?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Routine infant immunization with 10-valent pneumococcal conjugate vaccine (PCV-10) began in Brazil in 2010. The impact of the PCV-10 on rates of invasive pneumococcal disease (IPD) at the population level was not yet evaluated. Serotype-specific IPD changes after PCV-10 introduction is still to be determined. Data from national surveillance system for notifiable diseases (SINAN) and national reference laboratory for S. pneumoniae in Brazil (IAL) were linked to enhance case ascertainment of IPD. An interrupted time-series analysis was conducted to predict trends in the postvaccination IPD rates in the absence of PCV-10 vaccination, taking into consideration seasonality and secular trends. PCVs serotype-specific distribution were assessed before (2008–2009) and after (2011–2013) the introduction of PCV-10 in the immunization program. A total of 9,827 IPD cases were identified from 2008–2013 when combining SINAN and IAL databases. Overall, PCV-10 types decreased by 41.3% after PCV-10 vaccination period, mostly in children aged 2–23 months, while additional PCV-13 serotypes increased by 62.8% mainly in children under 5-year of age. For children aged 2–23 months, targeted by the immunization program, we observed a 44.2% (95%CI, 15.8–72.5%) reduction in IPD rates. In contrast, significant increase in IPD rates were observed for adults aged 18–39 y (18.9%, 95%CI 1.1–36.7%), 40–64 y (52.5%, 95%CI 24.8–80.3%), and elderly ≥ 65 y (79.3%, 95%CI 62.1–96.5%). This is the first report of a time-series analysis for PCV impact in IPD conducted at national level data in a developing country. We were able to show significant impact of PCV-10 on IPD for age groups targeted by vaccination in Brazil, 3 y after its introduction. No impact on other age groups was demonstrated.
Abbreviations
| IAL | = | Adolfo Lutz Institute |
| IPD | = | invasive pneumococcal disease |
| PCV | = | pneumococcal conjugate vaccine |
| PCV-10 | = | 10-valent pneumococcal conjugate vaccine |
| SINAN | = | National Surveillance System for Notifiable Diseases |
Introduction
Pneumococcal diseases are infections caused by Streptococcus pneumoniae. Invasive pneumococcal disease (IPD) comprises severe infections, such as bacteraemia, sepsis, and meningitis, in which S. pneumoniae can be isolated from blood stream, cerebrospinal fluid (CSF), and others (pleural fluids, abscess). Despite the availability of effective pneumococcal conjugate vaccines, IPDs remains a major cause of morbidity and mortality globally, with the highest burden of disease found in young children in the first 2 y of life.Citation1,2
Pneumococcal conjugate vaccines (PCVs) have been extensively used in infants in developed and developing countries. Universal vaccination programs have significantly impacted the burden of IPD in different population.Citation3,4 When evaluating PCV impact, the main challenge lies in the fact that we do not know what would disease incidence be in the absence of vaccination in the same study population. Therefore, one should estimate the IPD incidence in the post vaccination period (if the population had not been vaccinated), and measure the incidence in vaccinated and unvaccinated population in an unbiased way to avoid inaccurate conclusions, or uninterpretable data, refraining from the temptation of seeing what we expect to see.
Classical methods to assess vaccine impact include the before-and-after ecological study design, in which results are estimated as percentage of difference between the average incidence rates before vaccine introduction when compared to post-vaccine introduction period. Notwithstanding, most before-and-after studies are susceptible to a number of plausible and potentially severe biases, and thus this methodology must be used with caution.Citation5 The vaccination impact, in turn, measures the overall effect of a vaccine program in a defined population.
In the last decade time-series design has emerged as a sound methodology to evaluate the impact of PCVs on hospitalization due to pneumonia.Citation6,7 More recently this methodology has been also applied to assess the PCV impact on IPD and also on serotypes,Citation8,9 to predict what the trend in the postvaccination observations would have been in the absence of PCV vaccination, taking into consideration seasonality and secular trends, which are most likely due to other reasons, into the analysis.Citation10
Currently, 2 vaccines, 10-valent pneumococcal conjugate vaccine (PCV-10) (Synflorix, GlaxoSmithKline), and 13-valent PCV (PCV-13) (Prevnar 13, Pfizer) are widely used in the national immunization programs of several countries. Brazil introduced PCV-10 in March 2010 in its government funded immunization program,Citation11 through which is offered free of charge for all infants in primary health care units. The effectiveness of PCV-10 for vaccine type IPD in children was estimated as 84% by a case-control study; in which almost 50% of cases were meningitis.Citation12 Similar result was demonstrated against pneumococcal meningitis in children ≤2 years of age using before-and-after methodology.Citation13 Both studies were conducted in Brazil between 2007 and 2012.
In Brazil, meningitis is of mandatory notification to the National Notifiable Diseases Surveillance System (SINAN).Citation14 All suspected meningitis and meningococcemia cases should be reported to the SINAN, irrespective of etiology. SINAN database includes case-based information on demographic, clinical and laboratory characteristics of each case. Some authors have reported that SINAN database for meningitis is a sensitive and representative surveillance system.Citation15
Adolfo Lutz Institute (IAL) is the National reference laboratory for S. pneumoniae in Brazil, located in the city of São Paulo.Citation16 As such, IAL receives isolates from a network of State reference public health laboratories and from private hospitals in all geographic regions of the country, performing serotyping, antimicrobial susceptibility testing, and molecular typing. In addition, IAL is also part of the Regional laboratory-based surveillance system for bacterial invasive diseases implemented in 1993 in the American Region – SIREVA. As such, IAL database of invasive pneumococcal diseases contains records of all isolates of invasive bacterial disease sent from a network of public and private hospitals and other public health laboratories in all geographical regions of the country.Citation17
Taking advantage of available nationwide data, we linked data from population-based meningitis surveillance and from S. pneumoniae national reference laboratory, to evaluate the impact of PCV-10 vaccination on IPD in Brazil, using an interrupted time-series analysis. We also described serotype-specific IPD changes after PCV-10 introduction.
Results
Descriptive analysis
From 2008 to 2013, SINAN had a total 187,483 records of meningitis and meningococcemia of all etiologies. After cleaning the database, 7,300 (3.9%) duplicate records were excluded. Of the remaining 180,183 meningitis cases, 6,622 (3.7%) were cases of pneumococcal meningitis. During the same period, IAL had a total of 11,928 records of invasive bacterial diseases. After cleaning the database, 1,399 (11.7%) duplicate records were excluded. Of the remaining 10,529 cases, 5,518 (52.4%) were cases due to S. pneumoniae. After excluding cases in children under 2 months of age from both databases, there were a total of 6,243 cases of confirmed pneumococcal meningitis in SINAN and a total of 5,457 IPD cases in IAL.
Out of the 5,457 IPD cases notified to the IAL database, 1,602 occurred in the prevaccination period (2008–2009), 817 in the transition period (2010), and 3,038 in the postvaccination period (2011–2013). Regarding specimen source, 2,598 of the 5,457 IPD cases (47.6%) were isolated from CSF, 2,372 (43.5%) from blood, 230 (4.2%) were from pleural fluid, 156 (2.9%) were from other normally sterile sites (e.g. ascites, intra-articular fluid), and 101 (1.9%) had no indication of source.
Regarding geographic location of cases, cases notified to the IAL database were mostly notified in São Paulo state (Southeast Region of Brazil) (n = 3,036 out of 5,457; 55.6%), many of them from the state capital - São Paulo city (n = 1,715, 31.4%). Differently, cases from SINAN were geographically more evenly distributed, being 2,524 of 6,243 (40.4%) cases from São Paulo state.
A total of 5,457 IPD cases in individuals aged 2 months and more notified to IAL during the study period were evaluated for serotype by age group. Of these 189 IPD cases did not have age information available and therefore were excluded from the analysis. A further 115 IPD cases were also excluded as they did not have information on serotype (n = 88) or isolates could not be typed (n = 27). Thus, 5,153 IPD cases had available serotype information, of which 1,493 cases were from the prevaccination, 2,885 from the post vaccination, and 775 from the transition period. This later, was not considered when estimating the PCV type percentage change.
In the vaccine target group, we observed a sharp decrease in number of PCV-10 types after PCV-10 introduction, whereas in adults an increase in number of PCV-13 minus PCV-10 types was identified, mainly in the ≥40 years old age groups. This trend was present before vaccine introduction and continued in the postvaccination period. Increase in non PCV-10 non PCV-13 types is evident in all age groups ().
Figure 1. Number of invasive pneumococcal disease by year according to vaccine serotypes and age group. Brazil, 2008 to 2013.
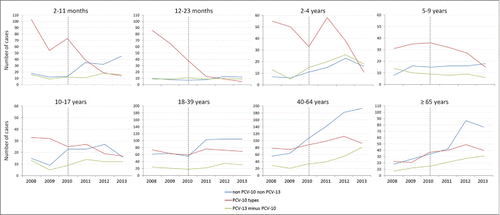
In the pre vaccination period, the contribution of PCV-10 types (878) among all IPD cases with serotype information analyzed (1,493) was higher for the younger age groups (2 months - 4 years) (74.1%, 80.7%, 77.2%) when compared to the older individuals (18 y and older) (44.6%, 47.5% and 40.6%). () Overall, PCV-10 types declined 41.3% after PCV-10 vaccine introduction, mostly in children aged 2–23 months, while PCV-13 minus PCV-10 types increased by 62.8% in all age groups. This increase was most significant in children under 5-year of age (data not shown).
Table 1. Distribution of PCV-10 and PCV-13 types by age groups before and after the introduction of PCV-10 in the immunization program. Brazil 2008 to 2013a.
SINAN and IAL data linkage
When cases from SINAN and IAL databases were combined by means of record-linkage, a total of 9,827 cases of IPD/meningitis were identified. Of these, 2,205 cases were common to both databases. While 3,308 cases from IAL had not been found in SINAN, 4,314 cases from SINAN had not been notified to IAL. Among the 3,308 cases identified only in the IAL database, 20.3% were from CSF, and 66.2% from blood. After excluding 189 records without age information, a total of 9,638 confirmed IPD cases were considered for time-series analysis.
Time-series analysis
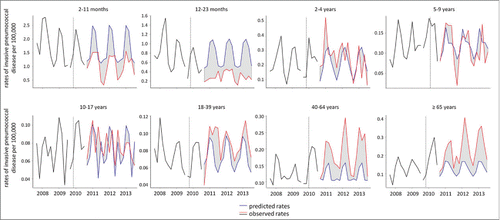
Analysis of time-series methodology stratified by age groups is presented in . During the postvaccination period, the observed IPD rates for children targeted for vaccination (aged 2–23 months) were significantly lower from the estimated rates based on rates from the prevaccination period (). Rate reduction was higher for children aged 12–23 months (61.1%) when compared to children aged 2–11 months (34.7%), however there was an overlap of their 95%CIs. In contrast, for individuals aged ≥18 years rates of IPD observed in the post vaccination period were statistically higher compared to the estimated rates.
Table 2. PCV-10 vaccination impact on invasive pneumococcal disease: time-series analysis, by age group. Brazil, 2008 to 2013.
Discussion
After 3 y of its introduction in Brazil, PCV-10 significantly reduced IPD burden in children in the vaccine target age groups. While a 44% reduction in IPD rates (95%CI 15.8–72.5) was found in children aged 2–23 months, no effect was observed in unvaccinated age groups. Instead, we observed an increase in IPD rates in 18 y of age and older, which was not expected; so, no evidence of indirect PCV-10 benefits could be showed.
The magnitude of the PCV impact on IPD can vary considerably among countries and according to data quality and method used to assess impact. Mostly, it has been demonstrated that a significant impact of PCV on IPD occurs shortly after its introduction, particularly in children.Citation7,9,18,19 Even when considering data from countries with varying prevaccination disease burden, and case definition and ascertainment methods, these results have been consistently demonstrated using time-series methodology.Citation7–9 Simonsen et al.Citation7 evaluated the impact of PCV-13 on IPD hospitalizations 2 y after its introduction in U.S. demonstrating a 40% reduction (95%CI 14–59) in children younger than 2 y of age, and a non-significant impact in children aged 2–4 y. Harboe et al.Citation8 found 71% reduction (95%CI 62–79) in children younger than 2 y of age after PCV-13 introduction in Danish. Moore et al.Citation9 reported a reduction 64% (95%CI 59–68) in IPD in children younger than 5 y of age 3 y after PCV-13 introduction, considering as the baseline IPD rates documented during PCV-7 use in the U.S. Both studies assessed population sub-groups within the US. More recent studies conducted in Finland by Jokinen et al.Citation18 and Palmu et al.Citation20 in cohorts of vaccine-eligible children reported similar results (80%; 95%CI 72–85 for IPD and 66%; 95%CI 59–73 for suspected IPD, respectively). These are the only 2 studies so far in the literature, which have assessed the impact of PCV-10 under routine immunization program, and no time-series PCV impact of IPD has been demonstrated in developing settings.
Vaccine impact studies can be conducted using various methods and data sources. Despite the available evidence of PCV impact, to date, few reports have used time-series analysis to assess PCV-10 impact on IPD. Most PCV impact studies consider data from existing surveillance system.Citation9 Such studies are possible when incident cases can be estimated in a defined population. Assessing the impact of PCV on pneumococcal disease, and particularly IPD, represents an additional challenge, as identification of S. pneumoniae from sterile site fluids requires laboratory confirmation of cases, which is not a simple task.
Most countries have passive surveillance systems of notifiable diseases. In Brazil, SINAN is such surveillance system. As a passive nationwide surveillance system, several data limitations should be taken into account. Laboratory based surveillance systems are in place in many countries, and provide valuable support for case management and surveillance networks. However, although serving well the purpose of laboratory confirmation of cases, identification of circulating serotypes, and antimicrobial resistance, such systems underestimates the full burden of disease.Citation21 Further, laboratory based systems may over-represent selected regions which are more likely to send specimens and isolates, either due to being located close to the reference laboratory, or due to logistical capabilities for shipment of biological specimens. Even considering such limitations, these data have been proved useful for PCV impact assessment in developing countries, particularly in South Africa where the laboratory-based surveillance has national representativeness and covers most laboratories in the country.Citation22
As one would expect, a sharp decrease in the number of PCV-10 type IPD cases was demonstrated in age groups targeted by vaccination confirming findings described by other investigators who have analyzed pneumococcal serotype distribution over time.Citation9,22,23 In the 2–4 y age group, decreased number of VT was only observed in 2013. Nonetheless, decreasing PCV-10 types were also noted in the prevaccination period in children under 5 y of age. As with most descriptive studies of serotypes, numbers are small and fluctuated over the years, precluding hypothesis testing. Thus, no further inference based on these results is possible, due to the relative small numbers and the fact that the isolates available may not represent all cases occurring in the country. It is worthwhile mentioning that time-series analysis have been nonetheless performed to assess PCV impact in IPD serotypes.Citation8,9 This has only been possible due to a great number of isolates with serotype information available over a long period of time. Unfortunately, such data is not usually available in most developing settings.
Our study has several limitations, which should be addressed. First, in the post vaccination period an enhanced surveillance of IPD was put in place all over the country in order to increase case detection for case control study to assess PCV-10 initiated shortly after PCV-10 introduction. This resulted in increased notification in all age groups, while during the pre-vaccination period no similar changes in the surveillance system where in place. As such, we have observed in our results an increase in the number of pneumococcal isolates detected in all age groups, most notably of non-vaccine types in age groups non targeted by the vaccine (adults and elderly), where the effect of the vaccine in reducing the number of IPD cases will not be observed. Thus, this limitation might have resulted in an underestimate of the true impact of PCV-10 in the age groups targeted by the vaccine by our time-series analysis. Changes in surveillance practices and how they can interfere and bias impact assessment studies are well described by Feikin et al.Citation24 Second, IAL data is not representative of the country as a whole. A vast proportion of cases in the IAL database were from São Paulo state (46.4%) and many were from São Paulo state capital city (25.4%), even though in 2013 only 21.7% and 5.9% of the Brazilian population lived in this state and city, respectively. We hypothesize that IAL data overly represents cases from São Paulo whereas cases from the rest of the country are underestimated. Third, SINAN reports meningitis cases and these represent the majority of cases included in our time-series analysis. One may hypothesize that PCV-10 impact on meningitis is different than that on non-meningitis IPD. However, other authors have demonstrated PCV impact in meningitis to be similar to that in overall IPD.Citation24 Finally, it was not possible to assess PCV impact on vaccine serotypes by means of time-series analysis, due to the small number of isolates and fluctuation over time. As reported by other authors, this is a limitation frequently encountered by investigators and for this reason time-series methodology is usually not adequate for impact assessment of PCV in serotypes when small number of isolates and short length of time is available, making it difficult to predict cases by serotypes over time.Citation24 In addition, our available serotype data are mostly from laboratory based surveillance systems, which are not population-based and thus we cannot estimate IPD serotype rates using this data source.
In conclusion, we were able to demonstrate significant impact of PCV-10 on IPD for age groups targeted by vaccine in Brazil, 3 years after its introduction. Through linkage of two available surveillance systems, we significantly increased case ascertainment, important for impact assessment, by means of time-series analysis. This is the first report of a time-series analysis for PCV impact in IPD conducted at national level data in a developing country. Considering the availability of notifiable diseases and laboratory based surveillance systems in most countries, such data should be better explored and used for assessment of PCV vaccination impact. This is of particular relevance for countries where the gold standard prospective population-based surveillance data over long periods of time are not available.
Methods
Study design
We assessed the impact of PCV-10 vaccination on IPD considering ecological analysis of trends in rates, using interrupted time-series analysis. We used SINAN and IAL databases from which notified cases were ascertained and case based-data were linked in order to enhance capture of cases. We also presented the number of IPD cases with serotype results by PCV types.
Study site and population
The study was conducted in Brazil in children from 2 months to 4 y of age, from 2008 to 2013. Brazil is the largest country in Latin America. In 2013, the Brazilian population was estimated as 201,032,714 inhabitants, which corresponds to all individuals under SINAN surveillance. The proportion of children under 5 y old was 7.5% (15,147,056) in 2013.Citation25
Intervention and study periods
Vaccination with PCV-10 was introduced by the National Immunization Program in all municipalities of Brazil from March to September 2010 in 3 primary doses at 2, 4, and 6 months plus a booster at 12 to 15 months of age (MS Brazil 2010). During the first year of vaccine introduction, a catch-up schedule was recommended for children between 7–11 months of age (2 doses plus booster) and for those between 12–23 months of age (single catch-up dose). The vaccine was not recommended for children aged 24 months of age and older. Three immunization periods were defined: 1) Pre vaccination period: defined as January 2008 to December 2009; 2) Transition period: defined as January to December 2010; and 3) Post vaccination period: defined as January 2011 to December 2013.
Study outcomes and case definitions
Study outcome was invasive pneumococcal disease, including pneumococcal meningitis and cases with pneumococcus isolated from any other sterile sites (i.e., blood, CSF, and others). Pneumococcal meningitis cases from SINAN were defined as the presence of diplococcus gram-positive in CSF, pneumococcal isolation by culture of CSF or blood, antigen detection by latex and contraimmunoeletrophoresis testings and real-time polymerase chain reaction. The latter test is mostly used in academic institutions.Citation16 IPD cases from IAL were those whose normally sterile samples (blood, CSF and others) or bacterial isolates were received by IAL for additional testing, including identification confirmation, antimicrobial susceptibility testing, and serotyping. In our analysis, when cases had several specimens with pneumococcus isolated, CSF was chosen over blood, which was chose over other specimens.
Isolates were serotyped by Quellung methodology and serotypes were categorized in either PCV-10 type (serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, or 23F), or PCV-13 minus PCV-10 types (serotypes 3, 6A, 19A), or non PCV-10 and non PCV-13 types.
Data sources
We obtained case based data from 2 national databases: meningitis cases from SINAN; and IPD cases, including meningitis, from IAL. Serotype data was only available for data from IAL.
Information of PCV-10 coverage was obtained from the National Immunization Program and estimated as 82%, 88%, and 92% for the years 2011, 2012 and 2013 (http://tabnet.datasus.gov.br/cgi/deftohtm.exe?pni/cnv/cpniuf.def).
Population estimates were calculated by interpolating a linear growth model using data from the Brazilian Census 2000 and 2010.Citation26 Bimonthly population estimates were considered for the interrupted time-series analysis.
Data analysis
Descriptive analysis
All cases of meningitis from SINAN and invasive bacterial disease from IAL were described by period, etiology, geographic location of case notification, type of sample, and age. The following age groups were considered: 2–11 months, 12–23 months, 2–4 years, 5–9 years, 10–17 years, 18–39 years, 40–64 years, and ≥ 65 y.
Number of IPD cases by age group and PCV types (PCV-10, PCV-13 minus PCV-10 types, and non PCV-10 and non PCV-13 types) was described by year for the 2008–2013 period.
Number and proportion of PCV types are presented by age group and vaccination periods; and PCV types percentage change were calculated, considering before and after PCV-10.
Bimonthly IPD rates per 100,000 population were calculated for the following age groups: 2–11 months, 12–23 months, 2–4 years, 5–9 years, 10–17 years, 18–39 years, 40–64 years, and ≥ 65 y.
SINAN and IAL data linkage
In order to enhance case caption from both SINAN and IAL databases, record-linkage procedures were performed to detect pairs of matched records in order to subtract copies of those that belonged to the same patient in the same episode of disease, so that there would not be duplications when adding up all the records from the 2 available sources of data. The record-linkage procedures used in-house deterministic algorithms tailored to the available data, similar to the one validated by Pacheco et al.Citation27 and used by us in Azevedo et al.Citation28
The definition of what constituted the same episode of disease for consecutive records belonging to the same patient varied for each database. For SINAN, it was defined as an interval of up to 30 d between the recorded dates of CSF collection and record entry. For IAL, it was defined as an interval of up to 6 months between the recorded dates of the arrival of samples for processing at IAL. For the linkage between SINAN and IAL data, we considered that consecutive records of the same patient with an interval of up to 6 months between the dates of CSF dates of entry in SINAN, and the dates of the arrival of samples for processing in IAL, belonged to the same episode of disease. This longer period of time when taking into consideration the IAL records was needed, because the only dates that are recorded in the IAL database are the month/year when samples arrived for processing at IAL and the year of the original sample collection, but in many cases these samples had been cultured and stored in the original laboratories for months before being finally sent for IAL for further processing. Importantly, matched records that were not identical for the variables name and dates were manually reviewed to confirm that they belonged to the same patient and episode of disease. Matched records with missing information in SINAN on disease etiology and serotyping had such details completed with IAL data. For the few matched records that had different disease etiology and/or serotyping information recorded in each database, priority was given to IAL data. After initial descriptive analysis, all cases from IAL that were due to other bacteria, and all cases from SINAN that were not confirmed as being due to S. pneumoniae were discarded for the remainder of the analysis.
Interrupted time-series analyses
In the interrupted time-series analysis, prevaccination, transition, and postvaccination periods were considered. The transition period was excluded from the time series analysis, since it represented the time period during which PCV-10 coverage was still rising to reach over 80% coverage nationwide. As we calculated bimonthly IPD incidence rates during the study period, the prevaccination period included 12 data points, while the postvaccination period included 18 data points. We used the observed postvaccination IPD rates to compare to that, which would have been expected if PCV-10 vaccination had not been introduced in the National Immunization Program. Bimonthly observed IPD rates were compared with predicted values for each age group, and the percentage changes were calculated with 95%CIs and respective p-values. The hypothesis tested was that the percentage difference calculated when comparing the estimated and observed rates in the postvaccination period represented the vaccine impact. The interrupted time-series analysis was based on exponential smoothing Holt-Winters additive modelCitation29 for rates of IPD. The explanatory variables in the model were calendar month, to control for seasonality, and linear trend over time, to control for preexisting trends (when these were found to be significant), although the time-series model implicitly considers all time-varying effects including changes in population size. The following formulae was used:Citation30
where:
α, β and γ are the smoothing parameters
is the smoothed level at time t
is the change in the trend at time t
is the seasonal smooth at time t
p is the number of seasons per year
= random error
The parameter estimates from the before PCV-10 time-series model and their variance provided a predicted distribution for time-series model parameters for after the introduction of PCV-10. Secular trends were assessed by linear regression and Cox-Stuart tests, and seasonal variations by Kruskal-Wallis tests. Residual analyses showed no substantial deviations from model assumptions. Analyses were done using Stata version 13 (www.stata.com/) and R (www.r-project.org/).
Ethical approval
This study was approved by the Research Ethics Committee, Hospital das Clínicas of the Federal University of Goias, Brazil (#162.532). Nominal information was used only for record-linkage purposes.
Disclosure of Potential Conflicts of Interest
ALA has received grants from GSK and Pfizer.
Funding
This investigation was supported by the Ministry of Health of Brazil (grant # 01/2012). ALA (#313286/2014–0), MCCB (#304211/2014–1) and CMT (#312532/2014–8) receive scientific productivity scholarships from the Brazilian National Council for Scientific and Technological Development (CNPq).
References
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet 2013; 381:1405-16; http://dx.doi.org/10.1016/S0140-6736(13)60222-6
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385:117-71; PMID:25530442; http://dx.doi.org/10.1016/S0140-6736(14)61682-2
- Conklin L, Loo JD, Kirk J, Fleming-Dutra KE, Deloria Knoll M, Park DE, Goldblatt D, O'Brien KL, Whitney CG. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type invasive pneumococcal disease among young children. Pediatr Infect Dis J 2014; 33 Suppl 2:S109-18; PMID:24336053; http://dx.doi.org/10.1097/INF.0000000000000078
- Ladhani SN, Slack MP, Andrews NJ, Waight PA, Borrow R, Miller E. Invasive pneumococcal disease after routine pneumococcal conjugate vaccination in children, England and Wales. Emerg Infect Dis 2013; 19:61-8; PMID:23259937; http://dx.doi.org/10.3201/eid1901.120741
- Robson LS, Shannon HS, Goldenhar LM, Hale AR. Guide to Evaluating the Effectiveness of Strategies for Preventing Work Injuries: How to Show Whether a Safety Intervention Really Works. Acessed October 16, 2015 at: http://www.cdc.gov/niosh/docs/2001-119/pdfs/2001-119.pdf
- Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007; 369:1179-86; PMID:17416262; http://dx.doi.org/10.1016/S0140-6736(07)60564-9
- Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: a time series analysis. Lancet Respir Med 2014; 2:387-94; PMID:24815804; http://dx.doi.org/10.1016/S2213-2600(14)70032-3
- Harboe ZB, Dalby T, Weinberger DM, Benfield T, Molbak K, Slotved HC, Suppli CH, Konradsen HB, Valentiner-Branth P. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis 2014; 59:1066-73; PMID:25034421; http://dx.doi.org/10.1093/cid/ciu524
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301-9; PMID:25656600; http://dx.doi.org/10.1016/S1473-3099(14)71081-3
- Reichardt CS, Mark MM. Quasi-experimentation. In: Wholey JS HH, Newcomer KE, ed. Handbook of Practical Program Evaluation: Jossey-Bass: A Wiley Imprint, 2004:126-49
- Ministry of Health of Brazil. National immunization program. [Technical Inform of 10-valent pneucococcal vaccine (conjugate)]. Acessed October 15, 2015 at: http://portal.saude.gov.br/portal/arquivos/pdf/intro_pneumococica10_val_04_02_10_ver_final.pdf
- Domingues CM, Verani JR, Montenegro Renoiner EI, de Cunto Brandileone MC, Flannery B, de Oliveira LH, Santos JB, de Moraes JC; Brazilian Pneumococcal Conjugate Vaccine Effectiveness Study Group. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med 2014; 2:464-71; PMID:24726406; http://dx.doi.org/10.1016/S2213-2600(14)70060-8
- Grando IM, Moraes C, Flannery B, Ramalho WM, Horta MA, Pinho DL, Nascimento GL. Impact of 10-valent pneumococcal conjugate vaccine on pneumococcal meningitis in children up to two years of age in Brazil. Cad Saude Publica 2015; 31:276-84; PMID:25760162; http://dx.doi.org/10.1590/0102-311X00169913
- Ministry of Health of Brazil. [Information System for Notifiable Diseases – SINAN]. Acessed October 15, 2015 at: http://dtr2004.saude.gov.br/sinanweb/
- Escosteguy CC, Medronho Rde A, Madruga R, Dias HG, Braga RC, Azevedo OP. [Epidemiologic surveillance and evaluation of meningitis hospital care]. Rev Saude Publica 2004; 38:657-63; PMID:15499436; http://dx.doi.org/10.1590/S0034-89102004000500007
- Ministry of Health of Brazil. [Guide to Health Surveillance]. Acessed October 16, 2015 at: http://portalsaude.saude.gov.br/images/pdf/2014/novembro/27/guia-vigilancia-saude-linkado-27-11-14.pdf
- Organización Panamericana de la Salud. SIREVA II (Sistema de Redes de Vigilancia de los Agentes Responsables de Neumonias y Meningitis Bacterianas). Acessed October 15, 2015 at: http://www.paho.org/hq/index.php?option=com _content&view=category &layout=blog &id=3609 &Itemid=3953
- Jokinen J, Rinta-Kokko H, Siira L, Palmu AA, Virtanen MJ, Nohynek H, Virolainen-Julkunen A, Toropainen M, Nuorti JP. Impact of ten-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in Finnish children–a population-based study. PLoS One 2015; 10:e0120290; http://dx.doi.org/10.1371/journal.pone.0120290
- Olarte L, Barson WJ, Barson RM, Lin PL, Romero JR, Tan TQ, Givner LB, Bradley JS, Hoffman JA, Hultén KG, et al. Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis in US Children. Clin Infect Dis 2015; 61(5):767-75; PMID:25972022; http://dx.doi.org/10.1093/cid/civ368
- Palmu AA, Kilpi TM, Rinta-Kokko H, Nohynek H, Toropainen M, Nuorti JP, Jokinen J. Pneumococcal Conjugate Vaccine and Clinically Suspected Invasive Pneumococcal Disease. Pediatrics 2015; 136:e22-7; PMID:26077477; http://dx.doi.org/10.1542/peds.2015-0458
- von Gottberg A, Cohen C, de Gouveia L, Meiring S, Quan V, Whitelaw A, Crowther-Gibson P, Madhi SA, Whitney CG, Klugman KP. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: South Africa, 2003-2008. Vaccine 2013; 31:4200-8; PMID:23684826; http://dx.doi.org/10.1016/j.vaccine.2013.04.077
- von Gottberg A, de Gouveia L, Tempia S, Quan V, Meiring S, von Mollendorf C, Madhi SA, Zell ER, Verani JR, O'Brien KL, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med 2014; 371:1889-99; PMID:25386897; http://dx.doi.org/10.1056/NEJMoa1401914
- Pirez MC, Algorta G, Cedres A, Sobrero H, Varela A, Giachetto G, Montano A. Impact of universal pneumococcal vaccination on hospitalizations for pneumonia and meningitis in children in Montevideo, Uruguay. Pediatr Infect Dis J 2011; 30:669-74; PMID:21407145; http://dx.doi.org/10.1097/INF.0b013e3182152bf1
- Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherian T, Levine OS, Whitney CG, O'Brien KL, Moore MR, et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med 2013; 10:e1001517; PMID:24086113; http://dx.doi.org/10.1371/journal.pmed.1001517
- IBGE. [Projeção da População do Brasil por sexo e idade: 2000-2060] Brazil's population projection by sex and age: 2000-2060. Acessed October 14, 2015 at: http://www.ibge.gov.br/home/estatistica/populacao/projecao_da_populacao/2013/default_tab.shtm
- IBGE. Brazilian demographic census. Acessed October 15, 2015 at: http://www.ibge.gov.br/home/mapa_site/mapa_site.php
- Pacheco AG, Saraceni V, Tuboi SH, Moulton LH, Chaisson RE, Cavalcante SC, Durovni B, Faulhaber JC, Golub JE, King B, et al. Validation of a hierarchical deterministic record-linkage algorithm using data from 2 different cohorts of human immunodeficiency virus-infected persons and mortality databases in Brazil. Am J Epidemiol 2008; 168:1326-32; PMID:18849301; http://dx.doi.org/10.1093/aje/kwn249
- Azevedo LC, Toscano CM, Bierrenbach AL. Bacterial Meningitis in Brazil: Baseline Epidemiologic Assessment of the Decade Prior to the Introduction of Pneumococcal and Meningococcal Vaccines. PLoS One 2013; 8:e64524; PMID:23823579; http://dx.doi.org/10.1371/journal.pone.0064524
- Snedecor GW, Cochran WG. Statistical Methods. 8th ed. Iowa State University Press, 1989
- Cowpertwait PSP, Metcalfe AV. Introductory Time Series with R. Springer, 2009