offered vaccination in-house and could objectively ascertain vaccine coverageCitation26,35,36,49,53,64,68,71; one study used an external occupational health service,Citation37 another used general practitioner claim forms,Citation34 3 studies used self-report via quesionnaires to ascertain vaccination,Citation41,47,58 and one study did not report the method of ascertainment.Citation51
Of the uncontrolled BnA studies, none described particular concurrent events that could influence the post-intervention vaccination coverage, except in the 2 studies that reported a vaccine shortage during the previous yearCitation43,56; although in these the comparison was made with the second-to-last year, the shortage might still have biased the post-intervention vaccination coverage. In five studies ascertainment of vaccination was via self-report,Citation29,44,46,61,62 and in 2 the method was unclearCitation59,67; the remaining studies used objective means to ascertain vaccination. Completeness or near-completeness of outcome data could be established for 6 studies,Citation27,28,39,45,62,69 while 3 studies used questionnaires and had a low (<50%) response rate, raising the possibility of selection bias. No study (including the controlled studies) had a reliable method of tracking participants that were vaccinated off-site or outside study arrangements.
Regarding calculation of effect measures, in 9 studiesCitation26,35,36,41,51,58,64,68,71 we employed the modified Poisson regression using GEECitation72 to calculate RRunvacc using the available study data, while for the remaining studies we reconstructed contingency tables. In another 9 studiesCitation26,34,37,41,47,51,58,64,71 we applied a correction for clustering to the RRunvacc by calculating the design effect; in six casesCitation26,34,41,51,64,71 the ICC was provided or could be indirectly estimated from the study data, and in 3 casesCitation37,47,58 we had to resort to an external ICC estimate of 0.1 (an approximate mean of the 6 known/estimable ICC coefficients).
Entering all comparisons into the meta-regression model (), we found “hard” mandates to be very effective for increasing influenza vaccine coverage (RRunvacc = 0.18, 95% CI: 0.08–0.45, p = 0.003). “Soft” mandates were also effective, but to a smaller extent (RRunvacc = 0.64, 95% CI: 0.45–0.92, p = 0.022). Statistically significant effectiveness was found for increased access (RRunvacc = 0.88, 95% CI: 0.78–1.00, p = 0.044) and increased awareness (RRunvacc = 0.83, 95% CI: 0.71–0.97, p=0.02), for incentives the difference did not reach statistical significance (RRunvacc = 0.89, 95% CI: 0.77–1.03, p = 0.12), while for education no effect was observed (RRunvacc = 0.96, 95% CI: 0.84–1.10, p=0.57).
Figure 2. Forest plot: results from individual studies and random-effects meta-regression model (logarithmic scale). Vertical bars before study names indicate comparisons that are clustered together.
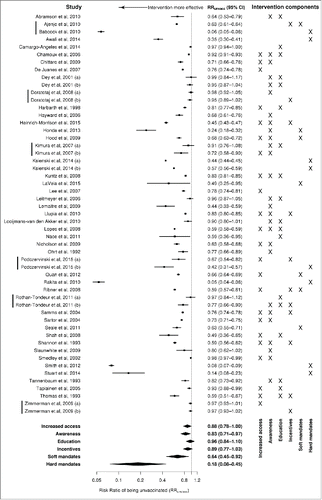
Substantial heterogeneity was identifiedCitation73; between-cluster variance τ2 was 0.083, while within-cluster variance ω2 was zero, indicating no clustering of effects between studies performed on the same population or using the same control group. The I2 statisticCitation74 was 99.5%, meaning that almost the entire variance was due to differences between studies and not due to sampling; the large I2 value is not unexpected though, given the large number of studies and the small standard errors for most of effect estimates.Citation75 Compared to an intercept-only model (τ2 = 0.116, ω2 = 0), a quarter of the variance was found to be explained by the moderators (pseudo-R2 = 28.9%).
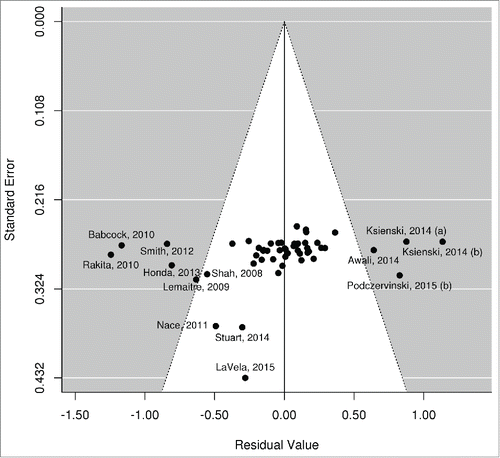
Visual inspection of the funnel plot of meta-regression residuals against their standard error identified a slight asymmetry (), while the Egger regression asymmetry test was statistically significant (p = 0.012), indicating possible publication bias or small-study effects. The funnel plot also highlights the studies contibuting most on the observed heterogeneity (those lying either side of the funnel).
Figure 3. Funnel plot of meta-regression residuals (observed–fitted log Relative Risk) against their standard errors.
The test of interaction with study type was not statistically significant for the first 4 intervention components, indicating that uncontrolled BnA studies did not produce different results compared to other designs; studies evaluating “soft” or “hard” mandates were all uncontrolled BnA, thus no interaction terms could be fitted. Regarding post- vs pre-pandemic studies, we did not observe statistically significant interaction for any intervention component but the term for “hard” mandates came very close to significance (p = 0.0506); indeed, among the studies assessing “hard” mandates, the 2 that had been conducted before the 2009 H1N1 pandemicCitation28,56 showed greater effectiveness than those conducted afterwards.Citation29,42,54,66,67 Finally, in the model that included an intercept and the intervention year, no temporal trend for the effectiveness of the reviewed interventions was identified (yearly change ratio of RRunvacc = 1.00, 95% CI: 0.98–1.03, p = 0.62).
Discussion
Our study indicates that it is possible to increase influenza vaccine coverage in HCWs, and there are various ways of doing so. We have found that mandating influenza vaccination, with consequences such as termination of employment for those refusing, is by far the most effective single intervention. There may be practical problems in its implementation, however, as it may cause resentment or opposition among HCWsCitation76; in one case, unions launched a successful legal challenge to the mandatory vaccination policy.Citation42 As a result, despite the high effectiveness of “hard” mandates, exploring alternative options is worthwhile and useful.
Declination statements (which formed the bulk of the “soft” mandates component) can be considered a form of mild pressure, a way to make hesitant or indifferent HCWs accept the vaccine, or think seriously about their reasons for refusing. In our review, declination statements were found to be highly effective, indicating that they can be an important element in any campaign to vaccinate HCWs against influenza. Other intervention components, such as increased access, increased awareness and incentives (for which the difference did not reach statistical significance) were found to be less effective; in combination though, their cumulative effect could match that of declination statements. Notably, educational interventions were not found to be effective on average; however, as different interventions may have variable impact on the various categories of HCWs,Citation46,77 the role of education should still be examined in more detail for particular HCW groups.
Based on the above findings, an “all of the above” approach can be suggested to increase influenza vaccine coverage of HCWs, as an alternative to mandatory vaccination. Declination statements should be the key intervention in such an approach, complemented by better on-site access to vaccination, active promotion of the campaign and possibly other targeted interventions such as incentives and education. Prerequisites for all that include managerial commitment, provision of the necessary resources, and engagement of all stakeholders in the goal of raising influenza vaccine coverage.
In our review we did not include studies assessing pandemic vaccination coverage of HCWs. However, the 2009 H1N1 pandemic could potentially also affect the willingness of HCWs to get vaccinated against seasonal influenza, modifying the effect of interventions to increase coverage. Our analysis did identify a trend toward lower effectiveness of “hard” mandates after 2009, indicating that HCWs may be more reluctant, or even suspicious against being pushed too hard on influenza vaccination. This needs to be considered by planners of vaccination campaigns, and investigated in future studies.
The risk of bias is thought to be greater for BnA studies without a control group; indeed the factors that have prompted the study and intervention may affect comparability with the pre-intervention year. Nevertheless we did include these studies in our review, knowing that they form the bulk of the available literature on the subject at hand (and for certain intervention components, i.e. mandates, the only available literature). In addition, systemwide interventions on entire populations of HCWs leave less potential for selection bias, and taking only the last previous season maximizes comparability of the population. The use of a control group in a BnA study may partly guard against hypothetical temporal trends (which we did not find in our particular analysis) or unexpected events, but raises the issue of comparability between groups. Even the randomized studies in our review had a high risk of bias in several domains. Thus we pooled all available evidence and a test of interaction was performed to assess whether uncontrolled BnA studies gave materially different estimates than other study types; no statistically significant interaction by study type was observed for any intervention component.
Our review has several advantages: a clear objective, a comprehensive systematic literature search with specific eligibility criteria, a risk-of-bias assessment for the included studies and a meta-analytic synthesis of results. Moreover, our methodology allows us to disentangle the effects of individual components across studies very heterogenous in terms of interventions implemented; this is a particular strength of our study. However, substantial heterogeneity still remains, as indicated by the large τ2 and modest R2 (the inflated I2 is rather misleading in our caseCitation75). Various study-specific factors can well account for this heterogeneity, namely: populations studied (all HCWs, HCWs with patient contact, or particular types of HCWs); clinical setting (nursing homes, hospitals, other); country (different cultures may impact intervention effectiveness); the specific details of each intervention (the 6 components are by definition not completely homogenous) and the way these were implemented in each study. Also, some studies used subjective methods (self-report) to ascertain vaccination, and no study could reliably track participants vaccinated off-site, thus contributing to overall heterogeneity. As a result, the pooled effect sizes for each intervention component should not be interpreted as a fixed and absolute truth, valid to all settings; rather they should be viewed as average effects across all reviewed studies, reflecting the sum of current knowledge on the subject. Furthermore, the funnel plot and Egger test result potentially indicate the existence of publication bias, a finding that also needs to be taken into account.
Another limitation in the analysis is the fact that we did not examine potential interaction effects between intervention components; doing so with sufficient statistical power would require far more studies than the 45 available. The assumption of additive effects in our meta-regression model is not unreasonable though; indeed the 3 studies in our review that directly evaluated the combination of 2 intervention components did not demonstrate a statistically significant interaction effect.Citation35,41,71 In addition, the data were not sufficient to calculate subgroup pooled effects for different clinical settings (hospitals, nursing homes, primary care) or different groups of HCWs (physicians, nurses, other HCWs); therefore we cannot conclude on whether certain interventions may be more effective in some settings or for particular groups of HCWs.
In conclusion, we quantified the average effectiveness of the different components that can be employed in a campaign to increase influenza vaccination coverage in HCWs. We demonstrated that mandates, either “hard” or “soft” (declination statements), are most effective, followed by other interventions such as increased access and increased awareness. Educational interventions did not appear effective on average, although they could still be useful for particular groups of HCWs. Further research is warranted on the issue, along with commitment by all stakeholders to promote influenza vaccination of HCWs as a patient safety and quality of care issue. In our opinion, however, increasing coverage is best done in a cooperative spirit, as part of a safety culture and without punitive measures,Citation78 especially not before having exhausted all available options first.
Materials and methods
Literature search and selection
We searched MEDLINE and Scopus databases for published articles using the following combination of keywords: vaccin* AND (influenza OR flu) AND (“healthcare worker(s)” OR “health worker(s)” OR “health personnel” OR “health staff” OR “physician(s)” OR “doctor(s)” OR “nurse(s)” OR “practitioner(s)”). We applied no date or language restrictions, using an automated translation service (Google Translate) when necessary. In addition, we searched the reference lists of relevant papers to identify additional studies. We did not consider conference abstracts or other data not published in the peer-reviewed literature.
Literature search and selection was performed by 4 reviewers (TL, FK, EM, DP) working in pairs, i.e., each abstract and full text was reviewed twice. Discrepancies were resolved by consensus. We selected studies evaluating a campaign or otherwise defined intervention to increase vaccination coverage in HCWs; interventions should be clearly defined and include one or more components, either separate or concurrent. To be eligible, a study had to compare as outcome the vaccination coverage between the intervention and control groups, and provide sufficient numerical data to calculate an appropriate effect measure for the intervention, as described below. We did not include studies assessing “intention to be vaccinated” as outcome, only studies comparing actual vaccination rates. Eligible study types were Randomized Controlled Trials (RCTs), Cluster Randomized Controlled Trials (cRCTs) and Before-and-After studies (BnA) both controlled and uncontrolled. For uncontrolled BnA studies, the comparison should be made between the intervention year and the immediately preceding year, unless the study authors provided a clear and specific reason to suggest non-comparability (e.g. vaccine shortage) in that particular year; in that case comparison was made with the next preceding year. For each selected study, we used the criteria suggested by the Cochrane Effective Practice and Organization of Care groupCitation79 to assess the risk of bias. Studies performed during the pandemic season 2009–2010 were excluded from our review.
Definition of interventions and effect measure
Two reviewers (TL, SB) abstracted the data and performed the analysis. For each study, intervention components were identified and codified into 6 broad categories (): increased access, increased awareness, education, incentives, “soft” mandates and “hard” mandates. Each intervention could consist of one or more of these components, evaluated concurrently (one intervention group, one comparison) or separately, as for example in a factorial design (multiple intervention groups, multiple comparisons).
Table 2. Intervention components to increase influenza vaccine coverage in HCWs.
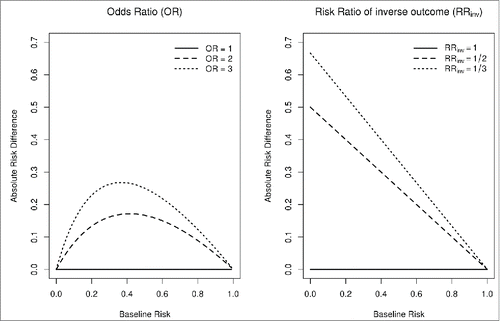
Different relative effect measures can be used to compare vaccination coverage between the intervention and control group: the Risk Difference, the Odds Ratio and the Risk Ratio. For rare outcomes the choice is inconsequential; it becomes important, however, when the probability of the outcome can vary widely, because different relative effect measures imply different absolute effects over the range of baseline risks.Citation80 In the case of influenza vaccination, a given intervention to increase coverage is expected to have a progressively smaller absolute benefit with increasing baseline coverage (). For this reason, we chose the Relative Risk of being unvaccinated (RRunvacc) as the most appropriate measure to consistently quantify the effect of an intervention across the entire range of baseline coverages; values of RRunvacc < 1 suggest that the intervention is effective in reducing the number of unvaccinated HCWs, i.e. improving vaccine coverage.
Figure 4. Relationship between absolute risk difference and baseline risks for different effect measures. Using OR as an intervention effect measure implies that, for a given intervention effectiveness, the absolute benefit (i.e., absolute risk difference) is maximal when baseline risks are near 50%. Using RRinv implies that the absolute benefit gets smaller as baseline risks increase. The latter is a much more reasonable assumption in the case of vaccination coverage, since if more HCWs are already vaccinated at baseline, fewer additional HCWs will get vaccinated for a given intervention effectiveness.
Statistical analysis
For every comparison in our review, we calculated the RRunvacc and its log standard error; for simpler designs (such as uncontrolled BnA) we used the available study data to reconstruct contingency tables, while for more complex designs (such as controlled BnA or factorial designs) we employed a modified Poisson regression using Generalized Estimating Equations (GEE).Citation72 In case of a clustered design, we also used the available study data to calculate the Intraclass Correlation Coefficient (ICC) and the design effect, in order to appropriately inflate the standard errors.Citation81
All effect sizes and standard errors were subsequently entered into a random-effects meta-regression model with 6 binary predictors, one for each intervention component (), and no intercept. The aim was not to derive a pooled effect estimate for all studies, but to assess the effectiveness of each intervention component in improving vaccination coverage. Some comparisons were made on the same population or with the same controls, within the same study or even across different studies. In order to account for this clustering, we used a robust meta-regression approach with hierarchical dependence structure as described by Hedges et al.Citation73 A big advantage of this method is that it does not require meta-regression covariates to be fixed, unlike in other meta-regression approachesCitation73; assuming fixed covariates makes little sense within a random-effects framework, particularly when the focus is on the regression coefficients and not on a pooled effect estimate.
Publication bias was assessed using a funnel plot of study meta-regression residuals against their standard errors, and with the Egger regression asymmetry test.Citation82 The risk of bias in uncontrolled BnA studies is considered to be higher than controlled BnA or cRCTs. To assess whether the results of uncontrolled BnA studies gave different results in our review, we did a test of interaction by re-fitting our model and including, for each predictor, an interaction term with study type (expressed as a binary variable: 1 = uncontrolled BnA, 0 = other study types). In similar fashion, in order to check whether the experience of the 2009 H1N1 influenza pandemic modified the effectiveness of each intervention component, we included interaction terms for post-pandemic vs pre-pandemic studies. In addition, we sought to identify a temporal trend in the effectiveness of the reviewed interventions by including in the model an intercept and the year that the intervention took place.
This review was conducted in accordance with the MOOSE guidelines.Citation83 All statistical analyses were performed with version 3.2 of the R software environmentCitation84 using the packages “robumeta,” “metafor”Citation85 and “geepack”.Citation86
Disclosure of potential conflicts of interest
The authors declare that they have no conflicts of interest.
Authors' contributions
TL conceived the idea for this review and designed the search strategy, inclusion criteria and analysis protocol. TL, FK, EM and DP searched the literature and selected the studies. TL and SB extracted and analyzed the data, interpreted the findings and drafted the initial version of the manuscript. All authors participated in the further development of the manuscript and approved the final version for publication.
References
- Kuster SP, Shah PS, Coleman BL, Lam P-P, Tong A, Wormsbecker A, McGeer A. Incidence of influenza in healthy adults and healthcare workers: a systematic review and meta-analysis. PLoS One 2011; 6:e26239; PMID:22028840; http://dx.doi.org/10.1371/journal.pone.0026239
- Voirin N, Barret B, Metzger M-H, Vanhems P. Hospital-acquired influenza: a synthesis using the Outbreak Reports and Intervention Studies of Nosocomial Infection (ORION) statement. J Hosp Infect 2009; 71:1-14; PMID:18952319; http://dx.doi.org/10.1016/j.jhin.2008.08.013
- Pearson ML, Bridges CB, Harper SA, Healthcare Infection Control Practices Advisory Committee (HICPAC), Advisory Committee on Immunization Practices (ACIP). Influenza vaccination of health-care personnel: recommendations of the Healthcare Infection Control Practices Advisory Committee (HICPAC) and the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1-16; PMID:16498385
- Macroepidemiology of Influenza Vaccination (MIV) Study Group. The macro-epidemiology of influenza vaccination in 56 countries, 1997–2003. Vaccine 2005; 23:5133-43; PMID:16039762; http://dx.doi.org/10.1016/j.vaccine.2005.06.010
- Mereckiene J, Cotter S, Nicoll A, Lopalco P, Noori T, Weber J, D'Ancona F, Levy-Bruhl D, Dematte L, Giambi C, et al. Seasonal influenza immunisation in Europe. Overview of recommendations and vaccination coverage for three seasons: pre-pandemic (2008/09), pandemic (2009/10) and post-pandemic (2010/11). Euro Surveill 2014; 19:20780; PMID:24786262; http://dx.doi.org/10.2807/1560-7917.ES2014.19.16.20780
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36-44; PMID:22032844; http://dx.doi.org/10.1016/S1473-3099(11)70295-X
- Carman WF, Elder AG, Wallace LA, McAulay K, Walker A, Murray GD, Stott DJ. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet 2000; 355:93-7; PMID:10675165; http://dx.doi.org/10.1016/S0140-6736(99)05190-9
- Potter J, Stott DJ, Roberts MA, Elder AG, O'Donnell B, Knight PV, Carman WF. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis 1997; 175:1-6; PMID:8985189; http://dx.doi.org/10.1093/infdis/175.1.1
- Salgado CD, Giannetta ET, Hayden FG, Farr BM. Preventing nosocomial influenza by improving the vaccine acceptance rate of clinicians. Infect Control Hosp Epidemiol 2004; 25:923-8; PMID:15566025; http://dx.doi.org/10.1086/502321
- Wilde JA, McMillan JA, Serwint J, Butta J, O'Riordan MA, Steinhoff MC. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA 1999; 281:908-13; PMID:10078487; http://dx.doi.org/10.1001/jama.281.10.908
- Van Buynder PG, Konrad S, Kersteins F, Preston E, Brown PD, Keen D, Murray NJ. Healthcare worker influenza immunization vaccinate or mask policy: Strategies for cost effective implementation and subsequent reductions in staff absenteeism due to illness. Vaccine 2015; 33:1625-8; PMID:25678243; http://dx.doi.org/10.1016/j.vaccine.2015.01.048
- Poland GA, Tosh P, Jacobson RM. Requiring influenza vaccination for health care workers: seven truths we must accept. Vaccine 2005; 23:2251-5; PMID:15755605; http://dx.doi.org/10.1016/j.vaccine.2005.01.043
- Blank PR, Schwenkglenks M, Szucs TD. Disparities in influenza vaccination coverage rates by target group in five European countries: trends over seven consecutive seasons. Infection 2009; 37:390-400; PMID:19768382; http://dx.doi.org/10.1007/s15010-009-8467-y
- Hollmeyer H, Hayden F, Mounts A, Buchholz U. Review: interventions to increase influenza vaccination among healthcare workers in hospitals. Influenza Other Respir Viruses 2013; 7:604-21; PMID:22984794; http://dx.doi.org/10.1111/irv.12002
- Lam P-P, Chambers LW, MacDougall DMP, McCarthy AE. Seasonal influenza vaccination campaigns for health care personnel: systematic review. CMAJ 2010; 182:E542-8; PMID:20643836; http://dx.doi.org/10.1503/cmaj.091304
- Hooper CR, Breathnach A, Iqbal R. Is there a case for mandating influenza vaccination in healthcare workers? Anaesthesia 2014; 69:95-100; PMID:24443849; http://dx.doi.org/10.1111/anae.12561
- Backer H. Counterpoint: in favor of mandatory influenza vaccine for all health care workers. Clin Infect Dis 2006; 42:1144-7; PMID:16575733; http://dx.doi.org/10.1086/501463
- Finch M. Point: mandatory influenza vaccination for all heath care workers? Seven reasons to say “no.” Clin Infect Dis 2006; 42:1141-3; PMID:16575732; http://dx.doi.org/10.1086/501466
- Zimmerman RK. Ethical analyses of institutional measures to increase health care worker influenza vaccination rates. Vaccine 2013; 31:6172-6; PMID:24188752; http://dx.doi.org/10.1016/j.vaccine.2013.10.066
- Helms CM, Polgreen PM. Should influenza immunisation be mandatory for healthcare workers? Yes. BMJ 2008; 337:a2142; PMID:18957466; http://dx.doi.org/10.1136/bmj.a2142
- Isaacs D, Leask J. Should influenza immunisation be mandatory for healthcare workers? No. BMJ 2008; 337:a2140; PMID:18957465; http://dx.doi.org/10.1136/bmj.a2140
- Thomas RE, Jefferson T, Lasserson TJ. Influenza vaccination for healthcare workers who care for people aged 60 or older living in long-term care institutions. Cochrane Database Syst Rev 2013; 7:CD005187; PMID:23881655
- Abramson ZH. What, in Fact, Is the Evidence That Vaccinating Healthcare Workers against Seasonal Influenza Protects Their Patients? A Critical Review. Int J Family Med 2012; 2012:205464; PMID:23209901; http://dx.doi.org/10.1155/2012/205464
- Bloch AB, Orenstein WA, Stetler HC, Wassilak SG, Amler RW, Bart KJ, Kirby CD, Hinman AR. Health impact of measles vaccination in the United States. Pediatrics 1985; 76:524-32; PMID:3931045
- Bock HL, Kruppenbacher J, Sänger R, Höbel W, Clemens R, Jilg W. Immunogenicity of a recombinant hepatitis B vaccine in adults. Arch Intern Med 1996; 156:2226-31; PMID:8885822; http://dx.doi.org/10.1001/archinte.1996.00440180088011
- Abramson ZH, Avni O, Levi O, Miskin IN. Randomized trial of a program to increase staff influenza vaccination in primary care clinics. Ann Fam Med 2010; 8:293-8; PMID:20644183; http://dx.doi.org/10.1370/afm.1132
- Ajenjo MC, Woeltje KF, Babcock HM, Gemeinhart N, Jones M, Fraser VJ. Influenza vaccination among healthcare workers: ten-year experience of a large healthcare organization. Infect Control Hosp Epidemiol 2010; 31:233-40; PMID:20055666; http://dx.doi.org/10.1086/650449
- Babcock HM, Gemeinhart N, Jones M, Dunagan WC, Woeltje KF. Mandatory influenza vaccination of health care workers: translating policy to practice. Clin Infect Dis 2010; 50:459-64; PMID:20064039; http://dx.doi.org/10.1086/650752
- Awali RA, Samuel PS, Marwaha B, Ahmad N, Gupta P, Kumar V, Ellsworth J, Flanagan E, Upfal M, Russell J, et al. Understanding health care personnel's attitudes toward mandatory influenza vaccination. Am J Infect Control 2014; 42:649-52; PMID:24837116; http://dx.doi.org/10.1016/j.ajic.2014.02.025
- Camargo-Ángeles R, Villanueva-Ruiz CO, García-Román V, Mendoza-García JL, Conesa-Peñuela FJ, Tenza Iglesias I, García Shimizu P, Sánchez-Payá J. [Evaluation of a novel flu vaccination campaign among health personnel for the 2011–2012 season]. Arch Prev Riesgos Labor 2014; 17:26-30; PMID:24458207; http://dx.doi.org/10.12961/aprl.2014.17.1.04
- Chamoux A, Denis-Porret M, Rouffiac K, Baud O, Millot-Theis B, Souweine B. [Impact study of an active antiflu vaccination programme on the Clermont-Ferrand Teaching Hospital staff]. Med Mal Infect 2006; 36:144-50; PMID:16581213; http://dx.doi.org/10.1016/j.medmal.2006.01.004
- Chittaro M, Turello D, Calligaris L, Farneti F, Faruzzo A, Fiappo E, Panariti M, Brusaferro S. Impact of vaccinating HCWs on the ward and possible influence of avian flu threat. Infection 2009; 37:29-33; PMID:19139813; http://dx.doi.org/10.1007/s15010-008-8002-6
- De Juanes JR, García de Codes A, Arrazola MP, Jaén F, Sanz MI, González A. Influenza vaccination coverage among hospital personnel over three consecutive vaccination campaigns (2001–2002 to 2003–2004). Vaccine 2007; 25:201-4; PMID:17011084; http://dx.doi.org/10.1016/j.vaccine.2005.10.057
- Dey P, Halder S, Collins S, Benons L, Woodman C. Promoting uptake of influenza vaccination among health care workers: a randomized controlled trial. J Public Health Med 2001; 23:346-8; PMID:11873900; http://dx.doi.org/10.1093/pubmed/23.4.346
- Doratotaj S, Macknin ML, Worley S. A novel approach to improve influenza vaccination rates among health care professionals: a prospective randomized controlled trial. Am J Infect Control 2008; 36:301-3; PMID:18455052; http://dx.doi.org/10.1016/j.ajic.2007.10.019
- Harbarth S, Siegrist CA, Schira JC, Wunderli W, Pittet D. Influenza immunization: improving compliance of healthcare workers. Infect Control Hosp Epidemiol 1998; 19:337-42; PMID:9613695; http://dx.doi.org/10.2307/30141375
- Hayward AC, Harling R, Wetten S, Johnson AM, Munro S, Smedley J, Murad S, Watson JM. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ 2006; 333:1241; PMID:17142257; http://dx.doi.org/10.1136/bmj.39010.581354.55
- Heinrich-Morrison K, McLellan S, McGinnes U, Carroll B, Watson K, Bass P, Worth LJ, Cheng AC. An effective strategy for influenza vaccination of healthcare workers in Australia: experience at a large health service without a mandatory policy. BMC Infect Dis 2015; 15:42; PMID:25656220; http://dx.doi.org/10.1186/s12879-015-0765-7
- Honda H, Sato Y, Yamazaki A, Padival S, Kumagai A, Babcock H. A successful strategy for increasing the influenza vaccination rate of healthcare workers without a mandatory policy outside of the United States: a multifaceted intervention in a Japanese tertiary care center. Infect Control Hosp Epidemiol 2013; 34:1194-200; PMID:24113604; http://dx.doi.org/10.1086/673452
- Hood J, Smith A. Developing a “best practice” influenza vaccination program for health care workers–an evidence-based, leadership-modeled program. AAOHN J 2009; 57:308-12; PMID:19728685; http://dx.doi.org/10.1177/216507990905700802
- Kimura AC, Nguyen CN, Higa JI, Hurwitz EL, Vugia DJ. The effectiveness of vaccine day and educational interventions on influenza vaccine coverage among health care workers at long-term care facilities. Am J Public Health 2007; 97:684-90; PMID:17329659; http://dx.doi.org/10.2105/AJPH.2005.082073
- Ksienski DS. Mandatory seasonal influenza vaccination or masking of British Columbia health care workers: Year 1. Can J Public Health 2014; 105:e312-6; PMID:25166135
- Kuntz JL, Holley S, Helms CM, Cavanaugh JE, Vande Berg J, Herwaldt LA, Polgreen PM. Use of a pandemic preparedness drill to increase rates of influenza vaccination among healthcare workers. Infect Control Hosp Epidemiol 2008; 29:111-5; PMID:18179365; http://dx.doi.org/10.1086/526434
- LaVela SL, Hill JN, Smith BM, Evans CT, Goldstein B, Martinello R. Healthcare worker influenza declination form program. Am J Infect Control 2015; 43(6):624-8; PMID:25798775
- Lee HY, Fong YT. On-site influenza vaccination arrangements improved influenza vaccination rate of employees of a tertiary hospital in Singapore. Am J Infect Control 2007; 35:481-3; PMID:17765562; http://dx.doi.org/10.1016/j.ajic.2006.10.007
- Leitmeyer K, Buchholz U, Kramer M, Schenkel K, Stahlhut H, Köllstadt M, Haas W, Meyer C. Influenza vaccination in German health care workers: effects and findings after two rounds of a nationwide awareness campaign. Vaccine 2006; 24:7003-8; PMID:16730866; http://dx.doi.org/10.1016/j.vaccine.2006.04.040
- Lemaitre M, Meret T, Rothan-Tondeur M, Belmin J, Lejonc J-L, Luquel L, Piette F, Salom M, Verny M, Vetel J-M, et al. Effect of influenza vaccination of nursing home staff on mortality of residents: a cluster-randomized trial. J Am Geriatr Soc 2009; 57:1580-6; PMID:19682118; http://dx.doi.org/10.1111/j.1532-5415.2009.02402.x
- Llupià A, García-Basteiro AL, Olivé V, Costas L, Ríos J, Quesada S, Varela P, Bayas JM, Trilla A. New interventions to increase influenza vaccination rates in health care workers. Am J Infect Control 2010; 38:476-81; http://dx.doi.org/10.1016/j.ajic.2010.01.013
- Looijmans-Van Den Akker I, Van Delden JJM, Verheij TJM, van der Sande M a. B, Van Essen GA, Riphagen-Dalhuisen J, Hulscher ME, Hak E. Effects of a multi-faceted program to increase influenza vaccine uptake among health care workers in nursing homes: A cluster randomised controlled trial. Vaccine 2010; 28:5086-92; PMID:20580740; http://dx.doi.org/10.1016/j.vaccine.2010.05.003
- Lopes MH, Sartori AMC, Mascheretti M, Chaves TSS, Andreoli RMM, Basso M, Barone AA. Intervention to increase influenza vaccination rates among healthcare workers in a tertiary teaching hospital in Brazil*. Infect Control Hosp Epidemiol 2008; 29:285-6; PMID:18257695; http://dx.doi.org/10.1086/528700
- Nace DA, Perera S, Handler SM, Muder R, Hoffman EL. Increasing influenza and pneumococcal immunization rates in a nursing home network. J Am Med Dir Assoc 2011; 12:678-84; PMID:21450182; http://dx.doi.org/10.1016/j.jamda.2010.05.002
- Nicholson MR, Hayes DM, Bennett AM. Partnering with nursing service improves health care worker influenza vaccination rates. Am J Infect Control 2009; 37:484-9; PMID:19361886; http://dx.doi.org/10.1016/j.ajic.2008.12.004
- Ohrt CK, McKinney WP. Achieving compliance with influenza immunization of medical house staff and students. A randomized controlled trial. JAMA 1992; 267:1377-80; PMID:1740861; http://dx.doi.org/10.1001/jama.1992.03480100083036
- Podczervinski S, Stednick Z, Helbert L, Davies J, Jagels B, Gooley T, Casper C, Pergam SA. Employee influenza vaccination in a large cancer center with high baseline compliance rates: comparison of carrot versus stick approaches. Am J Infect Control 2015; 43:228-33; PMID:25728148; http://dx.doi.org/10.1016/j.ajic.2014.11.025
- Quan K, Tehrani DM, Dickey L, Spiritus E, Hizon D, Heck K, Samuelson P, Kornhauser E, Zeitany R, Mancia S, et al. Voluntary to mandatory: evolution of strategies and attitudes toward influenza vaccination of healthcare personnel. Infect Control Hosp Epidemiol 2012; 33:63-70; PMID:22173524; http://dx.doi.org/10.1086/663210
- Rakita RM, Hagar BA, Crome P, Lammert JK. Mandatory influenza vaccination of healthcare workers: a 5-year study. Infect Control Hosp Epidemiol 2010; 31:881-8; PMID:20653445; http://dx.doi.org/10.1086/656210
- Ribner BS, Hall C, Steinberg JP, Bornstein WA, Chakkalakal R, Emamifar A, Eichel I, Lee PC, Castellano PZ, Grossman GD. Use of a mandatory declination form in a program for influenza vaccination of healthcare workers. Infect Control Hosp Epidemiol 2008; 29:302-8; PMID:18462141; http://dx.doi.org/10.1086/529586
- Rothan-Tondeur M, Filali-Zegzouti Y, Golmard J-L, De Wazieres B, Piette F, Carrat F, Lejeune B, Gavazzi G. Randomised active programs on healthcare workers' flu vaccination in geriatric health care settings in France: the VESTA study. J Nutr Health Aging 2011; 15:126-32; PMID:21365166; http://dx.doi.org/10.1007/s12603-011-0025-5
- Samms D, Reed K, Lee T, Barill S, Branham D. Achieving a corporate goal for influenza vaccination using nurse champions. Am J Infect Control 2004; 32:E7-8; http://dx.doi.org/10.1016/j.ajic.2004.04.011
- Sartor C, Tissot-Dupont H, Zandotti C, Martin F, Roques P, Drancourt M. Use of a mobile cart influenza program for vaccination of hospital employees. Infect Control Hosp Epidemiol 2004; 25:918-22; PMID:15566024; http://dx.doi.org/10.1086/502320
- Seale H, Leask J, Macintyre CR. Awareness, attitudes and behavior of hospital healthcare workers towards a mandatory vaccination directive: two years on. Vaccine 2011; 29:3734-7; PMID:21458607; http://dx.doi.org/10.1016/j.vaccine.2011.03.050
- Shah SI, Caprio M. Availability of trivalent inactivated influenza vaccine to parents of neonatal intensive care unit patients and its effect on the healthcare worker vaccination rate. Infect Control Hosp Epidemiol 2008; 29:309-13; PMID:18462142; http://dx.doi.org/10.1086/527515
- Shannon SC. Community hospitals can increase staff influenza vaccination rates. Am J Public Health 1993; 83:1174-5; PMID:8342732; http://dx.doi.org/10.2105/AJPH.83.8.1174
- Slaunwhite JM, Smith SM, Fleming MT, Strang R, Lockhart C. Increasing vaccination rates among health care workers using unit “champions” as a motivator. Can J Infect Control 2009; 24:159-64; PMID:19891169
- Smedley J, Palmer C, Baird J, Barker M. A survey of the delivery and uptake of influenza vaccine among health care workers. Occup Med (Lond) 2002; 52:271-6; PMID:12181376; http://dx.doi.org/10.1093/occmed/52.5.271
- Smith DR, Van Cleave B. Influenza vaccination as a condition of employment for a large regional health care system. WMJ 2012; 111:68-71; PMID:22616475
- Stuart RL, Gillespie EE, Kerr PG. A pilot study of an influenza vaccination or mask mandate in an Australian tertiary health service. Med J Aust 2014; 200:83-4; PMID:24484101; http://dx.doi.org/10.5694/mja13.10947
- Tannenbaum TN, Thomas D, Baumgarten M, Saintonge F, Rohan I. Evaluation of an influenza vaccination program for nursing home staff. Can J Public Health 1993; 84:60-2; PMID:8500061
- Tapiainen T, Bär G, Schaad UB, Heininger U. Influenza vaccination among healthcare workers in a university children's hospital. Infect Control Hosp Epidemiol 2005; 26:855-8; PMID:16320981; http://dx.doi.org/10.1086/502508
- Thomas DR, Winsted B, Koontz C. Improving neglected influenza vaccination among healthcare workers in long-term care. J Am Geriatr Soc 1993; 41:928-30; PMID:8409179; http://dx.doi.org/10.1111/j.1532-5415.1993.tb06757.x
- Zimmerman RK, Nowalk MP, Lin CJ, Raymund M, Fox DE, Harper JD, Tanis MD, Willis BC. Factorial design for improving influenza vaccination among employees of a large health system. Infect Control Hosp Epidemiol 2009; 30:691-7; PMID:19489716; http://dx.doi.org/10.1086/598343
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702-6; PMID:15033648; http://dx.doi.org/10.1093/aje/kwh090
- Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Res Synth Method 2010; 1:39-65; http://dx.doi.org/10.1002/jrsm.5
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557-60; PMID:12958120; http://dx.doi.org/10.1136/bmj.327.7414.557
- Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol 2008; 8:79; PMID:19036172; http://dx.doi.org/10.1186/1471-2288-8-79
- Winston L, Wagner S, Chan S. Healthcare workers under a mandated H1N1 vaccination policy with employment termination penalty: a survey to assess employee perception. Vaccine 2014; 32:4786-90; PMID:24996124; http://dx.doi.org/10.1016/j.vaccine.2014.06.001
- Edelstein M, Pebody R. Can we achieve high uptakes of influenza vaccination of healthcare workers in hospitals? A cross-sectional survey of acute NHS trusts in England. Epidemiol Infect 2014; 142:438-47; PMID:23672975; http://dx.doi.org/10.1017/S095026881300112X
- Yassi A, Lockhart K, Buxton JA, McDonald I. Vaccination of health care workers for influenza: promote safety culture, not coercion. Can J Public Health 2010; 101 Suppl 1:S41-5; PMID:20629446
- Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors. [Internet]. Oslo: Norwegian Knowledge Centre for the Health Services; 2015 [cited 2015 May 30]. Available from: http://epoc.cochrane.org/epoc-specific-resources-review-authors
- Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med 2002; 21:1575-600; PMID:12111921; http://dx.doi.org/10.1002/sim.1188
- Higgins JP, Green S. Cluster-randomized trials. In: Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration; 2011; pp. 493-498.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629-34; PMID:9310563; http://dx.doi.org/10.1136/bmj.315.7109.629
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008-12; PMID:10789670; http://dx.doi.org/10.1001/jama.283.15.2008
- R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2013. Available from: http://www.R-project.org/
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Statistical Software [Internet] 2010 [cited 2013 May 28]; 36:1-48. Available from: http://www.jstatsoft.org/v36/i03/paper
- Højsgaard S, Halekoh U, Yan J. The R Package geepack for generalized estimating equations. J Statistical Software 2005; 15:1-11