ABSTRACT
Vi polysaccharide typhoid vaccines cannot be used in children <2 years owing to poor immunogenic and T cell independent properties. Conjugate vaccine prepared by binding Vi to tetanus toxoids (Vi-TT) induces protective levels even in children <2 years. We evaluated efficacy and safety following vaccination with a Vi-TT vaccine in children 6 months to 12 years of age. Overall, 1765 subjects were recruited from two registered municipal urban slums of southern Kolkata. Most of the children of the slum dwellers attended the schools in the locality which was selected with permission from the school authority. Schools were randomly divided into vaccinated (Test group) and unvaccinated group (Control group). Children and their siblings of test group received 2-doses of PedaTyph™ vaccine at 6 weeks interval. Control group received vaccines as per national guidelines. Adverse events (AEs) were examined after 30 minutes, 1 month and clinical events were observed till 12 months post-vaccination. Incidence of culture positive typhoid fever in the control group was 1.27% vis-a-vis none in vaccine group during 12 months. In subgroup evaluated for immunogenicity, an antibody titer value of 1.8 EU/ml (95% CI: 1.5 EU/ml, 2.2 EU/ml), 32 EU/ml (95% CI: 27.0 EU/ml, 39.0 EU/ml) and 14 EU/ml (95% CI: 12.0 EU/ml, 17.0 EU/ml) at baseline, 6 weeks and 12 months, respectively was observed. Sero-conversion among the sub-group was 100% after 6 weeks of post-vaccination and 83% after 12 months considering 4-fold rise from baseline. The efficacy of vaccine was 100 % (95% CI: 97.6%, 100%) in the first year of follow-up with minimal AEs post vaccination. Vi conjugate typhoid vaccine conferred 100% protection against typhoid fever in 1765 children 6 months to 12 years of age with high immunogenicity in a subgroup from the vaccine arm.
Introduction
Typhoid fever is a communicable disease caused by Salmonella enterica serotype Typhi (S. typhi) that resulted in an approximately 21 million cases and 222,000 deaths annually worldwide.Citation1 It is an important public health problem in developing countries especially in India. The disease is endemic in almost all parts of the country with periodic outbreaks of water borne or food borne diseases.Citation2 In children ≤15 years, the sero-positivity rates in 1998–2002 were 32.66% ± 13.79% which increased to 50.04% ± 9.61% in 2007–2011.Citation3 Incidents occurring among children under 2 years to 4 years of age were 478/100,000 annually.Citation4
Previously, whole cell vaccines were available that conferred substantial protection against typhoid. However, because these vaccines commonly elicit debilitating adverse reactions, they were rarely used to control typhoid fever.Citation5-7 Modern typhoid vaccines are safe and highly effective in preventing typhoid. Two types of vaccines are available - live attenuated vaccine that is administered orally and contains live S.typhi and Vi capsular polysaccharide vaccine, that contains surface extracts of S.typhi bacteria and is administered intramuscularly.Citation8
Vi polysaccharide typhoid vaccines, because of their poor immunogenic and T cell independent properties, cannot be used in children less than two years of age.Citation9,10 These limitations were overcome by conjugating the Vi capsular polysaccharide vaccines to tetanus toxoid (Vi-TT).Citation11 Vi-TT conjugate vaccines stimulate specialized T cells in the human body leading to protection against typhoid.Citation11 The safety and immunogenicity of Vi-TT vaccines has already been examined in children less than 2 years of age.Citation12
The Vi-TT conjugate typhoid vaccine, PedaTyph™ (manufactured by BIO MED Pvt. Ltd, India) is the first commercially available conjugate typhoid vaccine in India. The pre-licensure study evaluated the safety and immunogenicity of vaccination in children aged up to five years and 2–16 years in separate studies.Citation10,13 We conducted this trial (CTRI no.: CTRI/2012/06/002719) to evaluate the efficacy, safety and immunogenicity of this Vi-conjugate typhoid vaccine in children aged 6 months to 12 years post vaccination for a period of one year.
Results
Study subjects
Overall, 970 and 950 subjects for the test and control group, respectively were screened. Of them, 905 and 860 subjects were enrolled for the test and control group, respectively. The demographic detail and lifestyle evaluation of both test and control groups is shown in .
Table 1. The demographic and lifestyle detail of test and control group.
Safety evaluation
All 905 subjects received the first dose of vaccine whereas only 765 subjects received the second dose of vaccine. The subjects were kept under surveillance for 30 minutes after vaccination. No remarkable AE was noticed immediately after vaccination on any of the two occasions (). Overall, AEs were reported in 172 subjects (19.0%) after the first vaccination and in 77 subjects (10.1%) after the second dose of vaccination. All the AEs were mild in nature. Two subjects aged >5 years – 6 years had a syncopal attack immediately following the vaccine but recovered after 5 minutes after lying down and taking some juices.
Table 2. Post vaccination adverse events within 30 minutes of duration.
In the follow-up period, no SAE related to the vaccine was recorded except for some episodes of fever when they were admitted to the hospital (). Thus, PedaTyph™ was found to be well tolerated in children of all age groups from 6 months to 12 years of age.
Table 3. Clinical events for a period of one year.
Efficacy evaluation
All the subjects were monitored for febrile episodes throughout the 12 months by conducting an active surveillance through weekly telephonic follow up and monthly school visit. Of all the subjects, 11 (1.27%) subjects from the control arm had BACTEC positive typhoid fever with an estimate relative risk of 0.0128 and none from the test arm had similar positivity among the febrile episodes subjects. Of the BACTEC positive cases, 72% were treated in the outpatient dispensary (OPD) of the hospital or the clinic of the site pediatrician and 27% required hospitalization with parenteral antibiotic. Among the vaccine group, 6 subjects were lost to follow-up as they migrated to their native place and none could be followed up over phone. Even though 140 subjects missed the second dose, but none of them had any febrile episodes without focus lasting for more than 3 days during the surveillance period.
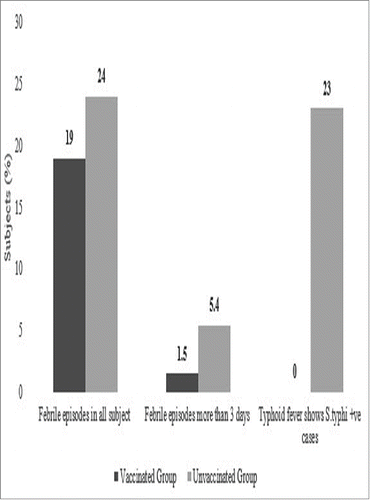
Comparison of the total number of febrile episodes without focus among the two groups showed that there were 174 subjects in the test group versus 207 in the control group having febrile episodes without focus (p = 0.015) ().
Table 4. Febrile episodes in test and control groups post vaccination.
Fever without focus for more than 3 days was reported in 7.14%, 42.86% and 50% of subjects aged 6 months to 2 years, >2–5 years and >5 years, respectively in the test group. In the control group, 27.66%, 38.30% and 34.04% of children belonging to age groups 6 months to 2 years, 2–5 years and >5 years, respectively had fever without focus for more than 3 days. All the subjects recovered from fever. Overall, febrile episodes in all subjects were 19% from vaccinated group and 24% in the unvaccinated group ().
The vaccine efficacy in the study was 100% (95%CI: 97.65%, 100%). The limitations in the study which could have affected the efficacy was migration of the 6 subjects in the vaccine arm. During the second half of the year of enrolment, the incidence of typhoid was higher probably due to the monsoon season which resulted in a higher incidence of typhoid fever in the study population.
Immunogenicity evaluation
While vaccine efficacy was planned to be done with the total population, considering some of the limitations of the efficacy study due to some confounding variables, a sub-group from the vaccine arm was selected for immunogenicity study to corroborate the efficacy findings with the rate of sero-conversion based on the GMT, fourfold rise and sero-protective cut-off titer as per the international standard of 3.52EU/ml.Citation14 Of 102 subjects, 76 subjects completed the second dose of the vaccine scheduled at 6 weeks, while 62 subjects came for the 12 month antibody titer evaluation. This resulted in a total of 40 dropouts. The sub-group of 62 subjects who came for all the three visits for the antibody titer estimation was considered for sero-conversion and immunogenicity analyses. The demographic details of this sub-group of patients are presented in .
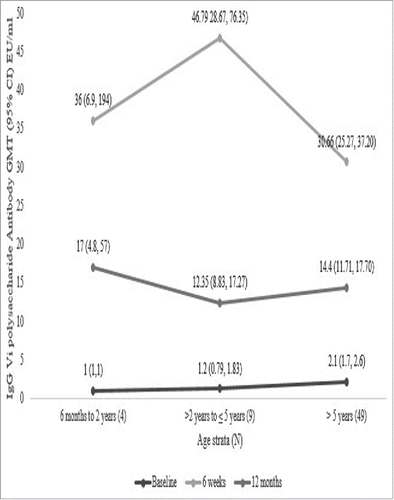
The antibody titer value was found to be 1.8 EU/ml (95% CI: 1.5 EU/ml, 2.2 EU/ml), 32 EU/ml (95% CI: 27.0 EU/ml, 39.0 EU/ml) and 14 EU/ml (95% CI: 12.0 EU/ml, 17.0 EU/ml) at baseline, 6 weeks and 12 months respectively. The GMT stratified according to age groups of 6 months to 2 years, >2 years to 5 years and >5 years at baseline, 6 weeks and 12 months post vaccination were evaluated (). The age stratified sero-protection rates at different time points post vaccination based on the sero-protective cut-off value of 3.52 EU/ml was also evaluated. It was seen that at younger age group below 5 years, the sero-protection was 100% and 98% for subjects more than 5 years of age.
Figure 2. Geometric Mean Titer of IgG Vi Polysaccharide Antibody at Baseline, 6 weeks and 12 months Post Vaccination.
Age stratified sero-conversion rates at different time points post vaccination showed that 100% of patients underwent sero-conversion (four fold or greater rise in IgG Vi polysaccharide antibody titer from baseline values) at 6 weeks post vaccination. At 12 months, it was seen that for age groups of 6 months to 2 years, 100% of the subjects were sero-converted while 88.88% subjects in the age group of 2 to 5 years and 81.63% subjects in the age group greater than 5 years (with a cut-off titer of 3.52EU/ml) were sero-converted. Overall, 100% of the subjects sero-converted at 6 weeks; while 83.87% subjects sero-converted at the 12 month follow up post vaccination.
Socio-economic status, life style (Excreta disposal and Source of Drinking water)
The subjects were categorized in to upper (Test- 2 {0.22%}, Sub-group- 0; p < 0.692), middle (Test- 87 {9.6%}, Sub-group- 13 {12.74%}; p < 0.692) and lower (Test- 816 {90.1%}, Sub-group- 89 {87.25%}; p < 0.692). The two groups were comparable statistically.
The lifestyles of the included subjects were evaluated in terms of type of toilets used for excreta disposal and source of drinking water. None used open fields for disposing excreta, a small population of 33 (3.64%) subjects in the test arm and 4 (3.92%) subjects in the control arm used public toilets.
Most of the subjects used private toilet either within (Test- 411 {45.41%}; Sub-Group- 39 {38.23%}; p < 0.301) or outside the house (Test- 461 {50.93%}; Sub-Group- 59 {57.84%}; p <0.301). Taking drinking water consumption into consideration, it was found that most of the subjects consumed tap water (Test- 882 {97.5%}, Sub-Group- 100 {98.03%}; p < 0.342) as against tube well water (Test- 21 {2.32%}, Sub-Group- 1{0.98%}; p < 0.342).
Discussion
Typhoid fever is a common serious disease in many parts of the world and remains a major health problem in developing countries. In highly endemic areas, children are at a particular risk with the peak age inversely proportional to the incidence in the community.Citation15 A study in Bangladesh showed that the most common age of infection in hospitalized children was 1–2 years.Citation16 To overcome this, polysaccharide vaccine was used but that only confers 70% protection in older children and adults. Re-vaccination is required at regular intervals as antibody levels decline, but reinjection at any age does not elicit a booster effect.Citation18-20 This leaves the most vulnerable population, i.e., the younger children and infants unprotected.
It is well known that conjugation to a carrier protein converts T cells-independent antigens into T cells-dependent ones, thereby providing a long lasting protection and enhancing memory responses. Antibody response is boosted by repeated immunization with polysaccharide conjugate vaccines which confer protection in children younger than 2 years of age.Citation17-19 Kossaczka et al.Citation4 conducted a study in children in Vietnam using Vi conjugate vaccine. The Vi conjugate vaccine in this study was prepared with Pseudomonas aeruginosa recombinant exoprotein A (rEPA) as the carrier, using either N-succinimidyl-3-(2-pyridyldithio)-propionate (SPDP; Vi-rEPA1) or adipic acid dihydrazide (ADH; Vi-rEPA2) as linkers. The authors reported that in age group of 2–4 years, a significantly higher anti-Vi IgG level of 69.9 EU was elicited with Vi-rEPA2 as compared with Vi-rEPA1 (28.9 EU; p < 0.0001). Further, the level of anti-Vi IgG elicited by 2 injections of Vi-rEPA2 in the 2–4 years age group was significantly higher than that elicited by Vi in 5–14 years age group (30.6 vs. 13.4; p< 0.0001). Canh and associates in a double-blind, placebo-controlled and randomized efficacy trial in children aged 2 to 5 years showed that Vi-rEPA offered a protective efficacy of 89%. Further, this protection lasted at least 4 years and the protective antibody titers persisted for over 8 years.Citation20
The present phase IV study primarily aimed at the field efficacy and safety of PedaTyphTM vaccine administered among healthy children aged 6 months to 12 years. The incidence of febrile episodes without focus in our study was approximately 10.34 % in 6 months to 2 years, 26.44 % in >2 years to 5 years and 63.21% in age more than 5 years children. Ochiai et al.Citation21 had conducted a study of typhoid fever in 5 Asian countries (China, India, Indonesia, Pakistan and Vietnam) and reported the incidence of febrile episodes in 0–1 years of children to be none except 12.39% and 87.60% in India and Indonesia respectively. Similarly, the incidences of febrile episodes in 2–4 years of children were 10.66%, 15.34% and 73.99% for India, Indonesia and Pakistan, respectively and none for others.
The efficacy of our Vi-TT conjugate typhoid vaccine in protecting against typhoid fever was 100% (95% CI: 97.6, 99.5%) among 1765 children aged 6 months to 12 years who received 2 doses of the vaccine after 6 weeks of post vaccination. The sero-conversion rate was 83% after 12 months of post vaccination considering 4 fold rise from the baseline. If 3.52 EU/ ml were considered as sero-protective level, then the sero-protection at 12 months among the sub-group was 98%. The GMT at baseline of the sub-group among the vaccine group was 1.8 EU/ml (95% Cl 1.5 to 2.2EU/ml) and it increased to 32 EU/ml (95 % Cl 27 to 39 EU/ml) at 6-week post the first dose. The GMT at 12 months was 14 EU/ml (95% Cl 12 to 17 EU/ml) and this value was well above the sero-protective value. Thus, Vi-TT conjugate vaccine is capable of eliciting an immune response for a longer time. Persistence of anti Vi IgG at levels that are significantly higher (>96% of the children had a > 8-fold rise; p < 0.0001) than pre-immune levels after vaccination with prototype Vi conjugate vaccine have been shown up to one year.Citation20 Mai et al.Citation22 reported an efficacy of 89% (95%CI: 76% to 96.9 %) in children aged 2–5 years after 46 months of vaccination with Vi conjugate vaccine. Lin et al.Citation23 had conducted a double blind randomized controlled study among 11,091 children of 2–5 years in Vietnam. We compared the results of GMT and protection level of both the studies and found that the results were similar with respect to each other. Szu et al.Citation24 conducted a phase I clinical trial of O-acetylated pectin conjugate typhoid vaccine in young children and observed >4-fold rise of anti-Vi IgG after 6-weeks of injection.
The incidence of culture positive typhoid fever in the control group was 1.27% vis-a-vis no typhoid incidence in the vaccine group. The overall incidence of febrile episodes was 19% in the vaccine group and 24% in the control group. During the post vaccination surveillance for 30 minutes, AEs were found including pain, redness, swelling etc. About 8.8% of test subjects complained of pain that was mild in nature. It is important to note that the number of events reduced drastically after the second dose among the same set of children. Occurrences of other AEs were less than 3% in all test subjects during the first dose which were reduced to 2% during second dose of injection.
The lifestyles of the subjects were evaluated in terms of excreta disposal and sources of drinking water. Drinking water and sanitation play an important role in the management and prevention of the disease. Both are directly proportional to the hygiene. According to a WHO report, 500 thousand typhoid cases are reported annually which includes 25 thousand deaths due to contaminated water.Citation25 Sur and associates in a study conducted in slums of Kolkata, India observed that lower literacy rates, economic status, hygienic food, type of water supply and toilet facility had a high risk for typhoid fever.Citation26
However, there are certain limitations of this study. It was an open labeled randomized control study. The sample size was only 1765, but it was nearly the total population of the children in the particular municipal ward within the studied age group. Though statistically significant differences (p < 0.001) were observed in the various socio-demographic variables between the two groups but as the sample size was large, even small differences were assumed as statistical significant. Secondly, as this was cluster randomized field study, perfect matching for age and weight was not feasible and the small differences though statistically significant may not bear any clinical significance on the end result.
Summarizing, the Vi conjugate typhoid vaccine conferred 100% protection against typhoid fever in the children 6 months to 12 years of age. The vaccine developed at the NIH used recombinant Pseudomonas aeruginosa exotoxin as a conjugate and tetanus toxoid was used in PedaTyph™. It is worth mentioning that the prototype conjugate vaccine used 25µg of Vi antigen where as PedaTyph™ contains only 5µg of Vi antigen but the 12 months follow-up data showed a comparable efficacy and immunogenicity in the two studies in spite of the various confounding variables present. But a long term post surveillance follow up data should be planned to examine the persistence of the protective efficacy and immunogenicity.
With the development of an effective conjugate Vi vaccine, it will be possible to prevent the incidence of typhoid in 6 months to 2 years old infants effective for whom no efficacious vaccine was available till now. The children between the ages of 2–5 years who were protected by Vi antigen polysaccharide vaccine conferring only 60% protection can now be vaccinated with conjugated Vi antigen typhoid for far more effective protection against typhoid. In 2008, WHO recommended typhoid vaccination for the control of endemic disease and outbreaks of typhoid.Citation27 However, the usage of typhoid vaccination programs still remains limited. There is a need to develop a system that these vaccines can be given to the infants less than two years and children of 2–5 years age group in the immunization programs.
Method
Ethical considerations
This school based study was conducted as per the Indian Council for Medical Research guidelines for Biomedical Research on Human subjects 2006 Citation28 and Schedule Y of the Drugs and Cosmetics Act.Citation29 The approval was obtained from the concerned Institutional Ethics Committee (IEC) before the initiation of the trial. Following the IEC approval, individual school permission was taken from the head of the institutes.
Study characteristics
This was an open label, randomized, controlled, post-marketing surveillance conducted in Indian children aged 6 months to 12 years in Kolkata, India. The primary objective of the study was to evaluate the field efficacy of the vaccine in healthy children aged 6 months to 12 years by comparing the rates of microbiologically proven (BACTEC positive) typhoid fever in the vaccinated group with the non-vaccinated group during a period of 1 year post-vaccination surveillance. The secondary objective of the study was to assess the immunogenicity of the vaccine in a sub-group of subjects by estimation of the anti-Vi IgG antibody titres. The subjects were given two doses at 6 weeks interval and their blood samples were taken at baseline, 6 weeks post first dose and 12 months post vaccination. The incidences of vaccine emergent local and systemic adverse events (AE) were evaluated for all the subjects.
Study site
The study was conducted among the school children from the two selected municipal ward. From this ward 12 schools were selected whose authority agreed for conducting the study. Most of the children from the slum areas go to these primary schools which were selected from that municipal ward. The siblings and the neighborhood children from 6 months to 3 years who did not go to school were enrolled in either test or control arm based on the randomized group in which the individual school child was enrolled. Each enrolled subject from the randomized school was assessed clinically and detailed clinical history was taken to assess the inclusion and exclusion criteria. The socioeconomic status, water source, and hygiene status of each household of the enrolled subject was surveyed.
Selection of study population
Randomization of Clusters
The 12 schools comprising of approximately 2000 students located in the municipal ward were randomized with each school considered as a cluster. A statistician who was unaware of the study-group assignments used graph pad software to generate the random numbers to assign clusters to vaccine and control group and the enrolled numbers in both the group comprised of 905 and 860 students, respectively. Even though some demographic difference was observed between the two groups, but being a large population, the statistical difference did not cause any significant impact in the outcome analysis.
Study population
The study included healthy children and teenagers of both genders from 6 months to 12 years of age. Parents/guardian of the subjects who gave voluntary written informed consent and agreed to comply with all trial related instructions were included in the study. Most part of the study was done in the school premises with the team of medical and paramedical staff after getting the written consent from the parents who were asked to come on the vaccine days. Few students from the vaccine group came to the hospital along with their parents to get enrolled.
Subjects having fever (>38.5°C) at the time of or in the 72 hours prior vaccination were excluded from the study. Subjects with a history of any undiagnosed fever/infection of more than 3 days duration within 1 month prior to vaccination and any established or clinically suspected immunosuppressive or immune compromised disorder/state (congenital or acquired- drug induced, neoplastic, tuberculosis etc.) were also excluded. Subjects who were administered typhoid vaccination in the last 3 years and with a known allergy to any of the components of PedaTyph™ were not included in the study.
Vaccines
The test group received two doses of PedaTyph™ vaccine–0.5 ml administered intramuscularly in the upper arm. The two doses were given at an interval of 6 weeks. Each dose of the vaccine contains 5 µg of Vi polysaccharide of S. typhi conjugated to 5 µg of TT. The vaccine was stored at 2–8°C in a refrigerator as per manufacturer's recommendations until used. The control group did not receive any study vaccine. However, they received all the scheduled vaccines as per national guidelines that were administered according to the age of the subject.
Statistical analysis
Sample Size: Previous studies reported that the risk of typhoid fever in the control group was 2 per 1000 population and that the test vaccine would reduce infection by 30% with a power of 90% at a level of 0.025 significance. Thus, the required number of subjects in test arm was found to be 970 and number of subjects in control arm was found to be 950. Therefore, with an assumption of certain drop out cases, it was decided to recruit about 2000 subjects for the study with 1000 subjects in each arm.
Descriptive statistics was used for summarizing the baseline demographic and socio economic parameters. Parametric numeric data was summarized by mean and standard deviation, non-parametric data as range, while categorical data as percentage. The 95% CI levels were computed for the variables and p < 0.05 was considered statistically significant.
Per protocol (PP) analysis was done for both efficacy and safety data. Chi Square or Fisher's exact test was used for the analysis of primary efficacy variable including typhoid fever between the test group (vaccinated) and the control group (non-vaccinated). For statistical analysis of the secondary variable, the GMT titers were evaluated. Comparison between the pre and post vaccination titres was done using paired t test.
For determination of IgG antibodies by ELISA method Citation30 standard curve was plotted using dilutions of (1:100, 1:200,1:400,1:800,1:1600,1:3200, and 1:12800) standard reference for Vi polysaccharide IgG antibodies. Preparation of standard curve of standard reference and calculation of concentration of IgG antibody from the optical density for the dilutions of test samples was done by program of ELISA for windows version 2.00 (Center for the Disease Control, U.S. Department of Health and Human Services, National Center for Infectious Diseases, Division of Bacterial and Mycotic Diseases, Atlanta, GA, USA).
Efficacy and safety variables
Efficacy data
All culture positive (BACTEC) cases of typhoid fever were identified as “cases” in each arm. The incidence of typhoid fever in each arm was compared between the test and control arm. For a sub-group of subjects from the test arm, immunogenicity was assessed; the Vi-IgG antibody titres were estimated at baseline, at 6 weeks post first dose of vaccination and after one year of vaccination. The geometric mean titer (GMT) and the mean fold rise of GMT from baseline values to 6 weeks post first dose and one year post-vaccination was computed. The sero-conversion rate (fourfold rise from baseline and sero-protection rate (with a cut-off titer of 3.52EU/ml) was also estimated.
Immunogenicity evaluation
A random subgroup comprising of 102 children from the vaccinated group was selected for immunogenicity study. This subgroup was demographically similar to the test group and hence representative of vaccine group. The IgG Vi antibody titers were measured by ELISA Citation30 (a modification of ELISA technique of the NIH, USA) at the microbiology department of the GTB Hospital, University College of Medical Sciences, New Delhi. From the 102 subjects, 62 subjects came for antibody estimation at all the different time points and were analyzed for the sero-protective level and GMT. Antibody titres were estimated at baseline, at 6 weeks post first dose of vaccination and after one year of vaccination. Human anti-Vi reference sera (lot no PUN/TYP/01) for S. typhi (118 ELISA units/ml) of anti Vi IgG was used to plot a standard curve. The IgG Vi antibody levels in the test subjects were calculated from this standard curve.
Safety evaluation
Each subject was observed for 30 minutes after vaccination. Active surveillance for AE was implemented for 1 month after vaccination. Serious adverse events (SAE) were evaluated by a local clinical monitor.Citation31 The parents were asked to report each febrile episode to the hospital or the site pediatrician. Each subject was given an identity number card which they were carrying to any local physician who were informed about the study and were asked to do a BACTEC culture from the Institute of Child Health hospital, Kolkata, if they suspected typhoid fever in the subjects with fever for more than 3 days without any focus (an acute febrile illness with rectal temperature of 38°C or higher in children younger than 36 months, without localizing signs or symptoms).
Evaluation of febrile episodes
For the entire duration of 12 months, active surveillance was done by the study team including the school authorized personnel. Telephonic follow-ups were done on a weekly basis for the subjects in both vaccine and control group. The social worker visited the school monthly and the key personnel selected from the school used to keep the record of the absenteeism and if any subject was sick the medical record was handed over to the social worker. The subject diary, which was given to the parents, also kept a record of the illness. The diary was collected from the parents by the school authority at the end of 12 months.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors acknowledge all the doctors, staff and school authority and the subjects from the Institute of Child Health and the respective schools. The authors also acknowledge Knowledge Isotopes Pvt. Ltd. (http://www.knowledgeisotopes.com) for the writing support.
References
- World Health Organization. Immunization, Vaccines and Biologicals. Typhoid. Accessed on June 04, 2015 Available at http://wwwwhoint/immunization/diseases/typhoid/en/
- Kanungo S, Dutta S, Sur D. Epidemiology of typhoid and paratyphoid fever in India. J Infect Dev Ctries 2008; 2:454-60; PMID:19745523
- Banerjee T, Shukla BN, Filgona J, Anupurba S, Sen MR. Trends of typhoid fever seropositivity over ten years in north India. Indian J Med Res 2014; 140:310-3; PMID:25297367
- Kossaczka Z, Lin FY, Ho VA, Thuy NT, Van Bay P, Thanh TC, Khiem HB, Trach DD, Karpas A, Hunt S, et al. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect Immun 1999; 67:5806-10; PMID:10531232
- Levine MM, Ferreccio C, Black RE, Tacket CO, Germanier R. Progress in vaccines against typhoid fever. Rev Infect Dis 1989; 11 Suppl 3:S552-67; PMID:2669099; http://dx.doi.org/10.1093/clinids/11.Supplement_3.S552
- Engels EA, Lau J. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev 2000; 2000(2):CD001261; PMID:10796623
- Fraser A, Goldberg E, Acosta CJ, Paul M, Leibovici L. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev 2007; 2000(2):CD001261; PMID:17636661
- Anwar E, Goldberg E, Fraser A, Acosta CJ, Paul M, Leibovici L. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev 2014; 1:CD001261; PMID:24385413
- Cadoz M. Potential and limitations of polysaccharide vaccines in infancy. Vaccine 1998; 16:1391-5; PMID:9711777; http://dx.doi.org/10.1016/S0264-410X(98)00097-8
- Chinnasami B, Mangayarkarasi V, Prema A, Sadasivam K, Davis MJ. Safety and Immunogenicity of Salmonella Typhi Vi conjugate vaccine (PedaTyph™) in children upto five years. Int J Scientific Res Pub 2013; 3:1-5
- Ochiai RL, Khan MI, Soofi SB, Sur D, Kanungo S, You YA, Habib MA, Sahito SM, Manna B, Dutta S, et al. Immune responses to Vi capsular polysaccharide typhoid vaccine in children 2 to 16 years old in Karachi, Pakistan, and Kolkata, India. Clin Vaccine Immunol 2014; 21:661-6; PMID:24599532; http://dx.doi.org/10.1128/CVI.00791-13
- Thiem VD, Lin FY, Canh do G, Son NH, Anh DD, Mao ND, Chu C, Hunt SW, Robbins JB, Schneerson R, et al. The Vi conjugate typhoid vaccine is safe, elicits protective levels of IgG anti-Vi, and is compatible with routine infant vaccines. Clin Vaccine Immunol 2011; 18:730-5; PMID:21411598; http://dx.doi.org/10.1128/CVI.00532-10
- Chinnasami B, Sadasivam K, Vivekanandhan A, Arunachalam P, Pasupathy S. A Study on Longevity of Immune Response after Vaccination with Salmonella Typhi Vi Conjugate Vaccine (Pedatyph™) in Children. J Clin Diagnostic Res 2015; 9:1-3; http://dx.doi.org/10.1111/crj.12091
- Lanh MN, Lin F-YC, Bay PV, Cong TT, Ho VA, Bryla DA, Chu C-Y, Shiloach J, Robbins JB, Schneerson R, et al. Persistence of antibodies and efficacy against typhoid fever 28–46 months following Vi conjugate vaccine (Vi-rEPA) in 2 to 5 years-old children. N Engl J Med 2003; 349:1390-91; PMID:14523155; http://dx.doi.org/10.1056/NEJM200310023491423
- Lin FY, Vo AH, Phan VB, Nguyen TT, Bryla D, Tran CT, Ha BK, Dang DT, Robbins JB. The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. Am J Trop Med Hyg 2000; 62:644-8; PMID:11289678
- Saha SK, Baqui AH, Hanif M, Darmstadt GL, Ruhulamin M, Nagatake T, Santosham M, Black RE. Typhoid fever in Bangladesh: implications for vaccination policy. Pediatr Infect Dis J 2001; 20:521-4; PMID:11368111; http://dx.doi.org/10.1097/00006454-200105000-00010
- Jones C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An Acad Bras Cienc 2005; 77:293-324; PMID:15895165; http://dx.doi.org/10.1590/S0001-37652005000200009
- Finn A. Bacterial polysaccharide-protein conjugate vaccines. Br Med Bull 2004; 70:1-14; PMID:15339854; http://dx.doi.org/10.1093/bmb/ldh021
- Vliegenthart JF. Carbohydrate based vaccines. FEBS Lett 2006; 580:2945-50; PMID:16630616; http://dx.doi.org/10.1016/j.febslet.2006.03.053
- Canh DG, Lin FY, Thiem VD, Trach DD, Trong ND, Mao ND, Hunt S, Schneerson R, Robbins JB, Chu C, et al. Effect of dosage on immunogenicity of a Vi conjugate vaccine injected twice into 2- to 5-year-old Vietnamese children. Infect Immun 2004; 72:6586-8; PMID:15501790; http://dx.doi.org/10.1128/IAI.72.11.6586-6588.2004
- Ochiai RL, Acosta CJ, Danovaro-Holliday MC, Baiqing D, Bhattacharya SK, Agtini MD, Bhutta ZA, Canh do G, Ali M, Shin S, et al. A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ 2008; 86:260-8; PMID:18438514; http://dx.doi.org/10.2471/BLT.06.039818
- Mai NL, Phan VB, Vo AH, Tran CT, Lin FY, Bryla DA, Chu C, Schiloach J, Robbins JB, Schneerson R, et al. Persistent efficacy of Vi conjugate vaccine against typhoid fever in young children. N Eng J Med 2003; 349:1390-1; PMID:14523155; http://dx.doi.org/10.1056/NEJM200310023491423
- Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, Kossaczka Z, Bryla DA, Shiloach J, Robbins JB, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med 2001; 344:1263-9; PMID:11320385; http://dx.doi.org/10.1056/NEJM200104263441701
- Szu SC, Lin KF, Hunt S, Chu C, Thinh ND. Phase I clinical trial of O-acetylated pectin conjugate, a plant polysaccharide based typhoid vaccine. Vaccine 2014; 32:2618-22; PMID:24657719; http://dx.doi.org/10.1016/j.vaccine.2014.03.023
- Meeting of the Immunization Strategic Advisory Group of Experts, November 2007–conclusions and recommendations. Wkly Epidemiol Rec 2008; 83:1-15; PMID:18175408
- Sur D, Ali M, von Seidlein L, Manna B, Deen JL, Acosta CJ, Clemens JD, Bhattacharya SK. Comparisons of predictors for typhoid and paratyphoid fever in Kolkata, India. BMC Public Health 2007; 7:289; PMID:17935611; http://dx.doi.org/10.1186/1471-2458-7-289
- Date KA, Bentsi-Enchill AD, Fox KK, Abeysinghe N, Mintz ED, Khan MI, Sahastrabuddhe S, Hyde TB, Centers for Disease C, Prevention. Typhoid Fever surveillance and vaccine use - South-East Asia and Western Pacific regions, 2009-2013. MMWR Morbidity Mortality Weekly Report 2014; 63:855-60; PMID:25275329
- Indian Council of Medical Research. Ethical Guidelines for Biomedical Research on Human Participants. Accessed on May 29, 2015 Available at http://icmrnicin/ethical_guidelinespdf 2006
- Drugs and Cosmetics (IInd Amendment) Rules. Schedule Y. Accessed on May 29, 2015 Available at http://dbtbiosafetynicin/act/schedule_ypdf 2005
- Vitale G, Librizzi R, Mocciaro C, Friscia I, Blandino E, Usticano V, Mansueto S, Di Fiore M, Reina G, Gambino G. An ELISA method in the diagnosis of typhoid fever. J Clin Lab Immunol 1990; 31:195-9; PMID:1967064
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline guideline for good clinical practice E6. Accessed on May 28, 2015 Available at http://wwwichorg/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guidelinepdf 1996