abstract
Rotavirus gastroenteritis accounts for an estimated 130,000 GP consultations and 13,000 hospitalisations for children under 5 y old each year in England and Wales. In July 2013, an oral live attenuated rotavirus vaccine (Rotarix®) was introduced into the UK infant immunisation program as a 2 dose schedule at 2 and 3 months of age. We collected vaccination uptake from October 2013 to March 2015 and laboratory confirmed cases data on children under the age of 5 y from 1 January 2004 to 31 May 2015. The vaccine uptake rates and laboratory confirmed cases were compared to provide evidence of the impact of this vaccination program. Vaccine uptake rates were available from sentinel data with between 91–98% of GP practices in Anglia and Essex providing data every month. These data showed from February 2014 to March 2015 between 90–92% of infants received the recommended 2 doses of Rotarix® each month. The numbers of rotavirus cases reported by laboratories decreased on average by 82% in the post vaccination seasons. The mean number of cases reported in weeks 1–22 for 2004–2013 in Anglia and Essex was 1,318. For the same period in 2014, 256 cases were reported and initial data for 2015 report 226 cases. In the first 5 months 2014 the greatest reduction in cases (89%) was seen in those under 1 yr (who would have been directly affected by vaccination) with case numbers falling to 59 from a mean 537 cases in the equivalent period for 2004–2013. Initially data suggests a 92% reduction in 2015 compared to the same pre-vaccination periods. For those aged 1 to <5 y who would not have been vaccinated, a reduction of 75% was also evident in 2014 and 77% in 2015, suggesting indirect protection in this group. In conclusion, initial results following the introduction of the Rotavirus vaccine clearly indicates a very good uptake of the vaccine and a significant reduction in the numbers of laboratory confirmed cases.
Introduction
Rotavirus is a common cause of acute gastroenteritis in children in England which occurs mostly in winter and early spring each year (January-March).Citation1 In England and Wales it is estimated to cause 130,000 GP consultations and around 13,000 hospitalisations for children under 5 y old each year.Citation2 It has been suggested that 45% of hospital admissions for gastroenteritis in those under-5 can be attributed to rotavirus.Citation1 The estimated annual cost of hospitalisation of cases under 5 in England and Wales in 2007 was ≤8.8 million.Citation3
On 1 July 2013, an oral live attenuated rotavirus vaccine (Rotarix®) was introduced into the UK infant immunisation program as a 2 dose schedule at 2 and 3 months of age.Citation3 Rotarix® contains the G1P[8] strain and has been shown to be 85% effective at protecting against severe rotavirus gastroenteritis in the first 2 y of life.Citation4
We aimed to describe the uptake of rotavirus vaccine and the effect of vaccination on the number of laboratory confirmed cases of rotavirus in children under 5 y in Anglia and Essex. Anglia and Essex includes the counties of Norfolk, Suffolk, Cambridgeshire and Essex. It had an estimated resident population of 4.2 million in 2013, of which around 250,000 where under the age of 5 y Following the implementation of the rotavirus vaccination program, we report on the change in rotavirus laboratory reports in under 5 y olds on the 2 rotavirus seasons following its introduction, providing evidence of the impact of this vaccination program.
Results
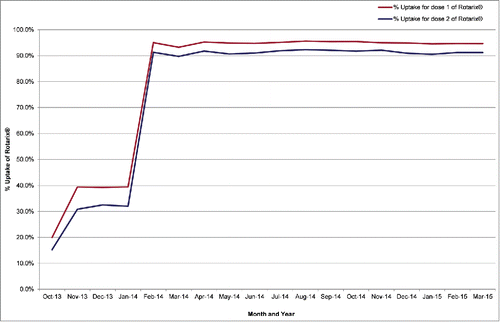
The rotavirus vaccine was introduced into the routine childhood immunisation schedule in July 2013 for infants born on or after 1 May 2013. Uptake rates were available from sentinel data for October 2013 to March 2015 with between 91–98% of GP practices in Anglia and Essex providing data every month. These data show from February 2014 to March 2015 between 90–92% of infants received the recommended 2 doses of Rotarix® each month (). Prior to February 2014 vaccine uptake was under 35% as the first cohorts of children to be routinely scheduled rotavirus vaccine alongside other primary vaccines was from January 2014.
Figure 1. Monthly sentinel rotavirus vaccination uptake data (ImmForm) at 25 weeks of age for 1st and 2nd dose, for Anglia and Essex, October 2013 to March 2015.
We ascertained the number of laboratory confirmed rotavirus cases in children aged under 5 y between 1 January 2004 and 31 May 2015 (). Data show that rotavirus has been highly seasonal with peaks of a comparable magnitude at a similar time each year. Comparing the seasonal peaks, shows a considerable attenuation in the number of cases in the early weeks of 2014 and 2015.
Figure 2. Laboratory reports of rotavirus in children aged 0<5 years, by week, Anglia and Essex, 2004–2015.
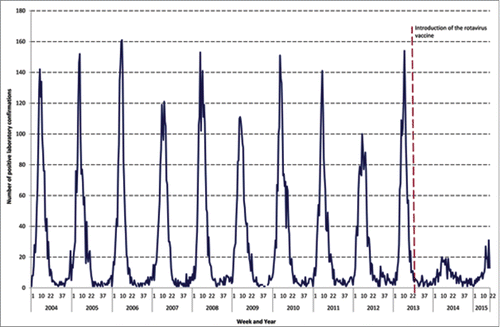
Following the inclusion of rotavirus vaccination in the childhood immunisation schedule in July 2013, the numbers of rotavirus cases reported by laboratories decreased on average by 82% [95% confidence interval (CI):80, 83] in the post vaccination seasons. The mean number of cases reported in weeks 1–22 for 2004–2013 in Anglia and Essex was 1,318. For the same period in 2014, 256 cases were reported and initial data for 2015 report 226 cases, which corresponds to an 81% [95% CI: 78, 83] and 83% [95% CI: 80, 85] reduction respectively compared to the pre-vaccination periods.
Our data show that rotavirus has a characteristic age distribution with the greatest number of cases in those aged under 2 years, with a peak in the number of cases around 12 months of age. In the first 5 months 2014 the greatest reduction in cases (89% [95% CI: 86, 92]) was seen in those under 1 yr (who would have been directly affected by vaccination) with case numbers falling to 59 from a mean 537 cases in the equivalent period for 2004–2013. Initially data suggests a 92% [95% CI: 89, 94] reduction in 2015 compared to the same pre-vaccination periods (). For those aged 1 to <5 y who would not have been vaccinated, a reduction of 75% [95% CI: 71, 78] was also evident in 2014 and 77% [95% CI: 73, 80] in 2015, suggesting indirect protection in this group.
Table 1. Number of laboratory reports of rotavirus in children aged 0 to < 5 y and percentage change by year for weeks 0–22, by age group, Anglia and Essex.
As we used laboratory data we were not able to reliably assign cases to a geographical area such as local authority or postcode, however our results showed that the reductions in the number of cases have come from all laboratories across Anglia and Essex.
Discussion
The rotavirus vaccination was introduced into the UK infant immunisation program in July 2013. The program in Anglia and Essex appears to have been well accepted with data showing uptake between 90–92% from February 2014 to March 2015. Our study provides an indication that the vaccination program has resulted in a significant reduction in the weekly number of rotavirus cases in children aged 0 to <5 y in both the first and second years following its implementation.
In Anglia and Essex, rotavirus incidence showed a clear seasonal trend with the highest number of cases between January and April each year. In line with this seasonality our results show that rotavirus activity fell significantly in the early months of both 2014 and 2015. The reduction was observed across all under 5 age groups, including those not covered by the updated immunisation schedule, suggesting both direct and indirect protection through vaccination.
Our findings support previous reports of high incidence of rotavirus gastroenteritis during the first 2 y of life and the need for long-term protection induced by rotavirus vaccination.Citation4
Prior to inclusion in the UK infant immunisation schedule, trials showed high efficacy for the rotavirus vaccine. Findings from a meta-analysis pooling results from 6 trialsCitation5 and 40,631 participants showed that Rotarix® prevented 86% of severe rotavirus cases compared to placebo (RR 0.14 95%CI 0.07 – 0.26) in infants under one year in low mortality (WHO strata 1 and 2) countries. Despite these results it is well known that vaccine effectiveness during routine use can differ from the observed efficacy under ideal conditions of a trial. The fact that our study showed comparable results is promising for on-going ‘real-world’ protection of children from rotavirus.
Following the introduction of the rotavirus vaccine in to the UK infant immunisation program, early findings showed a significant reduction in rotavirus cases in the 2014 rotavirus season along with a reduction in presentations of gastroenteritis to healthcare providers.Citation6,7 Lower rotavirus activity was also demonstrated in the Netherlands during the same season in the absence of a national rotavirus vaccination program.Citation8 Suggested factors contributing to the decrease seen in the Netherlands were a mild winter, low birth rate, high rotavirus incidence in previous years in the Netherlands, and rotavirus vaccination programs in neighboring countries.Citation8 While England did experience a mild winter during 2013/14 Atchison et al could not identify additional factors to explain the significant decline in rotavirus disease in England other than the infant immunisation program.Citation6 Initial virological surveillance data from the Dutch Working Group for Clinical Virology (NWKV) for 2015 indicate that they have seen a significant increase in the number of positive rotavirus cases compared to 2014,Citation9 while preliminary data for England does not show a similar increase.Citation10 Our early data for 2015 suggests that the rotavirus vaccinations has continued to have a positive effect in reducing the burden of rotavirus disease, with a 83% reduction of cases in 2015 compared to the 2004–2013 pre-vaccination seasons following a 81% reduction in 2014.
While the rotavirus vaccination program has had an impact on reducing the number of cases during the winter season, where the seasonal peak occurs, the number of cases during the rest of the year post-vaccination implementation appears to be relatively similar to the pre-vaccination period. Currently only one full years' worth of data post-vaccination implementation is available for 2014, and due to the relatively small numbers of cases that occur out of the winter season it is difficult to determine what the impact has been. Further investigation is needed to determine the impact of the vaccine program on rotavirus cases throughout the year and not just during the winter season.
The strengths of our study include the fact that our time series analysis included data spanning an 11 and a half year period. This allowed us to take into account the highly seasonal trend of laboratory reported cases of rotavirus in young children. These initial results have been published in a timely manner to inform public health professionals, we await confirmation of these trends from data in following post-vaccination years.
An additional strength of the study is that we used laboratory confirmed detection of cases as our outcome measure which would have minimised the chance of reporting bias. However, it is possible that this method could potentially have been affected by testing bias as medical professionals may have been less likely to test for rotavirus following the introduction of vaccination. National reports on norovirus, which is predominantly a winter pathogen causing gastroenteritis like rotavirus, reported a 47% reduction in the 2013/14 season (week 27 2013 to week 26 2014) following the implementation of the rotavirus vaccine program compared to the average for the same period in the seasons 2007/2008 to 2011/2012. A 69% reduction was seen in national rotavirus laboratory reports for the same period in 2013/14 compared to the 10 season average for the same period in the seasons 2003/2004 to 2012/2013.Citation11 This may suggest that testing bias has contributed to the reduction in both norovirus and rotavirus laboratory confirmations. In addition, by using this laboratory data, it is not possible to infer the impact of vaccination on clinical outcomes.
The laboratory confirmed data used in this analysis is from the Public Health England (PHE) Second generation surveillance system (SGSS) which became the primary source of infectious disease laboratory surveillance data for England from the 1st December 2014. Prior to this date an alternative reporting system was in place. All historic laboratory reports from the previous system have been incorporated into SGSS to ensure data completeness and allow trends to be analyzed. Laboratory reports are subject to reporting delay with the most recent data reported likely to increase as more laboratory reports are received, and as a dynamic system reports can be updated as additional information is submitted. SGSS provides information on where samples were submitted from but it does not include information on hospitalisation therefore this was not explored as part of this study.
Another limitation of our study was that vaccination uptake was based on sentinel data collection systems, which means that not all practices report their vaccine uptake; however this was the best data source available for analysis at this early stage. Whether practices with lower vaccine coverage are less likely to take part in the sentinel data collection is unknown. As the vaccine data collection involves automatic data extractions via general practice IT software suppliers it is possible that practices would be less likely to take part due to IT software functionality issues rather than a decision to opt-out of submitting data. In conclusion, the introduction of the rotavirus vaccination into the UK childhood immunisation program has protected a significant number of children from rotavirus and is likely to have reduced the burden of gastroenteritis in under 5 y olds in the UK.
Methods
We undertook a time series analysis in Anglia and Essex from 1 January 2004 to 31 May 2015. We collected vaccination uptake and laboratory confirmed case data on children under the age of 5 y and compared vaccine uptake rates to laboratory confirmed cases to provide evidence of the impact of this vaccination program.
Rotavirus vaccination uptake data was obtained from ImmForm. ImmForm is a website used to collect data on vaccine uptake for immunisation programmes in England. Monthly automatic data uploads from sentinel GP practices are submitted to the ImmForm website and monitored, validated and analyzed by PHE before being published on a regular basis.Citation12 Rotavirus vaccination data is not cumulative, but based on calendar month. The denominator for vaccine uptake of both one and 2 doses of Rotarix® is the number of infants in a GP practice who, in the survey month, reach 25 weeks of age. The numerator is the number of infants in the denominator who received a) a first dose and b) a second dose of Rotarix® from 6 weeks of age up to 24 weeks of age, including vaccinations given by other healthcare providers.Citation12 As it is a sentinel surveillance program not all practices provide data,
The vaccine uptake data included in this analysis covers the period from October 2013 to March 2015 Data after March 2015 has yet to be validated and published by PHE. Data prior to October 2013 is not presented as the first monthly cohort eligible by age for the vaccine would be children evaluated in November 2013, as they were aged less than 15 weeks on 1st July when the program started. The first cohort of children to be routinely scheduled rotavirus vaccine alongside other primary vaccines is from January 2014. Vaccinations prior to this point would have been opportunistically or via a specific appointment.
The laboratory confirmed data used in this analysis is from the PHE Second generation surveillance system (SGSS) which became the primary source of infectious disease laboratory surveillance data for England from the 1st December 2014. Prior to this date an alternative reporting system was in place. All historic laboratory reports from the previous system have been incorporated into SGSS to ensure data completeness and allow trends to be analyzed. Data for the number of laboratory confirmed rotavirus cases aged 0 <5 y was extracted from the SGSS. Positive laboratory tests from all laboratories across Anglia and Essex, reported since 1 January 2004, were included. The most widely available method for detection of rotavirus antigen in stool is an enzyme immunoassay (EIA) directed at an antigen common to all group A rotaviruses. Several commercial EIA kits are available that are inexpensive, easy to use, rapid, and highly sensitive (approximately 90–100%), making them suitable for rotavirus surveillance and clinical diagnosis.
Abbreviations
| EIA | = | Enzyme Immuno Assay |
| GP | = | General Practitioner |
| PHE | = | Public Health England |
| SGSS | = | Second Generation Surveillance System |
| UK | = | United Kingdom. |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Harris JP, Jit M, Cooper D, Edmunds WJ. Evaluating rotavirus vaccination in England and Wales. Part I. Estimating the burden of disease. Vaccine 2007 May 16; 25(20):3962–70; http://dx.doi.org/10.1016/j.vaccine.2007.02.072
- Djuretic T, Ramsey M, Gay N, Wall P, Ryan M, Fleming D. An estimate of the proportion of diarrhoeal disease episodes seen by general practitioners attributable to rotavirus in children under 5 yrs of age in England and Wales. Acta Paediatr Oslo Nor 1992 Suppl. 1999 Jan; 88(426): 38–41
- Joint letter from DH, PHE and NHS England (30th April 2013). Important changes to the national immunisation programme in 2013/14: introduction of rotavirus vaccination for babies at 2 and 3 months. Available at:https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/193055/130429_Rotavirus_tripartite_letter_FINAL.pdf [accessed 13/05/2014]
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised double-blind controlled study. Lancet 2007 Nov 24; 370(9601):1757–63; http://dx.doi.org/10.1016/S0140-6736(07)61744-9
- Soares-Weiser K, MacLehose H, Bergman H, Ben-Aharon I, Nagpal S, Goldberg E, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No.: CD008521. http://dx.doi.org/10.1002/14651858.CD008521.pub3. Available at http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008521.pub3/pdf (accessed 26 Jan 2016)
- Atchison C, Collins S, Brown D, Ramsay ME, Ladhani S. Reduction in rotavirus disease due to the infant immunisation programme in England; evidence from national surveillance. J Infect 2015 July; 71(1):128–46; http://dx.doi.org/10.1016/j.jinf.2015.01.007
- Bawa Z, Elliot AJ, Morbey RA, Ladhani S, Cunliffe NA, O'Brien SJ, Regan M, Smith GE. Assessing the Likely Impact of a Rotavirus Vaccination Program in England: The Contribution of Syndromic Surveillance. Clini Infect Dis 2015 July; 61(1):77–85
- Hahne S, Hooiveld M, Vennema H, van Ginkel A, de Melker H, Wallinga J, van Pelt W, Bruijning-Verhagen P. Exceptionally low rotavirus incidence in the Netherlands in 2013/14 in the absence of rotavirus vaccination. Euro Surveill 2014; 19(43) (pii):20945; PMID:25375899; http://dx.doi.org/10.2807/1560-7917.ES2014.19.43.20945
- Rijksinstituut voor Volksgezondheid en Milieu (RIVM). [The National Institute for Health and Environment]. Weekly virological reports. Dutch. Available at: http://www.rivm.nl/Onderwerpen/V/Virologische_weekstaten/Rapportages/Besloten_rapportages_virologische_weekstaten
- Public Health England. PHE Monthly National Norovirus and Rotavirus Report.. 12 June 2015. Available at: https://www.gov.uk/government/publications/norovirus-national-update
- Public Health England. PHE Monthly National Norovirus and Rotavirus Report. 10 July 2014. Available at: https://www.gov.uk/government/publications/norovirus-national-update
- Public Health England. Rotavirus infant immunisation programme 2014/15: Vaccine uptake report on the temporary sentinel data collection for England. June 2015. Available at: https://www.gov.uk/government/publications/rotavirus-vaccine-uptake-report-for-england
