ABSTRACT
Rotavirus is the leading cause of hospitalization due to acute gastroenteritis (AGE) in infants and toddlers. However, rotavirus vaccination has been associated with a decline in hospitalization rates due to rotavirus AGE.
A descriptive retrospective study was conducted to analyze the impact of rotavirus vaccination on the rate of hospitalizations due to AGE among children ≤2 years old in 2 areas of the province of Almería, Spain. After eight years of rotavirus vaccination, rates of hospitalizations due to rotavirus AGE are diminished. This decline is closely related to vaccine coverage in the studied areas.
Introduction
Rotavirus is the most frequent cause of severe diarrhea in children <5 years, causing a high number of hospitalizations due to acute gastroenteritis (AGE),Citation1-4 In high income countries, 65% of first episodes of rotavirus occur among infants <1 y old.Citation5
Rotavirus AGE accounted for a large public health burden in the European Union before vaccination era. It has been estimated that rotavirus caused 3.6 million episodes, 231 deaths and more than 87,000 hospitalizations among 23.6 million children <5 y in Europe.Citation6 In Spain, rotavirus AGE represents 14% to 30% of all cases of AGE and requires hospitalization in 25% of cases.Citation7
As of January, 2015, 13 countries in the WHO European Region (Armenia, Austria, Belgium, Estonia, Finland, Georgia, Germany, Israel, Luxembourg, Moldova, Norway, United Kingdom, and Uzbekistan) had already included rotavirus vaccines into their national immunization programs.Citation8 Reductions in hospitalizations due to rotavirus AGE have been reported in many countries in EuropeCitation9-16 and mathematical models have calculated a huge impact on the burden of rotavirus disease.Citation17 In the United States, where the rotavirus vaccine is recommended for routine immunization of infants, the rate of hospitalizations due to rotavirus AGE is also reduced.Citation18,19
Currently licensed rotavirus vaccines include a pentavalent rotavirus vaccine (RotaTeq®, Sanofi Pasteur MSD) and a monovalent rotavirus vaccine (Rotarix®, GlaxoSmithKline). Since 2013, the World Health organization (WHO) states that all national immunization programs should include rotavirus vaccines.Citation20,21
Rotavirus vaccines were licensed in Spain in late 2006. Since 2008, the Advisory Board of Vaccines of Spanish Association of Pediatrics is recommending vaccination against rotavirus in all infants.Citation22 However, rotavirus vaccine is not included in the national vaccination schedule and it is not financed by the public health system. As a result of this health policy, there are large differences in vaccine coverage in Spain.
In 2010 the Spanish Drugs and Health Products Agency (AEMPS) suspended temporarily the release of both vaccines due to the detection of DNA fragments of porcine circovirus. Although in September 2010 the European Medicines Agency (EMA) confirmed a positive risk-benefit balance,Citation23 AEMPS kept the suspension of monovalent vaccine. Only pentavalent vaccine is currently available in Spain. However, the suspension of both vaccines for 5 months was associated with an increase in hospitalizations due to rotavirus AGE in Spain,Citation24 showing the first reverse evidence of effectiveness of rotavirus vaccines.
From an epidemiological point of view, it is important to know the impact of vaccination on general population. Rotavirus vaccination may have indirect benefits by herd immunity in unvaccinated population, including older children and adults.Citation25-27
This is a descriptive retrospective study to analyze the impact of rotavirus vaccination on the rate of hospitalizations due to AGE among children <2 y old in 2 areas of the province of Almería, Spain, that could help to guide rotavirus vaccine policies from a public health point of view
Results
Between 2005 and 2013, there were 1,298 hospitalizations due to AGE (634 in Almeria Hospital and 664 in El Ejido hospital) in children <2 y. Among them, 197 cases (31 %) were caused by rotavirus infection, in the Hospital Torrecárdenas (located in Almería city, the provincial capital) and 204 (30.5%) in El Ejido. A decline in hospitalizations due to rotavirus AGE, with an accompanying decline in hospitalizations due to all-cause of AGE, were observed since 2007 in both areas (). However, the reduction of both all-cause AGE and Rotavirus AGE was lower in El Ejido Hospital than in the hospital in Almería city ( and ). Furthermore, an increase in the incidence rates of rotavirus hospitalizations was observed in El Ejido coinciding with a decrease in rotavirus vaccine coverage ().
Figure 1. Rate of all causes AGE hospitalizations 2005–2013 (95% CI) Hospitalizations due to AGE (cases per 1,000 in children <2 y old population) in Hospital Torrecárdenas, Almería city and Hospital Poniente (El Ejido city) between 2005 and 2013. AGE, acute gastroenteritis. Annual rate of hospitalization was estimated using as denominator the number of newborns in birth cohorts during the present year and the previous year, and the obtained figure was divided by 2 CI: Confidence Interval.
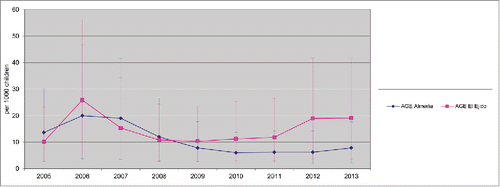
Figure 2. Rate of Rotavirus hospitalizations 2005–2013 (95% CI) Hospitalizations due to Rotavirus (cases per 1,000 in children <2 y old population) in Hospital Torrecárdenas, Almería city and Hospital Poniente (El Ejido city) between 2005 and 2013. AGE, acute gastroenteritis. Annual rate of hospitalization was estimated using as denominator the number of newborns in birth cohorts during the present year and the previous year, and the obtained figure was divided by 2 CI: Confidence Interval.
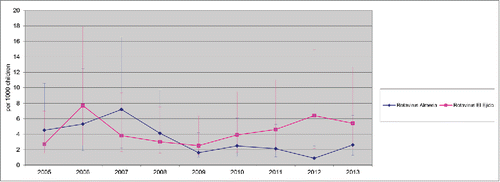
Table 1. Changes in hospitalizations due to rotavirus AGE (2005–2013) and Coverage of rotavirus vaccine***
Use of rotavirus vaccine significantly reduced the rates per 1000 children < 2 y of hospitalizations due to AGE between the 2005–2006 and 2007–2013 periods (before and after the introduction of Rotavirus Vaccines), with a decline of 30.4% of all-causes AGE (p < 0.0001) and 23% of Rotavirus AGE (p < 0.0001) studying combined data from both hospitals (Poisson regression). In addition, the reduction of Rotavirus hospitalizations rates was higher in Almeria hospital (RR= 1.67, 1.24–2.25 CI 95% shift) than in El Ejido hospital (RR= 1.29, 0.89–1.71 CI 95% shift) ().
Table 2. Incidence Rate per 1000 in children < 2 y old population comparing pre-vaccine period (2005–2006) and After introduction period (2007–2013) in both Hospitals.
Uptake of rotavirus vaccines reached its peak in 2007 in Almería (77.1%) and in 2009 in El Ejido (44.8%). In 2010, both areas had the lowest coverage (32.6% and 23.3% respectively) and since then they never have raised above 50% (). Since 2010, an increase in the rate of rotavirus hospitalizations was observed in El Ejido hospital. However, in the Almería hospital those rates remained stable the following years.
Discussion
Introduction of rotavirus vaccines was associated with a reduced rate of hospitalizations due to rotavirus AGE in both hospitals. However, higher vaccine coverage in the hospital of Almería was associated with a lower rate of hospitalizations due to rotavirus AGE compared to the hospital in El Ejido.
The differences in coverage between the 2 hospitals could be influenced by the different social and economic situation of both areas with a higher level of immigration in El Ejido area.
Limited and variable coverage has also been found in other studies in Spain, including differences in coverage between Spanish and foreigner children.Citation28 It has been estimated that the implementation of universal rotavirus vaccination in Spain could reduce hospitalizations due to rotavirus AGE by 76% to 95%.Citation7 Martinón-Torres et al. aimed to determine the effectiveness of rotavirus vaccines in Spain. From October 2008 to June 2009, among 467 consecutive children <2 y with AGE included in a pediatric research network (ReGALIP, www.regalip.org), 32.3% were rotavirus positive and 35.0% had received at least one dose of vaccine. The effectiveness of rotavirus vaccines to prevent hospitalization due to rotavirus AGE was 95.6% (85.6–98.6%).Citation29
In both areas, the uptakes for rotavirus vaccines reached their lowest level in 2010 related to the temporary suspension of both vaccines. The coverage increased after the suspension in 2010, but it has never achieved the levels found previously. This fact was related to an increase in the rates of AGE and rotavirus hospitalization in El Ejido hospital, where the coverage of rotavirus vaccine has never been above 30% until 2013. However, in Almería, where the coverage was around 50%, AGE and rotavirus hospitalizations rates has remained low, and even diminished in 2012.
This study only assessed rotavirus AGE cases, but not cases of AGE of different etiology or AGE due to unspecified viral infections. As Gil-Prieto et al.Citation17 pointed out, rotavirus AGE is not a reportable disease in Spain and many pediatricians do not ask for specific tests because the treatment does not significantly change. These authors found a 24% of rotavirus-coded hospitalizations in children <5 years, but also a 15% of undetermined etiology diarrhea, and they suggested that part of diarrhea cases coded as non-rotavirus AGE could be non-diagnosed rotavirus AGE.
The descriptive and retrospective design of this study means some limitations. Data on vaccination state of individuals were not available. With a better knowledge of rotavirus epidemiology and the introduction of new rotavirus vaccines, test for Rotavirus have become a routine test in the last years, so presumably number of rotavirus detected has increased which could be influence negatively the decrease of hospitalizations. Uptakes were computed using data from sales of rotavirus vaccines and the cohorts of newborns in the areas that might have some bias. On the other hand, hospitalizations rates were calculated using numbers of hospitalizations and newborns in the area. However, the data analyzed showed a strong relation between the rotavirus vaccines uptakes and the rates of hospitalization due to rotavirus.
Conclusion
After eight years of rotavirus vaccination in Spain, hospitalizations due to rotavirus AGE are diminished. This decline is closely related to vaccine coverage in the studied areas.
Patients and methods
Study design
A retrospective chart review was performed looking for hospitalizations due to AGE in patients <2 y old between January 2006 and December 2013 in 2 hospitals in Almería province in Spain (Hospital Torrecárdenas in Almería covering around 300.000 inhabitants and Hospital Poniente in El Ejido covering around 225.000 inhabitants). Records of patients hospitalized due to AGE were reviewed. Diagnosis codes for Acute Gastroenteritis or Enteritis were selected. Rotavirus diagnosis was made using immunochromatography test in stools. A positive test was classified as rotavirus AGE. Most AGE patients had a stool test performed. Few cases without Rotavirus test were due to difficulties to obtain the specimen within the hospitalization stay. Time periods compared were 2005–2006 (before introduction of rotavirus vaccines) and 2007–2013 (after introduction of rotavirus vaccines).
Statistical analysis
Descriptive statistical analyses were performed. To find out whether the decline in hospitalizations due to rotavirus was statistically significant over time, an overdipersion Poisson regression model was used to estimate a time trend. Number of hospitalizations and time were used as dependent and independent variables, respectively. Significance level was set at 0.05.
To estimate the annual rate of hospitalization due to rotavirus AGE per 1,000 children ≤2 years, the number of newborns in birth cohorts during the present year and the previous year were added, and the obtained figure was divided by 2. To estimate the annual vaccine coverage, the number of vaccine doses sold in the area was divided by the birth cohort, and the obtained figure was divided by 3 for RotaTeq® and by 2 for Rotarix®. Data on birth cohorts were from Statistical Institute of Andalucía (http://www.juntadeandalucia.es/institutodeestadisticaycartografia/) and data on sold vaccines were obtained from IMS Health España http://www.imshealth.com).
All statistical analysis was performed by using IBM SPSS Statistics 22.0.
Abbreviations
| AGE | = | acute gastroenteritis |
| AEMPS | = | Agencia Española de Medicamentos y Productos Sanitarios |
| DNA | = | deoxyribonucleic acid |
| EMA | = | European Medicines Agency |
| WHO | = | World Health Organization |
Disclosure of potential conflicts of interest
None of the authors has received honoraria related to this manuscript. FGS has received honoraria as consultant or conferences from Pfizer, GSK, Novartis and Sanofi Pasteur MSD in the past
Authors' contributions
Study design: Francisco Gimenez-Sanchez. Data collection: all authors. Analysis and interpretation of data: all authors. Drafting of the manuscript: Francisco Gimenez-Sanchez. Revision of the manuscript: all authors. Final manuscript was reviewed and approved by all authors before submission.
Acknowledgments
Editorial/medical writing support was provided by Marta Iglesias and Noelia Lopez. We thank Carmen Rosa Garrido for the statistical support.
References
- Giaquinto C, van Damme P. Age distribution of paediatric rotavirus gastroenteritis cases in Europe: the REVEAL study. Scand J Infect Dis 2010; 42:142-7; PMID:19916900; http://dx.doi.org/10.3109/003655-40903380495
- Giaquinto C, Van Damme P, Huet F, Gothefors L, Maxwell M, Todd P, da Dalt L. Clinical consequences of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL study. J Infect Dis 2007; 195 Suppl:S26-35; PMID:17387649; http://dx.doi.org/10.1086/516717
- Forster J, Guarino A, Parez N, Moraga F, Román E, Mory O, Tozzi AE, de Aguileta AL, Wahn U, Graham C, et al. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics 2009; 123:e393-400; PMID:19254975; http://dx.doi.org/10.1542/peds.2008-2088
- Alcalde Martín C, Gómez López L, Carrascal Arranz MI, Blanco Del Val A, Marcos Andrés H, Bedate Calderón P, González Pérez A, Jiménez Mena E. [Acute Gastroenteritis in hospitalized children. 14-Year evolution]. An Pediatr (Barc). 2002; 56:104-10; http://dx.doi.org/10.1016/S1695-4033(02)78939-X
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9:565-72; PMID:12737740; http://dx.doi.org/10.3201/eid0905.020562
- Soriano-Gabarró M, Mrukowicz J, Vesikari T, Verstraeten T. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J 2006; 25:S7-11; http://dx.doi.org/10.1097/01.inf.0000197622.98559.01
- Álvarez Aldeán J, Aristegui J, López-Belmonte JL, Pedrós M, Sicilia JG. Economic and psychosocial impact of rotavirus infection in Spain: a literature review. Vaccine 2014; 32:3740-51; http://dx.doi.org/10.1016/j.vaccine.2014.04.058
- PATH. National Rotavirus Vaccine Introduction by WHO region. [cited 2014 Dec 16]. Available from: http://sites.path.org/rotavirusvaccine/files/2014/12/PATH-Worldwide-Rotavirus-Vaccine-Introduction-Map-WHO.jpg
- Paulke-Korinek M, Kollaritsch H, Aberle SW, Zwazl I, Schmidle-Loss B, Vécsei A, Kundi M. Sustained low hospitalization rates after four years of rotavirus mass vaccination in Austria. Vaccine 2013; 31:2686-91; PMID:23597718; http://dx.doi.org/10.1016/j.vaccine.2013.04.001
- Raes M, Strens D, Vergison A, Verghote M, Standaert B. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J 2011; 30:e120-5; PMID:21436757; http://dx.doi.org/10.1097/INF.0b013e318214b811
- Vesikari T, Karvonen A, Ferrante SA, Ciarlet M. Efficacy of the pentavalent rotavirus vaccine, RotaTeq→, in Finnish infants up to 3 years of age: the Finnish Extension Study. Eur J Pediatr 2010; 169:1379-86; PMID:20559656; http://dx.doi.org/10.1007/s00431-010-1242-3
- Uhlig U, Kostev K, Schuster V, Koletzko S, Uhlig HH. Impact of Rotavirus Vaccination in Germany: Rotavirus Surveillance, Hospitalization, Side Effects and Comparison of Vaccines. Pediatr Infect Dis J 2014; 33:e299-304; PMID:24911897; http://dx.doi.org/10.1097/INF.0000000000000441
- Impact of first infant vaccination programme in England for rotavirus confirmed. HPR2014 [cited 2014 Dec 16]; 8. Available from: https://www.gov.uk/government/publications/health-protection-report-volume-8-2014/hpr-volume-8-issue-37-news
- Gagneur A, Nowak E, Lemaitre T, Segura J-F, Delaperrière N, Abalea L, Poulhazan E, Jossens A, Auzanneau L, Tran A, et al. Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: the IVANHOE study. Vaccine 2011; 29:3753-9; PMID:21443962; http://dx.doi.org/10.1016/j.vaccine.2011.03.035
- Trimis G, Koutsoumbari I, Kottaridi C, Palaiologou N, Assimakopoulou E, Spathis A, Lebessi E, Konstantopoulos A, Kafetzis D, Karakitsos P, et al. Hospital-based surveillance of rotavirus gastroenteritis in the era of limited vaccine uptake through the private sector. Vaccine 2011; 29:7292-5; PMID:21816195; http://dx.doi.org/10.1016/j.vaccine.2011.07.092
- Gil-Prieto R, Gonzalez-Escalada A, Alvaro-Meca A, Garcia-Garcia L, San-Martin M, González-López A, Gil-de-Miguel A. Impact of non-routine rotavirus vaccination on hospitalizations for diarrhoea and rotavirus infections in Spain. Vaccine 2013; 31:5000-4; PMID:23911782; http://dx.doi.org/10.1016/j.vaccine.2013.05.109
- Panatto D, Amicizia D, Giacchino R, Tacchella A, Natalizia AR, Melioli G, Bandettini R, Di Pietro P, Diana MC, Gasparini R. Burden of rotavirus infections in Liguria, Northern Italy: hospitalisations and potential savings by vaccination. Eur J Clin Microbiol Infect Dis 2011; 30:957-64; PMID:21293899; http://dx.doi.org/10.1007/s10096-011-1180-7
- Boom JA, Tate JE, Sahni LC, Rench MA, Hull JJ, Gentsch JR, Patel MM, Baker CJ, Parashar UD. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics 2010; 125:e199-207; PMID:20083525; http://dx.doi.org/10.1542/peds.2009-1021
- Kilgore A, Donauer S, Edwards KM, Weinberg GA, Payne DC, Szilagyi PG, Rice M, Cassedy A, Ortega-Sanchez IR, Parashar UD, et al. Rotavirus-associated hospitalization and emergency department costs and rotavirus vaccine program impact. Vaccine 2013; 31:4164-71; PMID:23845802; http://dx.doi.org/10.1016/j.vaccine.2013.06.085
- WHO Rotavirus vaccines: an update. Wkly Epidemiol Rec 2009; 18:533-40
- Rotavirus vaccines. WHO position paper – January 2013. Wkly Epidemiol Rec 2013; 88:49-64; PMID:23424730
- Moreno-Pérez D, Alvarez García FJ, Arístegui Fernández J, Cilleruelo Ortega MJ, Corretger Rauet JM, García Sánchez N, Hernández Merino A, Hernández-Sampelayo Matos T, Merino Moína M, Ortigosa Del Castillo L, et al. [Immunisation schedule of the Spanish Association of Paediatrics: 2014 recommendations]. An Pediatr (Barc) 2014; 80:55.e1-55.e37
- European Medicines Agency confirms positive benefit-risk balance of RotaTeq. Press release. 23/09/2010 [cited 2014 Aug 14]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2010/09/news_detail_001121.jsp&murl=menus/news_and_events/news_and_events.jsp&mid=WC0b01ac058004d5c1&jsenabled=true
- Martinón-Torres F, Aramburo A, Martinón-Torres N, Cebey M, Seoane-Pillado MT, Redondo-Collazo L, Martinón-Sánchez JM. A reverse evidence of rotavirus vaccines impact. Hum Vaccin Immunother 2013; 9:1289-91; http://dx.doi.org/10.4161/hv.24182
- Paulke-Korinek M, Kundi M, Rendi-Wagner P, de Martin A, Eder G, Schmidle-Loss B, Vecsei A, Kollaritsch H. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine 2011; 29:2791-6; PMID:21320539; http://dx.doi.org/10.1016/j.vaccine.2011.01.104
- Lopman BA, Payne DC, Tate JE, Patel MM, Cortese MM, Parashar UD. Post-licensure experience with rotavirus vaccination in high and middle income countries; 2006 to 2011. Curr Opin Virol 2012; 2:434-42; PMID:22749491; http://dx.doi.org/10.1016/j.coviro.2012.05.002
- Mameli C, Fabiano V, Zuccotti GV. New insights into rotavirus vaccines. Hum Vaccin Immunother 2012; 8:1022-8; PMID:22699445; http://dx.doi.org/10.4161/hv.20295
- Hernández Pascual M, Ruiz Serrano A, Rodríguez Ortiz de Salazar MI, Casado López M, López de Andrés A. [Rotavirus vaccine coverage in children population from Area 8 of Comunidad de Madrid]. Vacunas 2008; 9:117-20; http://dx.doi.org/10.1016/S1576-9887(08)75579-1
- Martinón-Torres F, Bouzón Alejandro M, Redondo Collazo L, Sánchez Lastres JM, Pértega Díaz S, Seoane Pillado MT, Martinón Sánchez JM. Effectiveness of rotavirus vaccination in Spain. Hum Vaccin 2011; 7:757-61; http://dx.doi.org/10.4161/hv.7.7.15576
