abstract
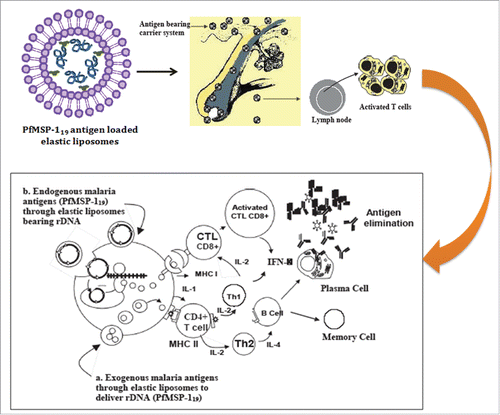
As transdermal immunization results in poor immunogenicity, which is attributed to poor permeability of antigens through the skin, we believed ultradeformable lipid vesicles (elastic liposome) might address the challenges encountered during transdermal immunization. The elastic liposome, versatile carrier, proves better vehicle for transcutaneous delivery of protein, peptide and nucleic acid antigens. Our recently published articleCitation1 is suggestive of improved immunogenicity of carboxyl-terminal 19 kDa fragment of merozoite surface protein-1 (PfMSP-119) of Plasmodium falciparum when administered subcutaneously via elastic liposomes ().
As is evident from the work done by malaria vaccinologists, erythrocytic merozoite invasion process is one of the most promising targets for the development of vaccine against malaria.Citation2,3 Merozoite surface protein-1 (MSP-1), a polypeptide of 190–230 kDa, present on the surface of all known Plasmodium spp., is a leading malaria vaccine candidate antigen.Citation4 At the time of rupturing the schizont, MSP-1 undergoes proteolytic cleavage to produce at least 4 fragments of variable molecular weights (83, 28–30, 38–45 and 42 kDa).Citation5 Further, during merozoite invasion, the carboxy terminal, cysteine rich, 42 kDa (MSP-142) is further processed (secondary processing of MSP-1 during the successful invasion of RBCs) to yield a 19-kDa fragment (MSP-119) that remains associated with merozoites.Citation6,7 The potential of MSP-119 and MSP-142 in terms of immunogenicity has already been explored and documented against the asexual stage of malaria parasite.Citation8,9 The scarcity of B and T cell dominant epitopes present on merozoite surface protein vaccine forced us to think and to come up with some innovative strategy to achieving humoral and cell-mediated immune (CMI) response elicited by topically applied PfMSP-119-loaded elastic liposomes. We discovered that effective immunoadjuvant property of this elastic liposomes justifies its potential for the delivery of soluble malaria antigen to achieving robust and heightened immunogenicity. This novel carrier shows its value to explore the feasibility of developing asexual blood stage malaria vaccine.
The effective vaccination against infectious diseases is one of the major achievements of the modern preventive medicine.Citation10 Vaccination stimulates specific immune response, and induces long-lasting immunologic memory to protect against subsequent infections.Citation11 Most of the available vaccines are of intramuscular administration, which could be painful and their proper administration requires aseptic technique, skilled and trained personnel. The suboptimal presentation of antigen to antigen presenting cells due to the absence of co-stimulatory molecules on myocytes leading to poor immunogenicity of surface antigen (MSP-119). Our findingsCitation1 has shown that transdermal delivery of PfMSP-119 through elastic liposomes has been accomplished as one of the promising alternative approaches to invasive routes of administration. Moreover, transdermal immunization has proven advantageous over parenteral routes in avoiding systemic adverse effects, maintaining uniform blood levels and increased patient compliance. We believe non-invasive topical immunization via skin may allow vaccination by individuals without needing medically trained personnel and makes wide spread vaccination cost effective and feasible.
Transcutaneous immunization (TCI) is an innovative technique, having both practical and immunological merits requiring simple introduction of antigens to host using a topical application to the intact skin.Citation12 Transcutaneous immunization is crucially important and is of interest because of ease of administration, and capability in eliciting robust immune responses when compared with conventional and invasive needle injections administered in equivalent doses.Citation13 The epidermal antigen presenting cells (LCs) and migratory T-lymphocytes, collectively termed as skin-associated lymphoid tissue (SALT), and constituting skin-immune system, are crucial players in eliciting cell-mediated and humoral immune responses.Citation14 Transcutaneous immunization activates potent antigen-presenting cells (LCs) by the adjuvants, and LCs rapidly migrate from epidermis to draining lymph nodes, carrying antigen(s) to induce robust systemic and cellular immune responses.Citation15–19
There has been advancing interest in the development of novel lipid-based vesicular approaches for effective transcutaneous immunization. The very recent development in vesicle designing for transcutaneous bioactive(s) delivery is the use of elastic liposomes that differ from conventional liposomes and niosomes by the virtue of their characteristic fluid membrane and high elasticityCitation20 of elastic liposomes.
We took advantage of this delivery vehicle to achieving significantly high immune response of asexual blood stage antigen of Plasmodium falciparum, PfMSP-119 (). In addition, we have reportedly shown to have achieved an adjuvant effect when immunized via transcutaneous route to induce immune responses at both systemic and mucosal sites. The immune responses mediated by MSP-119 are largely antibody-dependent on high antibody titers essentially required to confer protectionCitation21,22 against asexual blood stage P. falciparum infection. We, based on our finding,Citation1,23 claim that elastic liposome-mediated topical delivery of well-characterized P. falciparum antigen (PfMSP-119) to achieving significantly higher, and perdurable humoral (specific IgG antibodies and isotypes, IgG 1 & IgG3) as well as cell-mediated immune response (IFNg). Our recent investigation suggests better efficacy of elastic liposomes in delivering PfMSP-119 in order to evoke immune responses against asexual blood stage falciparum malaria protection (). Malaria antigen-loaded elastic liposomes following transdermal route has proven better vehicle than conventional liposomes when delivered via intramuscular route. The better immune responses mounted by the elastic liposomes loaded malaria antigen is due to preservation of immunogenicity of less number of B and T cell dominant epitope available on widely used surface antigen (MSP-1) of P. falciparum.
Concluding remark
In addition to the effective transdermal delivery of PfMSP-119, elastic liposomes formulation reported might also prove to be a valuable carrier towards pathological tissues such as cancer, inflammation and infection sites, and ischemic areas where delivery of antigen has always been a problem compared to normal tissues. Moreover, the enhanced immunogenicity and protective efficacy of PfMSP-119 when used as vaccine formulation upon being delivered via elastic liposomes is one of the glaring advantages of this carrier. The novel and ultradeformable carriers (elastic liposomes) overcome the skin permeability barrier and deliver antigenic payload to immunologically active cells of skin and draining lymph nodes. Elastic liposomes through their enhanced elasticity, better antigen presentation, controlled antigen release, immunoadjuvant properties and greater entrapment efficiency could be further explored as an effective tool for transdermal vaccination. Although non-invasive administration of elastic liposomes offering needle-free vaccine delivery, giving rise to higher antibody titer, and strong cellular responses for clearing asexual blood stage infections of P. falciparum, further studies with elastic liposome and more Plasmodium antigens might enhance the vaccine development effort against malaria.
Disclosure of potential conflict of interest
The authors have declared that no competing interest exists.
Funding
This work was funded by a grant from Department of Biotechnology (DBT), New Delhi, India and Fellowship (SRF) provided by Council of Scientific and Industrial Research (CSIR HRDG) New Delhi, India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
References
- Tyagi RK, Garg NK, Jadon R, Sahu T, Katare OP, Dalai SK, Awasthi A, Marepally SK. Elastic liposome-mediated transdermal immunization enhanced the immunogenicity of P. falciparum surface antigen, MSP-119. Vaccine 2015; 33:4630-8; PMID:26141014; http://dx.doi.org/10.1016/j.vaccine.2015.06.054
- Berzins K. Merozoite antigens involved in invasion. Chem Immunol 2002; 80:125-43; PMID:12058636; http://dx.doi.org/10.1159/000058843
- Cowman AF, Baldi DL, Healer J, Mills KE, O'Donnell RA, Reed MB, Triglia T, Wickham ME, Crabb BS. Functional analysis of proteins involved in Plasmodium falciparum merozoite invasion of red blood cells. FEBS Lett 2000; 476:84-8; PMID:10878256; http://dx.doi.org/10.1016/S0014-5793(00)01703-8
- Miller LH, Hoffman SL. Research toward vaccines against malaria. Nat Med 1998; 4:520-4; PMID:9585203; http://dx.doi.org/10.1038/nm0598supp-520
- Holder AA, Freeman RR. Protective antigens of rodent and human bloodstage malaria. Philos Trans R Soc Lond B Biol Sci 1984; 307:171-7; PMID:6151681; http://dx.doi.org/10.1098/rstb.1984.0117
- Blackman MJ, Holder AA. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol Biochem Parasitol 1992; 50:307-15; PMID:1741018; http://dx.doi.org/10.1016/0166-6851(92)90228-C
- Holder AA, Blackman MJ, Burghaus PA, Chappel JA, Ling IT, McCallum-Deighton N, Shai S. A malaria merozoite surface protein (MSP1)-structure, processing and function. Mem Inst Oswaldo Cruz 1992; 87:37-42; PMID:1343716; http://dx.doi.org/10.1590/S0074-02761992000700004
- Ahlborg N, Ling IT, Holder AA, Riley EM. Linkage of exogenous T-cell epitopes to the 19-kgdalton region of Plasmodium yoelii merozoite surface protein 1 (MSP1(19)) can enhance protective immunity against malaria and modulate the immunoglobulin subclass response to MSP1(19). Infect Immun 2000; 68:2102-9; PMID:10722607; http://dx.doi.org/10.1128/IAI.68.4.2102-2109.2000
- Tian JH, Kumar S, Kaslow DC, Miller LH. Comparison of protection induced by immunization with recombinant proteins from different regions of merozoite surface protein 1 of Plasmodium yoelii. Infect Immun 1997; 65:3032-6; PMID:9234750
- Glenn GM, Rao M, Matyas GR, Alving CR. Skin immunization made possible by cholera toxin. Nature 1998; 391:851; PMID:9495336; http://dx.doi.org/10.1038/36014
- Foged C, Sundblad A, Hovgaard L. Targeting vaccines to dendritic cells. Pharm Res 2002; 19:229-38; PMID:11934227; http://dx.doi.org/10.1023/A:1014474414097
- Glenn GM, Scharton-Kersten T, Alving CR. Advances in vaccine delivery: transcutaneous immunisation. Expert Opin Investig Drugs 1999; 8:797-805; PMID:15992132; http://dx.doi.org/10.1517/13543784.8.6.797
- Shi Z, Curiel DT, Tang DC. DNA-based non-invasive vaccination onto the skin. Vaccine 1999; 17:2136-41; PMID:10367946; http://dx.doi.org/10.1016/S0264-410X(98)00488-5
- Singh RP, Singh P, Mishra V, Prabhakaran D, Vyas SP. Vesicular systems for non-invasive topical immunization: rationale and prospects. Indian J Pharmacol 2002; 34:301-10.
- Baca-Estrada ME, Foldvari M, Ewen C, Badea I, Babiuk LA. Effects of IL-12 on immune responses induced by transcutaneous immunization with antigens formulated in a novel lipid-based biphasic delivery system. Vaccine 2000; 18:1847-54; PMID:10699333; http://dx.doi.org/10.1016/S0264-410X(99)00379-5
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245-52; PMID:9521319; http://dx.doi.org/10.1038/32588
- Silberberg-Sinakin I, Thorbecke GJ, Baer RL, Rosenthal SA, Berezowsky V. Antigen-bearing langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell Immunol 1976; 25:137-51; PMID:782728; http://dx.doi.org/10.1016/0008-8749(76)90105-2
- Eagling EM. Immediate management of perforating injuries: lacerations with anterior and posterior segment damage. Trans Ophthalmol Soc U K 1982; 102:225-6; PMID:6963513
- Hammond SA, Guebre-Xabier M, Yu J, Glenn GM. Transcutaneous immunization: an emerging route of immunization and potent immunostimulation strategy. Crit Rev Ther Drug Carrier Syst 2001; 18:503-26; PMID:11763499; http://dx.doi.org/10.1615/CritRevTherDrugCarrierSyst.v18.i5.30
- Cevc G, Blume G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim Biophys Acta 1992; 1104:226-32; PMID:1550849; http://dx.doi.org/10.1016/0005-2736(92)90154-E
- Daly TM, Long CA. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J Immunol 1995; 155:236-43; PMID:7602100
- Hirunpetcharat C, Tian JH, Kaslow DC, van Rooijen N, Kumar S, Berzofsky JA, Miller LH, Good MF. Complete protective immunity induced in mice by immunization with the 19-kgdalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J Immunol 1997; 159:3400-11; PMID:9317139
- Tyagi RK, Garg NK, Sahu T. Vaccination Strategies against Malaria: novel carrier(s) more than a tour de force. J Control Release 2012; 162:242-54; PMID:22564369; http://dx.doi.org/10.1016/j.jconrel.2012.04.037