abstract
Even after CD4 count recovery on antiretroviral therapy, HIV infection is associated with decreased response to most vaccines compared to the general population. Chronic infections with viruses such as cytomegalovirus (CMV), hepatitis B virus (HBV), and hepatitis C virus (HCV), which are more prevalent in HIV-infected populations, have been linked to immune dysfunction and decreased vaccine response in the general population. However, whether co-infection with these other viruses contributes to the decreased vaccine response seen in adults with well-controlled HIV infection is unknown. We conducted a secondary analysis of data and serum from adults with well-controlled HIV infection from an inactivated polio vaccine trial (224 subjects) and a pneumococcal conjugate vaccine study (128 subjects). We evaluated the association of CMV, HBV, or HCV co-infection with post-vaccination antibody levels using both univariate and multivariate analyses, controlling for factors such as age, race, CD4 count, comorbidities, smoking status, and baseline antibody levels. Ninety-three percent, 7%, and 14% of subjects were co-infected with CMV, HBV, and HCV respectively. On both univariate and multivariate analysis, neither CMV nor HCV co-infection were significantly associated with post-vaccination antibody levels to either vaccine. HBV co-infection was significantly associated with post-vaccination antibody concentrations for pneumococcal serotype 7F on univariate analysis and 6A on multivariate analysis, but the association was with higher antibody concentrations. In conclusion, co-infection with CMV, HBV, or HCV does not appear to contribute to the decreased vaccine response seen in adults with well-controlled HIV infection.
Keywords:
Introduction
Adults infected with the human immunodeficiency virus (HIV) are at substantially increased risk from vaccine-preventable infections compared to the general population. Even with the widespread use of effective combination antiretroviral therapy (cART), HIV-infected adults still have a 35-fold higher rate of invasive pneumococcal disease,Citation1 a 73-fold higher rate of influenza-related mortality,Citation2 a 10-fold higher rate of invasive meningococcal disease,Citation3 and a 19-fold higher rate of chronic hepatitis B virus (HBV) infection.Citation4
At the same time, HIV-infected adults have a suboptimal immunologic response to most vaccines. Although this improves with cART and CD4 count recovery, the vaccine response in HIV-infected adults with CD4 counts in the normal range remains lower than in uninfected individuals.Citation4-6 For example, 80% of HIV-infected adults with CD4 counts >500 cells/mm3 achieved seroprotection against hepatitis A virus (HAV) after a 2-dose HAV vaccine series, versus >94% of the general population.Citation7,8 Likewise, 59% of HIV-infected adults with CD4 counts >500 cells/mm3 responded to the H1N1 vaccine, vs. 80% of uninfected adults.Citation5 A significantly decreased vaccine response in adults with well-controlled HIV infection has also been demonstrated for the pneumococcal conjugate vaccineCitation6 and the HBV vaccine series.Citation4
Chronic infections with viruses such as cytomegalovirus (CMV), HBV, and hepatitis C virus (HCV) have been linked to immune dysfunction and decreased vaccine response in the general population.Citation9-16 Given similar behavioral risk factors for acquisition, HIV-infected adults generally have high rates of CMV, HBV, and HCV co-infection. Whether co-infection with these other viruses contributes to the decreased vaccine response in adults with well-controlled HIV infection is unknown.
To explore this question, we analyzed whether CMV, HBV, or HCV co-infection were associated with decreased response to either the inactivated polio vaccine or the pneumococcal conjugate vaccine in subjects with well-controlled HIV infection from our 2 recent vaccine studies.
Materials and methods
We conducted a secondary analysis of data and serum from adults with well-controlled HIV infection who participated in either of 2 prior vaccine studies conducted at Eastern Virginia Medical School (EVMS) in Norfolk, VA. The first was a clinical trial conducted from 2012–2013 which measured polio neutralizing antibodies before and one month after a booster of the inactivated polio vaccine (224 subjects).Citation17 The second was an observational study conducted from 2013–2014 which measured antibody concentration against 4 pneumococcal serotypes before and one month after receipt of the pneumococcal conjugate vaccine, Prevnar 13 (128 subjects). For both studies, inclusion criteria included documented HIV infection, age >18 years, and an HIV viral load <400 copies/ml on the most recent test.
Both original studies and the secondary analysis received approval from the EVMS institutional review board. All subjects underwent informed consent for the original studies. We limited serum testing to detect CMV coinfection to subjects who consented to future use of their excess serum for additional studies (207 and 107 subjects from the polio and pneumococcal studies respectively).
CMV seropositivity was determined on stored serum samples through a commercial IgG ELISA assay (GenWay Biotech, Inc., San Diego, CA). Co-infection with HBV or HCV, which are routinely tested for in clinic, were determined by chart review as part of the original studies. For both original studies, co-infection was considered to be present if the clinic notes reported that the subject was HBV or HCV infected, or if the subject had a positive HBVsAg or HBV viral load or a positive HCV Ab or HCV viral load, for HBV or HCV co-infection respectively. Of note, all subjects co-infected with HBV were taking antiretroviral therapy also active against HBV, and the database did not differentiate between treated versus untreated HCV infection. HCV viral load was not collected as part of the original studies. However, both studies occurred prior to the widespread use of the direct-acting antivirals against HCV, and most HCV co-infected patients at the HIV clinic where subjects were recruited had not been treated for HCV at the time of the original studies because they were waiting for the more effective direct-acting antivirals to become available. Methods to quantitate polio neutralizing antibodies have been described elsewhere.Citation17 For the pneumococcal study, serum was tested via Indirect ELISA for IgG antibody levels against serotypes 3, 6A, 7F, and 19A according to the WHO protocol,Citation18 using United States reference pneumococcal antiserum 007sp. Serotypes 3, 7F, and 19A were chosen as they are the most prevalent serotypes in the United States since 2008 that are included in Prevnar-13, and 6A is the only serotype included in Prevnar-13 but not Pneumovax.Citation19
Descriptive statistics were reported. Continuous variables included age, CD4 count, and log-transformed baseline antibody titer or concentration. Categorical variables included CMV co-infection, HBV co-infection, HCV co-infection, gender, race, statin use, AIDS diagnosis, diabetes, history of homelessness, currently smoking, dose and route of inactivated polio vaccine (polio trial only), history of international travel (polio trial only), 1–3 vs. >3 y since Pneumovax receipt (pneumococcal study only), and history of pneumonia (pneumococcal study only). The primary outcomes were log-transformed post-booster antibody titers for each of the 3 polio serotypes for the polio study, and log-transformed post-booster antibody concentrations for pneumococcal serotypes 3, 6A, 7F, and 19A for the pneumococcal study. For univariate analyses, we used t-test, ANOVA, or simple linear regression to analyze the association between the primary outcomes and each of the covariates. For multivariate analyses, various multiple regression models were examined. Since CMV, HBV, and HCV co-infection were the 3 primary variables of interest, they were kept in all models, as well as all variables that were significant on univariate analysis for the purpose of adjustment. Two-sided statistical tests were conducted at an α level of 0.05. Statistical analysis was performed using SAS version 9.3 (SAS institute, Cary, North Carolina).
Results
In the polio trial, 93% (193/207), 7% (16/224), and 15% (33/224) of the subjects were co-infected with CMV, HBV, and HCV respectively. Mean CD4 count for the 224 subjects was 649 cells/mm3 (SD = 339). The demographics of the subjects by co-infection status are shown in . Significant demographic differences by co-infection status included smoking status, age, and CMV co-infection by HCV co-infection status, and HCV co-infection by CMV co-infection status.
Table 1. Demographics of the subjects by co-infection status.
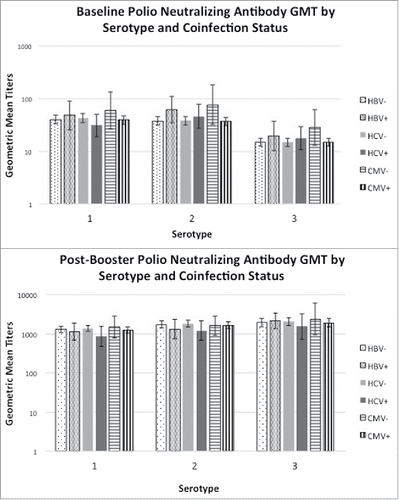
CMV, HBV, and HCV co-infection were not associated with post-booster polio neutralizing antibody titers for any serotype by univariate or multivariate analysis. shows the baseline and post-booster polio neutralizing antibody geometric mean titers separated by co-infection status. There were only 3 subjects in the polio trial co-infected with CMV, HBV, and HCV; co-infection with all 3 viruses was also not associated with post-booster polio neutralizing antibody titers compared to other subjects. Variables that were significantly associated with post-booster polio neutralizing antibody titers on both univariate and multivariate analysis included CD4 count (all 3 serotypes), baseline antibody titers (all 3 serotypes), AIDS diagnosis (serotypes 2 and 3), and race (serotype 3).
Figure 1. Polio neutralizing antibody geometric mean titer (GMT) for each serotype (1, 2, and 3) grouped by CMV, HBV, and HCV coinfection status for adults with well-controlled HIV infection. 1a shows the GMTs prior to an inactivated polio vaccine booster, and 1b shows the GMTs one month after receiving the booster. Bars represent 95% confidence intervals.
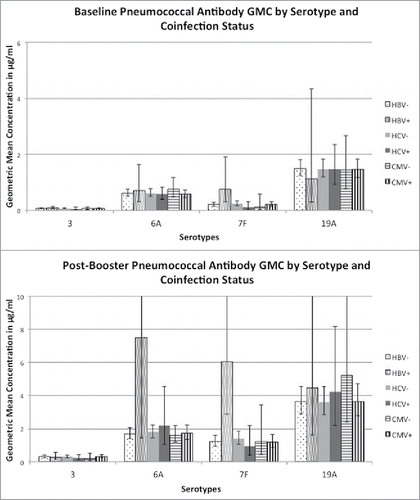
In the pneumococcal study, 92% (98/107), 5% (7/128), and 12% (16/128) of the subjects were co-infected with CMV, HBV, and HCV respectively. Mean CD4 count for the 128 subjects was 641 cells/mm3 (SD = 329). The demographics of the subjects by co-infection status are shown in . The only significant demographic difference by co-infection status was age for HCV co-infection.
CMV and HCV co-infection were not associated with post-booster pneumococcal antibody concentrations for any serotype on univariate or multivariate analysis. HBV co-infection was significantly associated with post-booster pneumococcal antibody concentrations for serotype 7F on univariate analysis only and for serotype 6A on multivariate analysis only. However, the associations were with higher concentrations. shows the baseline and post-booster pneumococcal antibody geometric mean concentrations separated by co-infection status. There were only 2 subjects in the pneumococcal study co-infected with CMV, HBV, and HCV; co-infection with all 3 viruses was not associated with post-booster pneumococcal antibody concentrations compared to other subjects. Variables that were significantly associated with post-booster pneumococcal antibody concentrations on both univariate and multivariate analysis included baseline antibody concentrations (all 4 serotypes), age (serotypes 3 and 19A), diabetes (serotypes 3 and 7F), current smoker (serotypes 3 and 7F), and race (serotype 7F).
Figure 2. Pneumococcal antibody geometric mean concentration (GMC) for each of 4 serotype (3, 6A, 7F, and 19A) grouped by CMV, HBV, and HCV coinfection status for adults with well-controlled HIV infection. 2a shows the GMCs prior to vaccination, and 2b shows the GMCs one month after receiving the pneumococcal conjugate vaccine Prevnar 13. Bars represent 95% confidence intervals.
Discussion
Our data suggests that CMV, HBV, and HCV co-infection do not significantly contribute to the decreased vaccine response seen in adults with well-controlled HIV infection. We found no association between CMV or HCV co-infection with post-vaccination antibody response after a dose of either the inactivated polio vaccine booster or the pneumococcal conjugate vaccine. HBV co-infection was significantly associated with post-vaccination antibody concentration to one pneumococcal serotype on univariate analysis only and a different pneumococcal serotype on multivariate analysis only. However, these associations were found with higher concentrations (), and were likely an artifact of the low number of HBV co-infected subjects in the pneumococcal study (7 out of 128 total).
Whether CMV infection has a positive or negative effect on immune function and vaccine response is not entirely clear. CMV infection has been hypothesized to be associated with immunosenescence, or immune aging, and chronic immune activation.Citation20 In CMV-infected individuals, CMV-specific T cells comprise around 10% of all memory CD4 and CD8 cells in middle-aged individuals,Citation21 around 20% of CD8 cells in adults with well-controlled HIV infection,Citation22 and up to 45% of the total CD8 population in the elderly.Citation23 However, recent CMV infection has also been shown to be protective against both bacterial infection and an influenza virus challenge in mice, likely due to increased levels of interferon-γCitation24-26. Studies of the effect of CMV infection on vaccine response in the general population have also had mixed and often conflicting results. In young adults, CMV infection has been associated with a lower antibody response to the 2009 H1N1 vaccine,Citation15 but a higher antibody response to the seasonal influenza vaccine.Citation26 In the elderly, CMV infection has been associated with decreased response to the influenza vaccine in some but not all studies,Citation16,26,27 and was not associated with antibody response to pneumococcal vaccines.Citation28
Chronic HBV and HCV infection have each been associated with immune exhaustion and increased expression of inhibitory lymphocyte receptors like PD-1.Citation9,29 It is well established that patients with chronic HCV infection have significantly reduced seroconversion rates after HBV vaccination,Citation9,11 and lower antibody titers after hepatitis A vaccination,Citation11 compared to the general population. Studies on vaccine response in patients with chronic HBV infection have been limited to studies of the hepatitis A vaccine, and in general have shown similar to slightly reduced seroconversion rates, but significantly lower antibody titers, after vaccination compared to the general population.Citation13,14,30-32 To our knowledge, the effect of chronic HBV or HCV infection on response to either polio or pneumococcal vaccines has not been studied previously.
Our study has several limitations. The sample sizes of subjects with HBV or HCV co-infection, or without CMV co-infection, were small. In addition, given the high rate of CMV co-infection, there were only a few subjects included who were only co-infected with HBV or HCV. Demographics and medical history were not uniform among our subjects. However, we did control for confounding factors on multivariate analysis. Our analysis was limited to HIV-infected subjects, as only HIV-infected subjects were involved in our vaccine studies. Finally, given previous vaccinations and likely prior pneumococcal exposure, the response to both vaccines likely represented a booster, rather than a primary, response.
Despite these limitations, we are the first to examine the associations between CMV, HBV, or HCV co-infection and vaccine response in adults with well-controlled HIV infection. We found no significant association between these co-infections and decreased vaccine response for either of the 2 vaccines evaluated. Other possible etiologies for the suboptimal vaccine response seen in adults with well-controlled HIV infection include residual immune dysfunction from the initial HIV infection, and/or chronic immune activation from HIV infection itself.Citation33 In addition, the CD4 count normal range, generally defined as 500–1500 cells/mm3, may be too broad. A recent study found that the median CD4 count in HIV-uninfected subjects was 900 cells/mm3, and that HIV-infected subjects who started antiretroviral therapy earlier were both more likely to achieve CD4 counts >900 cells/mm3, and more likely to respond to HBV vaccination.Citation34 Further studies are needed to explore whether this is also true for other vaccines.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was presented at the 18th Annual Conference on Vaccine Research April 13–15, 2015, in Bethesda, Maryland (Poster P15).
Funding
This work utilized data and serum from studies funded by the Doris Duke Charitable Foundation: (Clinical Scientist Development Award 2012061 [principal investigator, S.B. Troy]). S. B. Troy and A. E. B. Rossheim also received salary support while working on this project from the US National Institutes of Health: (Career Development Award 5K23AI093678 [principal investigator, S. B. Troy.]). However, neither funding agency was involved in the decision to conduct the secondary analysis, the writing of the manuscript, or the decision to submit for publication.
References
- Heffernan RT, Barrett NL, Gallagher KM, Hadler JL, Harrison LH, Reingold AL, Khoshnood K, Holford TR, Schuchat A. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995–2000. J Infect Dis 2005; 191:2038-45; PMID:15897989; http://dx.doi.org/10.1086/430356
- Cohen C, Simonsen L, Sample J, Kang JW, Miller M, Madhi SA, Campsmith M, Viboud C. Influenza-related mortality among adults aged 25–54 years with AIDS in South Africa and the United States of America. Clin Infect Dis 2012; 55:996-1003; PMID:22715173; http://dx.doi.org/10.1093/cid/cis549
- Miller L, Arakaki L, Ramautar A, Bodach S, Braunstein SL, Kennedy J, Steiner-Sichel L, Ngai S, Shepard C, Weiss D. Elevated risk for invasive meningococcal disease among persons with HIV. Ann Intern Med 2014; 160:30-7; PMID:24166695; http://dx.doi.org/10.7326/P14-9011
- Laurence JC. Hepatitis A and B immunizations of individuals infected with human immunodeficiency virus. Am J Med 2005; 118 Suppl 10A:75S-83S; PMID:16271546; http://dx.doi.org/10.1016/j.amjmed.2005.07.024
- Crum-Cianflone NF, Eberly LE, Duplessis C, Maguire J, Ganesan A, Faix D, Defang G, Bai Y, Iverson E, Lalani T, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in an immunocompromised population: a prospective study comparing HIV-infected adults with HIV-uninfected adults. Clin Infect Dis 2011; 52:138-46; PMID:21148532; http://dx.doi.org/10.1093/cid/ciq019
- Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, Ganesan A, Patel S, Landrum ML, Weintrob A, Agan BK, Medina S, Rahkola J, et al. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis 2010; 202:1114-25; PMID:20795819; http://dx.doi.org/10.1086/656147
- Kourkounti S, Papaizos V, Leuow K, Kordosis T, Antoniou C. Hepatitis A vaccination and immunological parameters in HIV-infected patients. Viral Immunol 2013; 26:357-63; PMID:24044625; http://dx.doi.org/10.1089/vim.2012.0100
- Murphy T, Feinstone S, Bell B. Hepatitis a vaccines. In: Plotkin O, and Offit, ed. Vaccines. China: Elsevier Saunders, 2013.
- Yao ZQ, Moorman JP. Immune exhaustion and immune senescence: two distinct pathways for HBV vaccine failure during HCV and/or HIV infection. Arch Immunol Ther Exp (Warsz) 2013; 61:193-201; PMID:23400275; http://dx.doi.org/10.1007/s00005-013-0219-0
- Le Saux S, Weyand CM, Goronzy JJ. Mechanisms of immunosenescence: lessons from models of accelerated immune aging. Ann N Y Acad Sci 2012; 1247:69-82; PMID:22224726; http://dx.doi.org/10.1111/j.1749-6632.2011.06297.x
- Isaguliants MG. Functionality of the immune system in patients with chronic hepatitis C: trial by superinfections and vaccinations. Expert Rev Vaccines 2007; 6:527-37; PMID:17669007; http://dx.doi.org/10.1586/14760584.6.4.527
- Ferrari C. HBV and the immune response. Liver Int 2015; 35 Suppl 1:121-8; PMID:25529097; http://dx.doi.org/10.1111/liv.12749
- Keeffe EB, Iwarson S, McMahon BJ, Lindsay KL, Koff RS, Manns M, Baumgarten R, Wiese M, Fourneau M, Safary A, et al. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology 1998; 27:881-6; PMID:9500723; http://dx.doi.org/10.1002/hep.510270336
- Lee SD, Chan CY, Yu MI, Wang YJ, Chang FY, Lo KJ, Safary A. Safety and immunogenicity of inactivated hepatitis A vaccine in patients with chronic liver disease. J Med Virol 1997; 52:215-8; PMID:9179771; http://dx.doi.org/10.1002/(SICI)1096-9071(199706)52:2%3c215::AID-JMV16%3e3.0.CO;2-J
- Wald A, Selke S, Magaret A, Boeckh M. Impact of human cytomegalovirus (CMV) infection on immune response to pandemic 2009 H1N1 influenza vaccine in healthy adults. J Med Virol 2013; 85:1557-60; PMID:23852679; http://dx.doi.org/10.1002/jmv.23642
- Derhovanessian E, Theeten H, Hähnel K, Van Damme P, Cools N, Pawelec G. Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine 2013; 31:685-90; PMID:23196209; http://dx.doi.org/10.1016/j.vaccine.2012.11.041
- Troy SB, Kouiavskaia D, Siik J, Kochba E, Beydoun H, Mirochnitchenko O, Levin Y, Khardori N, Chumakov K, Maldonado Y. Comparison of the immunogenicity of various booster doses of inactivated polio vaccine delivered intradermally versus intramuscularly to HIV-infected adults. J Infect Dis 2015; 211:1969-76; PMID:25567841
- Nahm MH, Goldblatt D. Training manual for Enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (Pn PS ELISA). (007sp Version). World Health Organization Pneumococcal Serology Reference Laboratories: WHO, 2011; Available at http://www.vaccine.uab.edu/ELISAProtocol(007sp).pdf
- Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999-2011(1.). Emerg Infect Dis 2013; 19:1074-83; PMID:23763847; http://dx.doi.org/10.3201/eid1907.121830
- Wittkop L, Bitard J, Lazaro E, Neau D, Bonnet F, Mercie P, Dupon M, Hessamfar M, Ventura M, Malvy D, et al. Effect of cytomegalovirus-induced immune response, self antigen-induced immune response, and microbial translocation on chronic immune activation in successfully treated HIV type 1-infected patients: the ANRS CO3 Aquitaine Cohort. J Infect Dis 2013; 207:622-7; PMID:23204178; http://dx.doi.org/10.1093/infdis/jis732
- Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr Opin Immunol 2009; 21:440-5; PMID:19535233; http://dx.doi.org/10.1016/j.coi.2009.05.012
- Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, Hecht F, Hsue P, McCune JM, Deeks SG. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 2010; 5:e8886; PMID:20126452; http://dx.doi.org/10.1371/journal.pone.0008886
- Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L, Rickinson AB, Moss PA. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol 2004; 173:7481-9; PMID:15585874; http://dx.doi.org/10.4049/jimmunol.173.12.7481
- Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW 4th. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 2007; 447:326-9; PMID:17507983; http://dx.doi.org/10.1038/nature05762
- Yager EJ, Szaba FM, Kummer LW, Lanzer KG, Burkum CE, Smiley ST, Blackman MA. gamma-Herpesvirus-induced protection against bacterial infection is transient. Viral Immunol 2009; 22:67-72; PMID:19210230; http://dx.doi.org/10.1089/vim.2008.0086
- Furman D, Jojic V, Sharma S, Shen-Orr SS, Angel CJ, Onengut-Gumuscu S, Kidd BA, Maecker HT, Concannon P, Dekker CL, et al. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med 2015; 7:281ra43; PMID:25834109; http://dx.doi.org/10.1126/scitranslmed.aaa2293
- den Elzen WP, Vossen AC, Cools HJ, Westendorp RG, Kroes AC, Gussekloo J. Cytomegalovirus infection and responsiveness to influenza vaccination in elderly residents of long-term care facilities. Vaccine 2011; 29:4869-74; PMID:21497631; http://dx.doi.org/10.1016/j.vaccine.2011.03.086
- O'Connor D, Trück J, Lazarus R, Clutterbuck EA, Voysey M, Jeffery K, Pollard AJ. The effect of chronic cytomegalovirus infection on pneumococcal vaccine responses. J Infect Dis 2014; 209:1635-41; PMID:24302755; http://dx.doi.org/10.1093/infdis/jit673
- Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis 2015; 6:e1694; PMID:25789969; http://dx.doi.org/10.1038/cddis.2015.42
- Cho HC, Kim YJ, Choi MS, Lee JH, Koh KC, Yoo BC, Paik SW. The seroconversion rate of hepatitis A virus vaccination among patients with hepatitis B virus-related chronic liver disease in Korea. Gut Liver 2011; 5:217-20; PMID:21814604; http://dx.doi.org/10.5009/gnl.2011.5.2.217
- Tsang SW, Sung JJ. Inactivated hepatitis A vaccine in Chinese patients with chronic hepatitis B infection. Aliment Pharmacol Ther 1999; 13:1445-9; PMID:10571600; http://dx.doi.org/10.1046/j.1365-2036.1999.00628.x
- Majda-Stanislawska E, Bednarek M, Kuydowicz J. Immunogenicity of inactivated hepatitis A vaccine in children with chronic liver disease. Pediatr Infect Dis J 2004; 23:571-4; PMID:15194843; http://dx.doi.org/10.1097/01.inf.0000130076.33497.6c
- Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013; 119:51-83; PMID:23886064; http://dx.doi.org/10.1016/B978-0-12-407707-2.00002-3
- Okulicz JF, Le TD, Agan BK, Camargo JF, Landrum ML, Wright E, Dolan MJ, Ganesan A, Ferguson TM, Smith DM, et al. Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals. JAMA Intern Med 2015; 175:88-99; PMID:25419650; http://dx.doi.org/10.1001/jamainternmed.2014.4010
