Abstract
The current recommendation for human papillomavirus (HPV) vaccination in the United States is for 3 doses to be administered over a 6 month period. In April 2014, the World Health Organization (WHO) recommended adoption of a 2-dose schedule, with doses spaced a minimum of 6 months apart, for teens who begin the series before age 15. We analyzed data from the 2013 National Immunization Survey-Teen to examine the timing of second and third dose receipt among US adolescents. All analyses were restricted to adolescents age 13–17 y who had adequate provider data. The Wilcoxon–Mann–Whitney test measured differences in time to receive vaccine doses among demographic and socioeconomic groups. Logistic regression identified socioeconomic characteristics associated with receiving the second dose of HPV vaccine at least 6 months after the first dose. The median time for teens to receive the second dose of HPV vaccine was 2.6 months after the first dose, and the median time to receive the third dose was 4.9 months after the second dose. Minority teens and teens living below the poverty level took significantly longer to receive doses. Among teens that initiated the HPV vaccine series before age 15 y, 28.6% received the second dose at least 6 months after the first dose. If these teens, who met the WHO criteria for up-to-date HPV vaccination, were classified as having completed the vaccination series, overall coverage in the US would increase 3.9 percentage points, with African American and Hispanic teens having the greatest increases in coverage.
Introduction
The Advisory Committee on Immunization Practices (ACIP) recommends human papillomavirus (HPV) vaccination for children age 11 or 12 y; vaccination is also recommended for adolescent girls age 13–26 y and adolescent boys age 13–21 y who have not previously received or completed the HPV vaccine series.Citation1-3 The recommended schedule is a 3-dose series, with the second dose administered 2 months after the first and the third dose administered 6 months after the first.Citation1 While bivalent, quadrivalent and 9-valent vaccines are licensed for females, only quadrivalent and 9-valent vaccines are licensed for boys.Citation4
US HPV vaccine uptake has been much slower than that of other vaccines recommended for adolescents, and HPV vaccination coverage is well below the Healthy People 2020 objective of 80% for girls and boys age 13 – 15.Citation5 In 2014, US HPV vaccination coverage for teens age 13–17 was 60.0% for at least 1 dose and 39.7% for 3 doses among girls and 41.7% for 1 dose and 21.6% for 3 doses among boys.Citation6
In 2014, the Strategic Advisory Group of Experts (SAGE) and the World Health Organization (WHO) recommended a 2-dose schedule of vaccine with an interval of at least 6 months between doses for girls who initiate the vaccine series before 15 y of age. A 3-dose schedule, identical to the US schedule, is recommended for girls 15 y of age or older. At this time, there is no WHO HPV vaccine recommendation for boys.Citation7,8 In its recommendation, WHO noted that, in addition to increasing vaccination coverage, this reduced dose schedule would result in cost savings, and programmatic advantages. Citation7
This study has 2 primary goals. First, it examines the current timing of second and third dose receipt among US adolescents, with comparisons made among groups of different demographics and socioeconomic status. Second, it determines the percentage of teens who received their second dose of HPV vaccine 6 months or more after the first dose and would be considered up-to-date according to the 2014 WHO recommendations.
Methods
Data from the 2013 National Immunization Survey-Teen (NIS-Teen), a cross-sectional, random-digit-dial telephone survey were analyzed. The NIS-Teen survey, administered every year since 2006 to households with an adolescent 13–17 y of age, uses landline and, since 2011, cell phones, and takes place in the 50 states, District of Columbia, and select urban areas. Sampling weights are adjusted to account for the dual-frame sampling, non-response rates, and overlap of cellphone and landline users. After survey participation and when consent is provided, a survey is sent to the teen's medical provider(s) to confirm dates and types of vaccination. Citation9,10
All analyses were restricted to adolescents (boys and girls) age 13–17 y who had adequate provider data compared with parental report (n = 18,264).Citation10 There was no distinction made between bivalent or quadrivalent vaccine received. All provider-confirmed doses of HPV vaccine were counted, regardless of whether they occurred before or after the interview date. Outcomes were determined by select demographic and socioeconomic characteristics, including age at vaccine initiation (before age 15 y, on or after age 15 y); sex; race (including Hispanic ethnicity); and economic status (at or above the poverty level, below poverty level). Race of the adolescent was self-reported by the survey respondent. Adolescents were classified as living below the federal poverty level if their family's total income was less than the federal poverty level specified for their family size and number of children age <18 y.
In order to evaluate the timing of HPV vaccination, the median interval between receipt of first and second dose of vaccine was calculated among all teens who had received at least 2 doses of HPV vaccine (n = 6,666) and the median interval between receipt of second and third dose of vaccine was calculated among all teens who had received at least 3 doses of HPV vaccine (n = 4,911). The Wilcoxon–Mann–Whitney test, adjusted for complex survey weighting measures, measured differences in time to receive vaccine doses, among demographic and socioeconomic groups.Citation11
The proportion of adolescents receiving the second dose of HPV vaccine at least 6 months after the first dose was assessed among adolescents who initiated the vaccine series before age 15 y and received at least 2 doses (n = 5,629). Logistic regression was performed to identify socioeconomic characteristics associated with receiving the second dose of HPV vaccine at least 6 months after the first dose.
The impact of including vaccinees who are up-to-date under WHO recommendations in the assessment of US HPV vaccination coverage was also considered. Original up-to-date HPV coverage was calculated as the percentage of all teens (n = 18,264) who had received 3 doses of HPV vaccine as recommended by ACIP. Up-to-date HPV vaccination coverage was recalculated among all teens with the numerator including both adolescents who met the criteria for the original up-to-date definition and adolescents who initiated the series before age 15 y and received at least 2 doses with the second dose at least 6 months after the first dose.
Significance level for all tests was set at p < 0.05. All analyses were performed using SAS 9.3 (SAS Institute) and SUDAAN 11.0.1 (RTI International).
Results
Among teens who received at least 2 doses of HPV vaccine, the median time between the first and second dose was 2.6 months; however, the distribution of time to receive the second dose of vaccine was highly right-skewed, with the 95th percentile at 19.5 months. (). Teens who started the vaccine series on or after age 15 y had a shorter interval between doses than teens who received the first HPV dose before age 15 y (2.3 vs 2.8 months, p < 0.01). While the time interval between the first and second dose was similar for boys and girls, non-white teens and teens of lower socioeconomic status had longer intervals (p < 0.01 for all groups).
Table 1. Interval between first and second dose of HPV vaccination for teens age 13–17 y, National Immunization Survey-Teen, United States, 2013.
The time between the second and third doses showed similar patterns by race, age, and socioeconomic status; however, boys took less time to receive the third dose (p = 0.03) (). For teens who started the series before age 15 y, the median interval was 5.0 months compared with 4.2 months for those age 15 y or older (p < 0.01). Compared with non-Hispanic white teens (4.7 months), the time interval between the second and third doses was longer for African American (6.5 months, p<0.01) and Hispanic teens (5.2 months, p = 0.03), although it was similar for teens of other races. Teens living below the poverty level also took longer time to receive the third dose than teens living at or above the poverty level (p < 0.01). shows the median time to receive the entire vaccine series by socioeconomic and demographic groups.
Figure 1. Median time to complete the HPV vaccination series among teens age 13–17 y. National Immunization Survey-Teen, United States, 2013. *Race of the adolescent was self-reported by the survey respondent. Adolescents of Hispanic ethnicity may be of any race. +Adolescents were classified as living below the federal poverty level if their family's total income was less than the federal poverty level specified for their family size and number of children age <18 y.
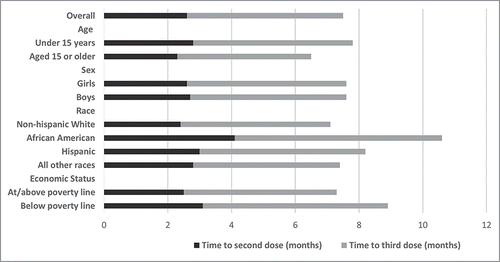
Table 2. Interval between the second and third dose of HPV vaccination for teens age 13–17 y, National Immunization Survey-Teen, United States, 2013.
Among adolescents who initiated the HPV vaccine series before age 15 y and received at least 2 doses, 28.6% received their second HPV dose 6 or more months after the first ( and ). African American teens and Hispanic teens were more likely to receive the second HPV vaccine dose at least 6 months after the first dose compared to non-Hispanic white teens (OR: 2.62 and 1.61, respectively, p < 0.01 for both groups), as were teens who lived below the poverty level (OR: 1.51, p < 0.01) ().
Figure 2. Interval (months) between first and second dose of HPV vaccine among teens who began the HPV vaccination series before age 15 y (n = 5,625), National Immunization Survey-Teen, United States, 2013.
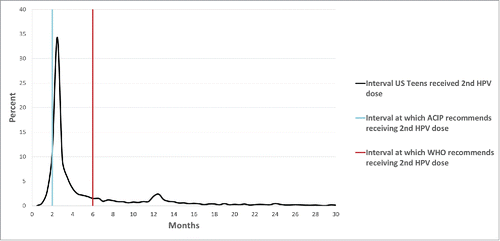
Table 3. Factors associated with an interval of greater than 6 months between first and second dose of HPV vaccination, among teens initiating vaccination before age 15 y (n = 5,629), National Immunization Survey-Teen, United States, 2013
Overall, 25.1% (95% CI: 23.9% – 26.3%) of teens received 3 doses of HPV vaccine as recommended by current ACIP HPV guidelines (). If teens who were not considered up-to-date with HPV vaccine by ACIP guidelines, but who completed the vaccination series according to WHO guidelines were included in coverage estimates, overall US HPV vaccine coverage would increase to 29.0% (95% CI: 27.7% – 30.3%), with minority teens showing the greatest increases in coverage ().
Table 4. Among teens 13-17 y, comparison of HPV vaccine coverage according to ACIP recommendations alone versus coverage when those up-to-date by WHO recommendations are also included.* National Immunization Survey-Teen, United States, 2013.
Discussion
Our analysis of national survey data from the 2013 NIS-Teen found that the majority of teens in the United States who initiated HPV vaccination received vaccine doses on a schedule consistent with ACIP recommendations. Of those who had only received 2 doses and initiated the series before age 15 y, 28.6% received doses at least 6 months apart. If these teens, who met the WHO criteria for up-to-date HPV vaccination, were included in current US assessments of HPV vaccination coverage, overall coverage would increase 3.9 percentage points.
There was variation among teens in the time to receive vaccine doses. We found that teens living below the poverty level received their second and third doses of HPV vaccine at longer time intervals than those who are living at or above the poverty level. Teens living below the poverty level were also more likely to receive their second dose of HPV vaccine more than 6 months after the first. We also found that African American and Hispanic teens were more likely than white teens to have received the second dose of vaccine after 6 months had passed; these teens took longer than non-Hispanic white teens to receive both second and third doses of vaccine. Of note, the overall median intervals between doses in our analysis were similar to those reported by Dorell et al. using the 2008–2009 NIS Teen data, which showed that the median time between first and second HPV doses was 2.3 months (compared to our 2.6 months) and that median time between second and third dose was 4.3 months (similar to our 4.9 months).Citation12
Although a 2-dose schedule has been recommended by WHO for reasons including possible programmatic and coverage advantages, it is acknowledged that there are gaps in knowledge about this schedule.Citation7,8 Current evidence shows non-inferior immunogenicity of a 2-dose schedule (given 6 months apart) in children aged 9–13, 9–10, and 9–14 compared with a 3-dose schedule in young adults aged 15 and older.Citation13-16 Much of these data are on the bivalent vaccine, which is not the vaccine most frequently used in the US. In addition, post-licensure quadrivalent HPV vaccine effectiveness data suggest similar protection from such 2-dose and 3-dose schedules against genital warts.Citation17 Other gaps in knowledge include whether long-term efficacy will differ between 2- and 3-dose schedules. Of note, at this time, there are no available data on 2-dose schedules with the 9-valent HPV vaccine, which was recommended for use in the United States by the ACIP in February 2015.Citation4
It is important to note that WHO recommended a 2-dose schedule in part because it is assumed that a 2-dose schedule is more cost-effective than 3-dose regimens, but this is based on assumptions about duration of protection.Citation7 Models based on levels of coverage higher than those currently achieved in the United States have found that there are few health benefits to having a third dose if a 2-dose schedule confers protection for 20 y; however, the 2-dose schedule may not be cost-effective if 2 doses provide protection for less than 10 y.Citation18,19
We evaluated the increase in coverage estimates that would result from classifying some people who only received 2 doses of HPV vaccine as fully vaccinated. This analysis has several limitations. First, this study is cross-sectional, and only reflects data from one year of data collection. Therefore, the numbers here do not reflect overall trends in vaccination rates, and may not be representative of current vaccination. Secondly, we cannot assess whether or not a reduced dose schedule would facilitate vaccine delivery or if it would increase HPV vaccine initiation in the United States. If a 2-dose schedule was adopted in the United States, a variety of programmatic issues would need to be addressed, including the education of healthcare providers and parents and the updating of office-based systems in order to facilitate scheduling healthcare visits at least 6 months in advance. Of note, in 2014, only 60.0% of girls and 39.7% of boys have received at least 1 dose of HPV.Citation9 Since most teens in the United States who initiate HPV vaccination complete the series, it will be necessary to continue addressing barriers to the initiation of HPV vaccinations, regardless of the HPV vaccinations schedule. There are multiple factors contributing to low HPV vaccine coverage in the United States, including parental attitudes toward HPV vaccination, missed opportunities for providers to vaccinate children, and lack of provider recommendations for vaccinations.Citation2,9 Even though changing the current HPV vaccination schedule could have programmatic advantages, it would not address any other of these known issues surrounding failure to initiate HPV vaccination. As a lack of strong provider recommendation has been consistently linked with parents not initiating the HPV vaccination series for their child, it is critical that pediatricians continue to encourage uptake of this vaccine if nationwide coverage rates are to improve.Citation20,21
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent human papillomavirus vaccine: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 2007; 56(Rr-2):1-24; PMID:17380109
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JA Jr, Unger ER; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(Rr-05):1-30; PMID:25167164
- Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males–Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60(50):1705-8; PMID:22189893
- Petrosky E, Bocchini JA Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, Unger ER, Markowitz LE; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015; 64(11):300-4; PMID:25811679
- Office of Disease Prevention and Health Promotion, US. Department of Health and Human Services. Healthy People 2020 Topics and Objectives: Sexually Transmitted Diseases. 2014 [ cited 2015 30 March 2015]
- Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Singleton JA, Curtis CR, MacNeil J, Markowitz LE, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2014. MMWR Morb Mortal Wkly Rep 2015; 64(29):784-92; PMID:26225476; http://dx.doi.org/10.15585/mmwr.mm6429a3
- World Health Organization. Meeting of the strategic advisory group of experts on immunization, April 2014 – conclusions and recommendations. Wkly Epidemiol Rec 2014; 89(21):221-36; PMID:24864348
- World Health Organization. Human papillomavirus vaccines: WHO position paper, October 2014. Wkly Epidemiol Rec 2014; 89(43):465-91; PMID:25346960
- Curtis CR, Dorell C, Yankey D, Jeyarajah J, Chesson H, Saraiya M, Gold R, Dunne EF, Stokley S; Centers for Disease Control and Prevention (CDC). National human papillomavirus vaccination coverage among adolescents aged 13–17 years-National Immunization Survey–teen, United States, 2011. MMWR Surveill Summ 2014; 63 Suppl 2:61-70; PMID:25208260
- US. Department of Health and Human Services (DHHS), National Center for Health Statistics. The 2013 National Immunization Survey - Teen. 2014, Hyattsville, MD: Centers for Disease Control and Prevention
- Natarajan S, Lipsitz SR, Fitzmaurice GM, Sinha D, Ibrahim JG, Haas J, Gellad W. An extension of the Wilcoxon Rank-Sum test for complex sample survey data. J R Stat Soc Ser C Appl Stat 2012; 61(4):653-64; PMID:23913985; http://dx.doi.org/10.1111/j.1467-9876.2011.01028.x
- Dorell CG, Stokley S, Yankey D, Markowitz LE. Compliance with recommended dosing intervals for HPV vaccination among females, 13–17 years, National Immunization Survey-Teen, 2008–2009. Vaccine 2012; 30(3):503-5; PMID:22119587; http://dx.doi.org/10.1016/j.vaccine.2011.11.042
- Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Behre U, et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum Vaccin Immunother 2014; 10(5):1155-65; PMID:24576907; http://dx.doi.org/10.4161/hv.28022
- Dobson SM, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al. Immunogenicity of 2 doses of hpv vaccine in younger adolescents vs 3 doses in young women: A randomized clinical trial. JAMA 2013; 309(17):1793-802; PMID:23632723; http://dx.doi.org/10.1001/jama.2013.1625
- Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, González P, Solomon D, Jiménez S, Schiller JT, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst 2011; 103(19):1444-51; PMID:21908768; http://dx.doi.org/10.1093/jnci/djr319
- Lazcano-Ponce E, Stanley M, Muñoz N, Torres L, Cruz-Valdez A, Salmerón J, Rojas R, Herrero R, Hernández-Ávila M. Overcoming barriers to HPV vaccination: non-inferiority of antibody response to human papillomavirus 16/18 vaccine in adolescents vaccinated with a two-dose vs. a three-dose schedule at 21 months. Vaccine 2014; 32(6):725-32; PMID:24355090; http://dx.doi.org/10.1016/j.vaccine.2013.11.059
- Blomberg M, Dehlendorff C, Sand C, Kjaer SK. Dose-related differences in effectiveness of human papillomavirus vaccination against genital warts: A nationwide study of 550 000 young girls. Clin Infect Dis 2015; 61(5):676-82; PMID:25944340; http://dx.doi.org/10.1093/cid/civ364
- Laprise JF, Drolet M, Boily MC, Jit M, Sauvageau C, Franco EL, Lemieux-Mellouki P, Malagón T, Brisson M. Comparing the cost-effectiveness of two- and three-dose schedules of human papillomavirus vaccination: a transmission-dynamic modelling study. Vaccine 2014; 32(44):5845-53; PMID:25131743; http://dx.doi.org/10.1016/j.vaccine.2014.07.099
- Jit M, Choi YH, Laprise JF, Boily MC, Drolet M, Brisson M. Two-dose strategies for human papillomavirus vaccination: how well do they need to protect? Vaccine 2014; 32(26):3237-42; PMID:24726246; http://dx.doi.org/10.1016/j.vaccine.2014.03.098
- Rahman M, Laz TH, McGrath CJ, Berenson AB. Provider recommendation mediates the relationship between parental human papillomavirus (HPV) vaccine awareness and HPV vaccine initiation and completion among 13- to 17-year-old US adolescent children. Clin Pediatr (Phila) 2015; 54(4):371-5; PMID:25238779; http://dx.doi.org/10.1177/0009922814551135
- Dorell C, Yankey D, Strasser S. Parent-reported reasons for nonreceipt of recommended adolescent vaccinations, national immunization survey: teen, 2009. Clin Pediatr (Phila) 2011; 50(12):1116-24; PMID:21856964; http://dx.doi.org/10.1177/0009922811415104
