abstract
Human enterovirus 71 (EV71) is a causative agent of hand, foot, and mouth disease (HFMD). In a previous phase III trial in children, a human diploid cell-based inactivated EV71 vaccine elicited EV71 specific immune responses and protection against EV71 associated HFMD. This study aimed to assess the factors influencing the severity of adverse events observed in this previous trial. This was a randomized, double-blinded, placebo-controlled, phase III clinical trial of a human diploid vaccine carried out in 12,000 children in Guangxi Zhuang Autonomous Region, China (ClinicalTrials.gov: NCT01569581). Solicited events were recorded for 7 days and unsolicited events were reported for 28 days after each injection. Age trend analysis of adverse reaction was conducted in each treatment group. Multiple logistic regression models were built to identify factors influencing the severity of adverse reactions. Fewer solicited adverse reactions were observed in older participants within the first 7 days after vaccination (P < 0.0001), except local pain and pruritus. More severe adverse reactions were observed after the initial injection than after the booster injection. Serious cold or respiratory tract infections (RTI) were observed more often in children aged 6–36 months than in older children. Only the severity of local swelling was associated with body mass index. Children with throat discomfort before injection had a higher risk of serious cold or RTI. These results indicated that the human diploid cell-based vaccine achieved a satisfactory safety profile.
Introduction
Hand, foot, and mouth disease (HFMD) is an acute viral skin disease primarily observed in children under the age of 5.Citation1,2 The symptoms of HFMD include low fever, followed by blistering and ulceration of the mouth, and rashes on the hands and feet. HFMD can also lead to complications such as central nervous system (CNS) injury and cardiopulmonary failure.Citation2-5 HFMD is caused by polioviruses, coxsackieviruses, echoviruses, and enteroviruses.Citation1,6 Although HFMD outbreaks are more commonly associated with coxsackievirus 16 (CA16),Citation5 enterovirus 71 (EV71) has been reported to be significantly associated with most global outbreaks, particularly with outbreaks with high mortality,Citation7-16 as was also observed in China.Citation1,6,7,14,15 The number of reported HFMD cases has sharply increased in almost all Chinese provinces since the first EV71 infection-related outbreak in Fuyang in 1995.Citation1,6,8,14,15 In addition to HFMD, EV17 infection can also cause herpangina, aseptic meningitis, febrile illness, viral exanthema, and airway infection.Citation2,16,17
As no pharmacological intervention has been found to prevent and control EV71, a vaccine is urgently sought to control EV71 epidemics.Citation18-22 Several candidate EV71 vaccines have been developedCitation10,23-25 and clinical trials have been carried out around the globe.Citation9, 13, 26-34 A 400U vero-based vaccine,Citation33 a 320U vero cell-based vaccine,Citation19 and a 100U human diploid cell-based vaccineCitation32 have demonstrated good efficacy and adequate safety in mainland China. The latter was developed by the Institute of Medical Biology, Chinese Academy of Medical Sciences and cultured in a human diploid cell line (KMB17 stain).Citation32,34 This inactivated EV71 vaccine was prepared from a genotype C4 strain isolated during a pandemic in Fuyan, China, in 2008.Citation35 In a randomized, double-blinded, placebo-controlled, phase III clinical trial of this vaccine in 12,000 children in the Guangxi Zhuang Autonomous Region, China (ClinicalTrials.gov: NCT01569581) neutralizing antibodies against EV71 were observed in all subjects, while evidence of protection against HFMD caused by other enteroviruses such as CA16 was not observed.Citation32
As no EV71 vaccine has been widely used in the population and since the vaccine recipients are young children, comprehensive safety assessments and targeted vaccination guidance are essential.Citation10,18,22 Within 7 days of immunization in the previously described trial, systemic adverse events occurred at a higher frequency in vaccine recipients compared to controls.Citation32 Commonly reported adverse events included fever, diarrhea, nausea, and vomiting. The frequency of local adverse events (including pain, redness, swelling, and itching at the injection site) was also higher in the vaccine group compared to the placebo group.Citation32 Therefore, we performed a comprehensive analysis of the safety data from this phase III trial to further probe the safety profile of this efficacious vaccine.
Results
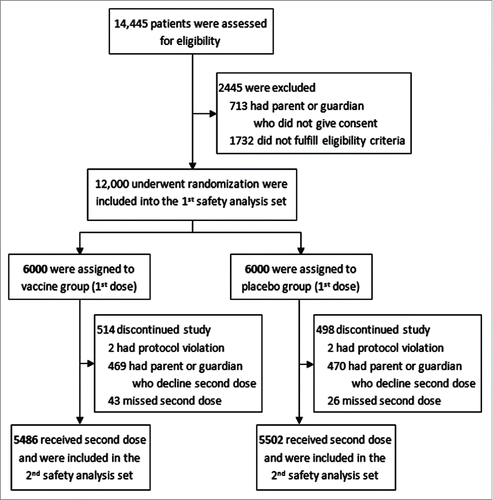
Of the 14,445 children assessed for eligibility, 12,000 (83.1%) were enrolled and randomly assigned to receive the vaccine (n = 6000) or placebo (n = 6000). Among them, 10,988 (91.6%) participants received the second dose of vaccine (n = 5486) or placebo (n = 5502). Therefore, the first safety set included 12,000 participants, and the second safety set included 10,988 participants (). Among the 6000 participants who received the first vaccine injection, there were 3151 (52.52%) males and 2849 (47.48%) females. Among the 6000 participants who received the first placebo injection, there were 3099 (51.65%) males and 2901 (48.35%) females. There were 514 participants in the vaccine group (protocol violation, n = 2; parents or guardians declined the second dose, n = 469; missed the second dose, n = 43) and 498 in the placebo group (protocol violation, n = 2; parents or guardians declined the second dose, n = 470; missed the second dose, n = 26) who discontinued the study; they were excluded from the analyses ().
Figure 1. Randomized and safety analysis set. A total of 14,445 children, 6–71 months of age, were assessed for eligibility. 12,000 eligible participants, who were randomly assigned to received vaccine or placebo, were defined as the 1st safety analysis population. 5486 of vaccine group and 5502 of placebo group were injected with successive second dose and eligibly included in the per-protocol analysis were defined as the 2nd safety analysis population.
Safety profile
Between March 2012 and February 2013, although more adverse events were found in the vaccine group (3878 (64.63%) vs. 3759 (62.65%), P = 0.025), significantly fewer serious adverse events were reported in the vaccine group than in the placebo group (68 (1.13%) vs. 125 (2.08%), P < 0.001). Within 7 days after each injection, significantly higher incidences of solicited events, systemic events, and local events were observed in the vaccine group compared to the placebo group (all P < 0.001) ().
Table 1. Safety profile.
The incidence of adverse reactions associated with injection was significantly higher in the vaccine group (2195 (36.58%) vs. 1584 (26.40%), P < 0.001). Two participants in each group reported serious adverse reactions. Within the first week after injection, significantly higher incidences of solicited reactions, systemic reactions, and local reactions were found in the vaccine group compared to the placebo group (all P < 0.001) ().
Unsolicited adverse events within 28 days were similar in 2 treatment groups (2507 (41.78%) vs. 2563 (42.72%), P = 0.309). Unsolicited adverse reactions within 28 days were also more frequent in the vaccine group compared to the placebo group (122 (2.03%) vs. 89 (1.48%), P = 0.026). More participants caught HFMD in the placebo group during the whole study (197 (3.28%) vs. 388 (6.47%), P < 0.001). In addition, fewer participants were hospitalized in the vaccine group (67 (1.12%) vs. 125 (2.08%), P < 0.001). In the vaccine group, 41 (0.68%) participants were hospitalized for HFMD, compared to 88 (1.47%) in the placebo group (P < 0.001) (). No HFMD in the vaccine group was found to be associated with EV71 virus, and all participants recovered within 3 days after hospitalization. One participant in the placebo group died because of severe EV71-associated HFMD. One patient in the vaccine group died because of a traffic accident.
Age trends in safety assessment
In the vaccine group, the incidence of each solicited reaction within 7 days after injection showed a significant trend with age except allergy (). Fewer participants in older age strata had reactions within 28 days after vaccination (Z = −8.654, P < 0.001). The incidence of solicited adverse reactions within a week after injection was significantly lower with increasing age (Z = −8.914, P < 0.001). The incidence of systemic reactions was usually lower with age, except allergy (). Although fewer local reactions were observed in older children, higher incidence of pain and pruritus was reported in older children (pain: Z = 7.274, P < 0.001; pruritus: Z = 2.500, P = 0.012) (). There was no trend of unsolicited reaction within 28 days in 4 age strata. However, participants aged 36–71 months had a significantly higher incidence of unsolicited reactions compared to participants aged 6–35 months (88 (1.76%) vs. 34 (3.40%), P = 0.002).
Table 2. Cochrane-Armitage age trend test for major adverse reactions in the vaccine and placebo groups.
In the placebo group, a similar trend for overall adverse reactions within 28 days after injection was observed, i.e. fewer adverse reactions were reported in older participants (Z = −4.470, P < 0.001). Systemic reactions were also more rare in older patients, aside from allergy (), and of all reported local reactions, only pain was reported significantly more often in older participants (Z = −4.425, P < 0.001). No significant age trend was found in other local or unsolicited reactions (). However, participants aged 36–71 months had a significantly higher incidence of unsolicited reactions compared to participants aged 6–35 months (65 (1.30%) vs. 24 (2.40%), P = 0.014).
Safety assessment in the 2 safety analysis populations
The relative risk (RR) of overall solicited adverse reactions within 7 days of the first injection was higher in the vaccine group than in the placebo group (27.75% vs. 18.23%, RR (95% confidence interval (CI)): 1.72 (1.58–1.88)), and the difference remained significant following the booster injection (15.97% vs. 11.67%, RR (95% CI): 1.44 (1.29-1.60)). Systemic adverse reactions were observed more frequently in the vaccine group than in the placebo group, except diarrhea and allergy (). Local reactions were also reported more frequently in the vaccine group (). No matter the safety analysis set, no significant differences were found in unsolicited reaction between the 2 treatment groups.
Table 3. Occurrence of major adverse reactions after the different injections.
In the vaccine group, significantly fewer solicited adverse reactions within 7 days were reported after the booster injection than after the initial injection (27.75% vs. 15.97%, RR (95% CI): 2.02 (1.84-2.22)). To each specific solicited adverse reaction (including systemic and local adverse reactions), higher morbidity was expected after the initial vaccination (), aside from allergy. However, the incidence of unsolicited adverse reactions after the second vaccination was similar to that after the first injection ().
Figure 2. Comparison of the 2 safety sets in the vaccine group or in the placebo group. The rate of adverse reactions in the first safety analysis population was compared to the rate of adverse reactions in the second safety analysis population in the vaccine group (A) and placebo group (B).
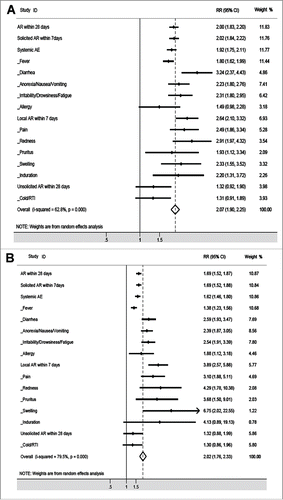
In the placebo group, a similar pattern was observed. Aside from induration, the relative risk of solicited adverse reaction within 7 days was higher after the initial injection than that after the booster injection (), and a similar incidence of unsolicited adverse reactions was reported in the first and second safety populations ().
Risk factors of adverse reaction severity
The severity of each major adverse reaction in the 2 treatment groups is presented in . Although adverse reactions were often observed after injection, the severity was mainly mild and moderate. Rare severe local reactions or unsolicited reactions were observed is both groups ().
Table 4. Severity of major adverse reaction in vaccine and placebo groups.
The severity of the adverse reactions within 28 days after injection was significantly affected by the different safety analysis populations (after initial injection or booster injection) and body mass index (BMI) (BMI<18.5 kg/m2 or >18.5 kg/m2). Specifically, more severe reactions were observed after the initial injection (odds ratio (OR) (95% CI): 0.86 (0.75, 0.98)) or in participants with a BMI <18.5 kg/m2 (OR (95% CI): 1.19 (1.03, 1.37). Although BMI affected the severity of overall systemic reactions (OR (95%CI): 1.17 (1.01, 1.35)), the pattern was not observed in specific reactions except swelling (OR (95%CI): 2.84 (1.21, 6.66)). More severe diarrhea and digestive discomforts (anorexia, nausea or vomiting, ANV) were reported after the initial injection (diarrhea (OR (95% CI): 0.58 (0.37, 0.92); ANV (OR (95% CI): 0.68 (0.47, 0.98)). The severity of local reactions was mainly affected by treatment except pruritus and induration (). Participants in placebo group had higher severity of local reactions (OR (95% CI): 0.31 (0.17, 0.56)). The severity of the unsolicited reactions within 28 days after injection was affected by the safety analysis population and age strata. Higher severity of unsolicited reactions was found after the initial injection (OR (95% CI): 0.27 (0.14, 0.51)) and younger age strata (OR (95%CI): 0.32 (0.16, 0.64)).
Table 5. Multiple stepwise cumulative odds logistic regression analysis of the adverse reaction severity.
In the vaccine group, no factor affected the occurrence of solicited reactions within 7 days except age strata on irritability, drowsiness, or fatigue (IDF) and BMI on swelling (). More severity of unsolicited reactions within 28 days were observed in younger participants (OR (95% CI): 0.27 (0.11, 0.65)) or those without throat discomfort before vaccination (OR (95% CI): 0.27 (0.11, 0.66)).
In the placebo group, the severity of adverse reactions within 28 days after injection was affected by the different safety analysis populations (OR (95% CI): 0.75 (0.61, 0.92)) and BMI (OR (95% CI): 1.26 (1.01, 1.57)). More serious diarrhea was observed after the initial injection (OR (95% CI): 0.46 (0.26, 0.85)). BMI affected the severity of ANV (OR (95%CL): 1.74 (1.01, 3.01)) and pain (OR (95% CI): 5.73 (1.02, 32.10)). Local reactions were not affected by any factor except pain. More severe unsolicited reactions within 28 days after injection were usually observed after the initial injection compared to the booster injection (OR (95% CI): 0.11 (0.04, 0.32)).
Discussion
The objective of this study was to assess the factors influencing the incidence and the severity of adverse events observed in a randomized, double-blinded, placebo-controlled, phase III clinical trial of a human diploid cell-based inactivated EV71 vaccine. The trial enrolled 12,000 children randomized to receive the vaccine or placebo. Of these, 10,988 received the second dose of vaccine or placebo. Reporting of local and systemic reactions was solicited within the 7 days after injection, and for a further 21 days.
Fever was the most common systemic reaction reported in the 2 arms, as has been previously reported for other inactivated vaccines.Citation27,28,36 Systemic reactions (excluding allergy) were reported significantly more frequently in younger vaccine recipients, suggesting better tolerance with increasing age.Citation37 Since a similar trend was observed in the placebo group, these reactions may be attributed to the adjuvant.Citation38 The most frequently reported local reaction (pain) was more common in older participants in both groups.Citation38 Pain and induration were reported more often in older children in the vaccine group, probably due to higher capacity to express this symptom in older children.Citation37,38 However, children over 36 months of age also reported a substantially higher rate of cold or RTI.
Within seven days of immunization systemic adverse events occurred in roughly half of the vaccine recipients, but significantly fewer placebo recipients, and the rate of local adverse events was also higher in the vaccine group than in the placebo group, suggesting that these adverse events occur in response to the vaccine antigen.Citation39 However, in both arms the rate of adverse events was dramatically lower following booster injections,Citation40,41 as has been previously reported for other vaccines.Citation39,42
The Cochrane-Armitage analysis showed that in the vaccine group age was associated with the occurrence of adverse effects. In general, solicited adverse reactions were more frequent in younger patients, including fever, diarrhea, ANV, IDF, redness, swelling, and induration. On the other hand, solicited adverse reactions like pain and pruritus and unsolicited adverse reactions like cold/RTI were more frequent in older children. Similar patterns were observed in the placebo group, except for cold/RTI that showed no difference between age groups. These results are supported by previous studies.Citation39-42
Factors for adverse reaction severity were assessed by multiple stepwise cumulative odds logistic regression. Gender did not affect the severity of any reaction. Severity of reactions after the initial injection was usually higher than that after the booster injection. Participants with a BMI < 18.5 kg/m2, who were weak and prone to diseases, did not show more serious reactions except swelling. Younger children had more severity of unsolicited reactions within 28 days after injection. Participants in the placebo group had less local reactions within 7 days after injection, but the severity was higher compared to those in the vaccine group, which also suggested that adjuvant itself might cause side effects.Citation38 Although children aged 36 to 71 months had a higher risk of cold and RTI, these symptoms were less severe, reflecting the improved immunity in children of this age. In this study, underweight children (suggesting poor immunity) tolerated the vaccine well except slightly higher severity of swelling. But in the placebo group underweight children were observed higher severity of ANV and pain.
These results demonstrate that this inactivated diploid vaccine had a favorable safety profile, and was well tolerated when administered to children aged <71 months.Citation32,34 This finding is consistent with previous reports of trials of other inactivated EV71 vaccines (320U/0.5 mL for Vigoo and 400U/0.5 mL for Sinovac).Citation19,33
While the safety profile of this vaccine appears to be favorable, this study has several limitations. Some participants were too young to express their discomfort with any precision, probably leading to imprecise results in younger children. In addition, if further demographic and clinical characteristics of participants were collected before immunization, the factors affecting the rate and severity of adverse events may have been better characterized. In the subsequent post-marketing phase IV clinical trial, a substantially larger group of participants will be involved and accompanied by a strong network of safety surveillance to characterize further the safety profile of this vaccine.
In conclusion, this inactivated human diploid cell-based EV71 vaccine showed satisfactory safety. Side effects after vaccination were normal and acceptable.
Methods
Study design
A randomized, double-blinded, placebo-controlled phase III trial (ClinicalTrials.gov number: NCT01569581) was conducted by the Institute of Medical Biology, Chinese Academy of Medical Science in 7 counties in the Guangxi Zhuang Autonomous Region, China. The study was approved by the Ethics Committee of the research center. Healthy children aged 6–71 months, whose parent or legal guardian provided written informed consent, were eligible for enrollment in this study.
Vaccine
This human cell-based inactivated EV71 vaccine was developed with the seed virus EV71 strain KMB17 (subgenotype C4), and contained 100U of inactivated viral antigen with aluminum hydroxide adjuvant. The placebo contained aluminum hydroxide diluents with no EV71 antigen; both were packaged in syringes (0.5 mL/vial). The vaccine and placebo were supplied in coded, identical-appearing, single-dose vials and were administered intramuscularly in the deltoid region on days 0 and 28. Both vaccine and placebo were prepared using Good Manufacturing Practices and tested by the Chinese National Institute for Food and Drug Control before the study began.
Participants
A total of 12,000 eligible healthy Chinese children between 6 and 71 months old were enrolled. Children with a history of HFMD disease or vaccination with EV71 vaccine and those with acute febrile disease on the day of enrollment were excluded. The participants were randomized in a 1:1 ratio in blocks of 8 to receive EV71 vaccine or placebo, according to a randomization list that was generated by an independent statistician.
Participants who were randomized to receive the first injection were included in the first safety analysis set and the participants who successfully received 2 injections were included in the second safety analysis set.
Safety assessment
Participant demographics (e.g., age, weight) were collected on recruitment. Adverse events occurring immediately after vaccine administration were reported by the participants' parents or legal guardians in diary cards. The solicited adverse events including systemic events (e.g., fever, irritability) and local events (e.g., pain, redness), were listed on the diary cards. Unsolicited adverse events were reported spontaneously by the participants' parents or guardians. An adverse event was defined as an adverse reaction when the association with injection was possible, probable, or definite.
A serious adverse event was defined as any health-related problem that was life-threatening, necessitated hospitalization, or resulted in death, disability or incapacity. Serious adverse events were recorded throughout the entire study period. Parents and legal guardians were asked to contact the investigator immediately in the event of any serious adverse event.
Adverse reaction was defined as an adverse event that was associated with the injection itself.
An independent data and safety monitoring board, whose members were blinded to the study assignments, periodically reviewed all reports of serious adverse events to assess the causality of events and to determine associated secondary diagnoses and other underlying conditions.
Statistical methods
A sample size of 6000 participants per group was required for 95% statistical power to detect vaccine efficacy of 75% between 2 arms at a 2-sided significance level of 0.05. In order to ensure an equal distribution of ages, the enrolled participants were divided into 4 age groups: 6–11, 12–23, 24–35, and 36–71 months.
The safety assessment was performed in the intention-to-treat population who received at least one injection (vaccine or placebo) and for whom safety data was available. Cases, incidence, and corresponding 95% confidence intervals (CIs) of adverse events in each safety analysis set were separately determined. The Fisher exact test was used for categorical data. The RR with 95% CI of each group was compared. The Cochrane-Armitage trend test was conducted to assess the influence of age strata (6–11, 12–23, 24–35, and 36–71 months) on the incidence of adverse reaction. Multiple stepwise cumulative odds logistic regression models were used to analyze the factors influencing the severity of reactions for all participants and also in the 2 different treatment groups. Criteria for entering and keeping a variable into the multivariate models were all set as P < 0.05. The dependent variable was the severity of each adverse reaction (mild, moderate, and severe), and the independent variables included the treatment groups (vaccine and placebo; only for all participants), the safety analysis population (after initial injection or booster injection), age strata (aged 6–35 or 36–71 months), BMI (<18.5 or ≥18.5 kg/m2), gender (male or female), and throat status (whether or not the participant experienced throat discomfort before injection).
Hypothesis testing was 2-sided with an alpha value of 0.05. All data were handled and analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81273176 and 81302509).
References
- Xiao G, Hu Y, Yu S, Ma J. Epidemiology of hand, foot and mouth disease in children aged 5 years in China, 2008–2011. Dis Surveill 2012; 27:932-6.
- Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol 2010; 9:1097-105; PMID:20965438; http://dx.doi.org/10.1016/S1474-4422(10)70209-X
- Lum LC, Wong KT, Lam SK, Chua KB, Goh AY. Neurogenic pulmonary oedema and enterovirus 71 encephalomyelitis. Lancet 1998; 352:1391; PMID:9802304; http://dx.doi.org/10.1016/S0140-6736(05)60789-1
- Chang LY, Lin TY, Hsu KH, Huang YC, Lin KL, Hsueh C, Shih SR, Ning HC, Hwang MS, Wang HS, et al. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 1999; 354:1682-6; PMID:10568570; http://dx.doi.org/10.1016/S0140-6736(99)04434-7
- Sun S, Jiang L, Liang Z, Mao Q, Su W, Zhang H, Li X, Jin J, Xu L, Zhao D, et al. Evaluation of monovalent and bivalent vaccines against lethal Enterovirus 71 and Coxsackievirus A16 infection in newborn mice. Hum Vaccin Immunother 2014; 10:2885-95; PMID:25483672; http://dx.doi.org/10.4161/hv.29823
- De W, Changwen K, Wei L, Monagin C, Jin Y, Cong M, Hanri Z, Jun S. A large outbreak of hand, foot, and mouth disease caused by EV71 and CAV16 in Guangdong, China, 2009. Arch Virol 2011; 156:945-53; PMID:21305327; http://dx.doi.org/10.1007/s00705-011-0929-8
- Ho M. Enterovirus 71: the virus, its infections and outbreaks. J Microbiol Immunol Infect 2000; 33:205-16; PMID:11269363
- WHO. Report on the Hand, Foot and Mouth Disease Outbreak in Fuyang City, Anhui Province and the Prevention and Control in China. 2008:1-25.
- Li YP, Liang ZL, Gao Q, Huang LR, Mao QY, Wen SQ, Liu Y, Yin WD, Li RC, Wang JZ. Safety and immunogenicity of a novel human Enterovirus 71 (EV71) vaccine: a randomized, placebo-controlled, double-blind, Phase I clinical trial. Vaccine 2012; 30:3295-303; PMID:22426327; http://dx.doi.org/10.1016/j.vaccine.2012.03.010
- Chong P, Liu CC, Chow YH, Chou AH, Klein M. Review of enterovirus 71 vaccines. Clin Infect Dis 2015; 60:797-803; PMID:25352588; http://dx.doi.org/10.1093/cid/ciu852
- McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev 2002; 26:91-107; PMID:12007645; http://dx.doi.org/10.1111/j.1574-6976.2002.tb00601.x
- Weng KF, Chen LL, Huang PN, Shih SR. Neural pathogenesis of enterovirus 71 infection. Microbes Infect 2010; 12:505-10; PMID:20348010; http://dx.doi.org/10.1016/j.micinf.2010.03.006
- Zhu FC, Liang ZL, Li XL, Ge HM, Meng FY, Mao QY, Zhang YT, Hu YM, Zhang ZY, Li JX, et al. Immunogenicity and safety of an enterovirus 71 vaccine in healthy Chinese children and infants: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet 2013; 381:1037-45; PMID:23352749; http://dx.doi.org/10.1016/S0140-6736(12)61764-4
- Tan X, Huang X, Zhu S, Chen H, Yu Q, Wang H, Huo X, Zhou J, Wu Y, Yan D, et al. The persistent circulation of enterovirus 71 in People's Republic of China: causing emerging nationwide epidemics since 2008. PLoS One 2011; 6:e25662; PMID:21980521; http://dx.doi.org/10.1371/journal.pone.0025662
- Zhang Y, Tan X, Cui A, Mao N, Xu S, Zhu Z, Zhou J, Shi J, Zhao Y, Wang X, et al. Complete genome analysis of the C4 subgenotype strains of enterovirus 71: predominant recombination C4 viruses persistently circulating in China for 14 years. PLoS One 2013; 8:e56341; PMID:23441179; http://dx.doi.org/10.1371/journal.pone.0056341
- Sun LM, Zheng HY, Zheng HZ, Guo X, He JF, Guan DW, Kang M, Liu Z, Ke CW, Li JS, et al. An enterovirus 71 epidemic in Guangdong Province of China, 2008: epidemiological, clinical, and virogenic manifestations. Jpn J Infect Dis 2011; 64:13-8; PMID:21266750
- Yi L, Lu J, Kung HF, He ML. The virology and developments toward control of human enterovirus 71. Crit Rev Microbiol 2011; 37:313-27; PMID:21651436; http://dx.doi.org/10.3109/1040841X.2011.580723
- Kung YA, Hung CT, Liu YC, Shih SR. Update on the development of enterovirus 71 vaccines. Expert Opin Biol Ther 2014; 14:1455-64; PMID:24989170; http://dx.doi.org/10.1517/14712598.2014.935330
- Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013; 381:2024-32; PMID:23726161; http://dx.doi.org/10.1016/S0140-6736(13)61049-1
- Li YP, Liang ZL, Xia JL, Wu JY, Wang L, Song LF, Mao QY, Wen SQ, Huang RG, Hu YS, et al. Immunogenicity, safety, and immune persistence of a novel inactivated human enterovirus 71 vaccine: a phase II, Randomized, double-blind, placebo-controlled Trial. J Infect Dis 2014; 209:46-55; PMID:23922377; http://dx.doi.org/10.1093/infdis/jit429
- Liang Z, Wang J. EV71 vaccine, an invaluable gift for children. Clin Transl Immunology 2014; 3:e28; PMID:25505956; http://dx.doi.org/10.1038/cti.2014.24
- Liang ZL, Mao QY, Wang YP, Zhu FC, Li JX, Yao X, Gao F, Wu X, Xu M, Wang JZ. Progress on the research and development of inactivated EV71 whole-virus vaccines. Hum Vaccin Immunother 2013; 9:1701-5; PMID:23744508; http://dx.doi.org/10.4161/hv.24949
- Jee YM, Cheon DS, Kim K, Cho JH, Chung YS, Lee J, Lee SH, Park KS, Lee JH, Kim EC, et al. Genetic analysis of the VP1 region of human enterovirus 71 strains isolated in Korea during 2000. Arch Virol 2003; 148:1735-46; PMID:14505086; http://dx.doi.org/10.1007/s00705-003-0133-6
- Hwa SH, Lee YA, Brewoo JN, Partidos CD, Osorio JE, Santangelo JD. Preclinical evaluation of the immunogenicity and safety of an inactivated enterovirus 71 candidate vaccine. PLoS Negl Trop Dis 2013; 7:e2538; PMID:24244774; http://dx.doi.org/10.1371/journal.pntd.0002538
- Cheng A, Fung CP, Liu CC, Lin YT, Tsai HY, Chang SC, Chou AH, Chang JY, Jiang RH, Hsieh YC, et al. A Phase I, randomized, open-label study to evaluate the safety and immunogenicity of an enterovirus 71 vaccine. Vaccine 2013; 31:2471-6; PMID:23541623; http://dx.doi.org/10.1016/j.vaccine.2013.03.015
- Liu CC, Chow YH, Chong P, Klein M. Prospect and challenges for the development of multivalent vaccines against hand, foot and mouth diseases. Vaccine 2014; 32:6177-82; PMID:25218294; http://dx.doi.org/10.1016/j.vaccine.2014.08.064
- Yun KW, Lee HJ, Kang JH, Eun BW, Kim YJ, Kim KH, Kim NH, Hong YJ, Kim DH, Kim HM, et al. Safety and immunogenicity of a freeze-dried, Vero cell culture-derived, inactivated Japanese encephalitis vaccine (KD-287, ENCEVAC(R)) versus a mouse brain-derived inactivated Japanese encephalitis vaccine in children: a phase III, multicenter, double-blinded, randomized trial. BMC Infect Dis 2015; 15:7; PMID:25567119; http://dx.doi.org/10.1186/s12879-014-0744-4
- Carmona Martinez A, Salamanca de la Cueva I, Boutet P, Vanden Abeele C, Smolenov I, Devaster JM. A phase 1, open-label safety and immunogenicity study of an AS03-adjuvanted trivalent inactivated influenza vaccine in children aged 6 to 35 months. Hum Vaccin Immunother 2014; 10:1959-68; PMID:25424805; http://dx.doi.org/10.4161/hv.28743
- Chou AH, Liu CC, Chang JY, Jiang R, Hsieh YC, Tsao A, Wu CL, Huang JL, Fung CP, Hsieh SM, et al. Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody against subgenotypes B1, B4, B5 and C4A in adult volunteers. PLoS One 2013; 8:e79783; PMID:24278177; http://dx.doi.org/10.1371/journal.pone.0079783
- Li JX, Mao QY, Liang ZL, Ji H, Zhu FC. Development of enterovirus 71 vaccines: from the lab bench to Phase III clinical trials. Expert Rev Vaccines 2014; 13:609-18; PMID:24621093; http://dx.doi.org/10.1586/14760584.2014.897617
- Chen YJ, Meng FY, Mao Q, Li JX, Wang H, Liang ZL, Zhang YT, Gao F, Chen QH, Hu Y, et al. Clinical evaluation for batch consistency of an inactivated enterovirus 71 vaccine in a large-scale phase 3 clinical trial. Hum Vaccin Immunother 2014; 10:1366-72; PMID:24633366; http://dx.doi.org/10.4161/hv.28397
- Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med 2014; 370:829-37; PMID:24571755; http://dx.doi.org/10.1056/NEJMoa1303224
- Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med 2014; 370:818-28; PMID:24571754; http://dx.doi.org/10.1056/NEJMoa1304923
- Liu L, Zhang Y, Wang J, Zhao H, Jiang L, Che Y, Shi H, Li R, Mo Z, Huang T, et al. Study of the integrated immune response induced by an inactivated EV71 vaccine. PLoS One 2013; 8:e54451; PMID:23372725; http://dx.doi.org/10.1371/journal.pone.0054451
- Ma SH, Liu JS, Wang JJ, Shi HJ, Yang HJ, Chen JY, Liu LD, Li QH. Genetic analysis of the VP1 region of human enterovirus 71 strains isolated in Fuyang, China, during 2008. Virologica Sinica 2009; 24:162-70; http://dx.doi.org/10.1007/s12250-009-3033-4
- Belshe RB, Frey SE, Graham IL, Anderson EL, Jackson LA, Spearman P, Edupuganti S, Mulligan MJ, Rouphael N, Winokur P, et al. Immunogenicity of avian influenza A/Anhui/01/2005(H5N1) vaccine with MF59 adjuvant: a randomized clinical trial. JAMA 2014; 312:1420-8; PMID:25291578; http://dx.doi.org/10.1001/jama.2014.12609
- Wysocki J, Brzostek J, Szymanski H, Tetiurka B, Toporowska-Kowalska E, Wasowska-Krolikowska K, Sarkozy DA, Giardina PC, Gruber WC, Emini EA, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine administered to older infants and children naive to pneumococcal vaccination. Vaccine 2015; 33:1719-25; PMID:25698485; http://dx.doi.org/10.1016/j.vaccine.2015.02.005
- Nolan T, Richmond PC, McVernon J, Skeljo MV, Hartel GF, Bennet J, Basser RL. Safety and immunogenicity of an inactivated thimerosal-free influenza vaccine in infants and children. Influenza Other Respir Viruses 2009; 3:315-25; PMID:19903213; http://dx.doi.org/10.1111/j.1750-2659.2009.00108.x
- Lambert SB, Chuk LM, Nissen MD, Nolan TM, McVernon J, Booy R, Heron L, Richmond PC, Walls T, Marshall HS, et al. Safety and tolerability of a 2009 trivalent inactivated split-virion influenza vaccine in infants, children and adolescents. Influenza Other Respir Viruses 2013; 7:676-85; PMID:23551933; http://dx.doi.org/10.1111/irv.12107
- Shenyu W, Jingxin L, Zhenglun L, Xiuling L, Qunying M, Fanyue M, Hua W, Yuntao Z, Fan G, Qinghua C, et al. A booster dose of an inactivated enterovirus 71 vaccine in chinese young children: a randomized, double-blind, placebo-controlled clinical trial. J Infect Dis 2014; 210:1073-82; PMID:24625805; http://dx.doi.org/10.1093/infdis/jiu113
- Hu YM, Wang X, Wang JZ, Wang L, Zhang YJ, Chang L, Liang ZL, Xia JL, Dai QG, Hu YL, et al. Immunogenicity, safety, and lot consistency of a novel inactivated enterovirus 71 vaccine in Chinese children aged 6 to 59 months. Clin Vaccine Immunol 2013; 20:1805-11; PMID:24108780; http://dx.doi.org/10.1128/CVI.00491-13
- Li L, Yin H, An Z, Feng Z. Considerations for developing an immunization strategy with enterovirus 71 vaccine. Vaccine 2015; 33:1107-12; PMID:25444807; http://dx.doi.org/10.1016/j.vaccine.2014.10.081
