 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.abstract
Rotavirus gastroenteritis is a highly contagious, acute viral disease that imposes a significant health burden worldwide. In Japan, rotavirus vaccines have been commercially available since 2011 for voluntary vaccination, but vaccine coverage and effectiveness have not been evaluated. In the absence of a vaccination registry in Japan, vaccination coverage in the general population was estimated according to the number of vaccines supplied by the manufacturer, the number of children who received financial support for vaccination, and the size of the target population. Patients with rotavirus gastroenteritis were identified by reviewing the medical records of all children who consulted 6 major hospitals in Saga Prefecture with gastroenteritis symptoms. Vaccination status among these patients was investigated by reviewing their medical records or interviewing their guardians by telephone. Vaccine effectiveness was determined using a screening method. Vaccination coverage increased with time, and it was 2-times higher in municipalities where the vaccination fee was supported. In the 2012/13 season, vaccination coverage in Saga Prefecture was 14.9% whereas the proportion of patients vaccinated was 5.1% among those with clinically diagnosed rotavirus gastroenteritis and 1.9% among those hospitalized for rotavirus gastroenteritis. Thus, vaccine effectiveness was estimated as 69.5% and 88.8%, respectively. This is the first study to evaluate rotavirus vaccination coverage and effectiveness in Japan since vaccination began.
Introduction
Rotavirus is the predominant cause of severe gastroenteritis among infants and young children, with most infections occurring from winter to early spring. Almost every child has been infected with rotavirus at least once by the age of 5 years, with subsequent infections becoming less severe because of increasing immunity. Severe dehydration resulting from rotavirus-induced diarrhea and vomiting can be fatal. In 2008, approximately 453,000 infants or children, mainly in developing countries, were estimated to have died from rotavirus gastroenteritis (RVGE).Citation1
Although death resulting from rotavirus infection is rare in developed countries, it has been reported that approximately 40–50% of hospitalizations due to infectious gastroenteritis of infants and young children (<5 y of age) are caused by rotavirus.Citation2 Infants of less than 11 months with RVGE often require intravenous hydration and hospitalization.Citation3 Moreover, severe complications such as encephalopathy,Citation4 myocarditis,Citation5 and sudden unexpected deathCitation6 have been reported.
In Japan, a study estimated that approximately 790,000 children aged 6 y or younger visited pediatric outpatient departments because of RVGE in a year.Citation7 Furthermore, limited studies indicate an estimated 26,500–78,000 people a year are hospitalized because of RVGE.Citation8-10 These estimates indicate the substantial health burden that rotavirus also presents in developed countries.
To date, 2 live oral vaccines, a monovalent human rotavirus vaccine (RV1, Rotarix®, GlaxoSmithKline Biologicals) and a pentavalent bovine-human reassortant vaccine (RV5, Rotateq®, Merck & Co., Inc.), have been licensed in more than 100 countries. These vaccines were recommended for global use by the World Health Organization in 2009 and had been integrated into national immunization programs in more than 50 countries by April, 2014.Citation11 After approval, both vaccines showed high effectiveness and safety for RVGE.
According to a systematic review, the effectiveness of vaccines RV1 and RV5 against severe RVGE was 94–71% and 83–75%, respectively, in high-income countries.Citation12 In Japan, RV1 and RV5 have been commercially available since November 2011 and July 2012, respectively. In large clinical trials, high efficacies against severe RVGE including hospitalization were shown (79–96%).Citation13,14
An observational study examining changes in disease burden over time, including seasons before and after the introduction of these vaccines, has been reported in Japan.Citation15 In Shibata, Niigata Prefecture, Japan, the incidence rates of severe RVGE among children aged less than 3 y were found to be reduced by 71.2%, 47.7%, and 81.1% for 2012, 2013, and 2014 compared with that in 2011 before the vaccine was introduced.Citation15
Although data regarding the efficacy of the vaccine in clinical trials and the impact of vaccination via an observational study have been reported in Japan, no studies assessing the current effectiveness of these vaccines have been published. To promote rotavirus vaccination, it is first important to establish the effectiveness of this vaccine since its introduction into Japan. The aim of this study was to investigate retrospectively changes in vaccine coverage and effectiveness since 2011 in Japan using a screening method.
Results
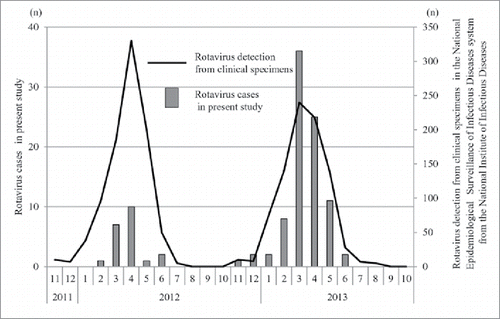
During the 2011/12 and 2012/13 seasons, 38 and 187 children, respectively, were consulted as part of a study of acute gastroenteritis patients. For this target group, the outbreak of disease peaked from March to April. According to the National Epidemiological Surveillance of Infectious Diseases system from the National Institute of Infectious Diseases, this trend correlated with the rotavirus outbreak data reported by a national infection research institute.Citation16
As shown in , during the 2011/12 season, target patients were limited and no patients with RVGE who had been vaccinated presented. Thus, vaccine effectiveness in the 2011/12 season could not be estimated. In the 2012/13 season, 87 children (46.5%) were diagnosed with RVGE using an immunochromatography kit. shows the characteristics of acute gastroenteritis patients in the 2012/13 season: the median age was 11 months (age range: 2–21 months), 109 children (58.3%) were male, and 120 children (64.2%) were inpatients. The median age of the rotavirus positive cases was 13 months with 36 cases (41.4%) being children under 1 y of age. In rotavirus positive cases, severe outcomes requiring intravenous rehydration and hospitalization were more prevalent than in the rotavirus negative cases.
Table 1. Baseline characteristics of cases in the 2012/13 season.
Figure 1. Monthly numbers of rotavirus cases in the 2011/12 and 2012/13 seasons (Bar graph) and those of rotavirus detection from clinical specimens, November 2011-October 2013 (Line graph).> The number of rotaviruses detected from clinical specimens was obtained from the Infectious Agents Surveillance Report.
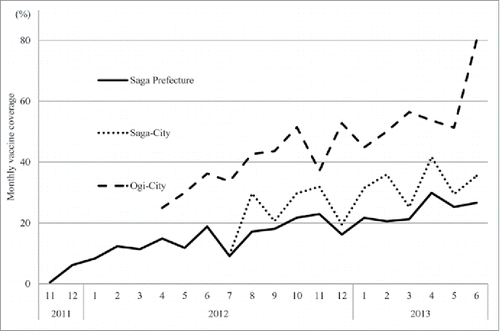
shows the change in monthly vaccine coverage following introduction of the vaccine for Saga Prefecture, Saga city, and Ogi city. In all areas, vaccine coverage increased with time (P for trend <0.01). Compared with Saga city, vaccine coverage in Ogi city, which supports the vaccination fee, was approximately 2-times higher and increased more sharply. The vaccine coverage in Saga prefecture during the 2012/13 season was 14.9%.
Figure 2. Monthly rotavirus vaccine coverage after vaccine introduction in Saga Prefecture (solid line), Saga city (dotted line) and Ogi city (dashed line). In all areas, vaccine coverage increased with time (P for trend <0.01).
shows vaccine coverage in rotavirus-positive cases and in the general population and the effectiveness of vaccination, as determined by a screening method, in the 2012/13 season. Among 87 rotavirus-positive cases, one case (1.1%) was medically examined the day after vaccination and 7 cases (8%) had an unknown vaccination status; these cases were excluded from the analysis. Four children among the remaining 79 cases received vaccine RV1. They were vaccinated with 2 doses (as recommended), and symptoms, such as vomiting and diarrhea, developed at least 1 month after the day of the last inoculation. Vaccine coverage during the 2012/13 season was estimated to be 5.1%. Vaccine coverage for rotavirus-positive cases within 12 months was 6.1% and for more than 12 months was 4.3%. According to these results, the effectiveness of vaccination for preventing RVGE in Saga for the 2012/13 season was estimated to be 69.5% (95% confidence interval [CI]: 37.1–98.9%). Vaccine effectiveness within 12 months was 71.7%, compared with 55.4% for more than 12 months. Additionally, the effectiveness in terms of reducing hospitalization owing to RVGE was estimated to be 88.8% (95% CI: 34.3–100.0%).
Table 2. Proportion of cases vaccinated and estimates of vaccine effectiveness in the 2012/13 season.
Discussion
This is the first study to investigate the effectiveness of the rotavirus vaccine during the first 2 y after it was introduced into Japan. The effectiveness of the vaccine in protecting against RVGE, as determined by the screening method, was estimated to be 69.5%. This level of efficacy was similar to that reported by a previous clinical trial (75–79% for RVGE, 96% for hospitalization owing to RVGE),Citation13,14 confirming the effectiveness of the rotavirus vaccine in Japan.
Most analyses of rotavirus vaccine effectiveness following its introduction have been case-control studies, reporting rates of 80%–90% effectiveness. According to reports from developed countries (Spain,Citation17,18 Germany,Citation19 Taiwan,Citation20 Portugal,Citation21 the United States of America,Citation22 and BelgiumCitation23), the effectiveness of the vaccine against severe RVGE requiring hospitalization was higher than that against cases not requiring hospitalization. An investigation in 2010 and 2011 in the United States of America, where the rotavirus vaccine was being used routinely, reported that the effectiveness of the vaccine against hospitalization owing to RVGE was 91% (95% CI: 80%–95%).Citation22 Similarly, in Taiwan, where rotavirus vaccination was voluntary, the effectiveness against severe RVGE was 90.4% (95% CI: 70.3–98.1%).Citation20 The effectiveness of the vaccine against hospital visits owing to RVGE was 83.7% (95% CI: 73.9%–89.8%) in PortugalCitation21 and 83.5% (95% CI: 45.5%–99.7%) in Spain.Citation18 Although, in our study, vaccine effectiveness against the total number of RVGE cases was estimated to be slightly lower compared with the results of previous clinical trials,Citation13,14 the effectiveness of the vaccine against hospitalization for RVGE was found to be almost the same.
The current study was conducted using a screening method. Vaccine effectiveness determined by this method was similar to the findings from case-control studies and will be helpful in verifying estimates in clinical trials. In Nicaragua, 2 case-control studies for rotavirus effectiveness were conducted.Citation24,25 The first study was conducted by the United States Centers for Disease Control and Prevention from 2007 to 2008. The vaccine effectiveness in 2008 against severe RVGE in children aged 8 to 11 months was 69% (95% CI: 24%–87%).Citation24 The second study was coordinated by the Ministry of Health in Nicaragua and Merck and Co., Inc. from 2007 to 2009. Using the data from 2008, the vaccine effectiveness to prevent severe RVGE was 92% (95% CI: 79%–97%) for cases <12 months of age.Citation25 Cardellino.A et al employed the screening method using these data in 2008 and estimated that vaccine effectiveness was 72% (95% CI: 62%–83%) to 92% (95% CI: 78%–100%). The results were relatively consistent with those of their 2 cases-control studies.Citation26 The screening method is therefore reliably consistent with the data from case-control studies.
The effectiveness of the vaccine among 12–22 months was lower than that among under 12 months, indicating the possibility that the effect of the vaccine lessened with time post-vaccination. However, the present study was conducted just after the introduction of the vaccine and the proportion of the population vaccinated (PPV) after 1 y was low; therefore, the effectiveness of the vaccine may have been underestimated. There are few reports about the effect of lasting immunity after rotavirus vaccination, but a follow-up survey of RV1 in high income countries of Asia reported that the protective effects against severe cases lasted at least 3 y after vaccination.Citation27
Interestingly, the rise in vaccination coverage since approval of the rotavirus vaccine was higher in areas where the vaccination fee was supported. It has previously been reported that financial assistance and information are necessary to motivate parents to voluntarily vaccinate their children in Japan.Citation28 It is possible that in areas in which financial support for vaccination is available, more information is publicized regarding the vaccine, both of which may contribute to the observed rise in vaccine coverage.
This study had some limitations. First, vaccine coverage was calculated by the number of births and the number of shipments of the vaccine; therefore, the PPV is an estimate. This estimation does not take into account infants who died after birth, those who were unable to be vaccinated because of underlying diseases, and those who only received one dose of the vaccine and were therefore not fully protected. In April, 2013, the Ministry of Health, Labour and Welfare published rotavirus vaccine coverage data by prefecture. Vaccine coverage in Saga Prefecture was 28%, which was in accordance with our findings for the same period, 29.9%. Second, using the screening method, estimations of vaccine effectiveness may fluctuate when the PPV and the proportion of cases vaccinated (PCV) are too low.Citation29 In the current study, vaccine coverage was estimated just after the introduction of the vaccine and would therefore include a period of time when vaccine coverage would be low. Because this variability in the PPV would be high, vaccine effectiveness may consequently be underestimated. Additionally, because this study was based on data from one prefecture, the limited population size may confer a degree of error in the PCV. Third, in this study the target hospitals were limited to only higher order medical institutions having hospitalization facilities and emergency lifesaving centers. The effectiveness of the vaccine against mild RVGE could therefore not be estimated because almost all of the children with initial symptoms of this disease would have presented at primary facilities. However, the primary purpose of the rotavirus vaccine is to prevent hospitalization and death caused by serious vomiting and diarrhea. The effectiveness in these situations was evaluated in this study. Finally, the screening method is a relatively simple and effective method for determining vaccine effectiveness, but does not take into account confounding factors such as incomplete vaccination or other underlying diseases. It is now necessary to confirm the authenticity of our data using a more elaborate method such as a case-control study.
In conclusion, this is the first study to evaluate the effectiveness of the rotavirus vaccine by the screening method in Japan. Vaccine coverage increased with time following introduction of the vaccine and the effectiveness of the vaccine was estimated to be 69.5% for clinically diagnosed RVGE and 88.8% for hospitalized RVGE cases. These results support promotion of the rotavirus vaccination program in Japan.
Patients and methods
Investigation area
The investigation was conducted in Saga Prefecture in Japan. This area has a stable population of approximately 850,000 inhabitants, and approximately 7,500 babies are born each year. Rotavirus vaccine is voluntary in Japan and costs 13,000–15,000 Japanese yen per inoculation. Among municipalities in this area, only Ogi city has public money to assist with the vaccine program, contributing 5,000 yen per inoculation. We requested the cooperation of 6 major hospitals with pediatric outpatient departments and hospitalization facilities in Saga (Saga University Hospital, Saga-Ken Medical Centre Koseikan, Saga National Hospital, Saga Chubu Hospital, Ureshino Medical Center, and Higashisaga Hospital). These are higher order medical institutions treating the seriously ill including those with acute gastroenteritis. The survey protocol was approved by the Ethical Committee of Saga University Faculty of Medicine, Saga-Ken Medical Centre Koseikan, and Saga National Hospital. Other hospitals were approved as cooperation facilities of Saga University Faculty of Medicine.
Rotavirus gastroenteritis patients and vaccine coverage
A case was defined as any child who was born between August 1, 2011 and April 30, 2013 (able to receive the rotavirus vaccine), displayed the clinical characteristics of gastroenteritis such as vomiting and diarrhea, and who tested positive for fecal rotavirus antigen using an immunochromatography kit. The investigation period included 2 seasons between November, 2011 and June, 2012 (2011/12 season), and November, 2012 and June, 2013 (2012/13 season), when an outbreak of rotavirus occurred according to the National Epidemiological Surveillance of Infectious Diseases system from the National Institute of Infectious Diseases.Citation16 Vaccination information, including whether a child had received the rotavirus vaccine, the type of vaccine, the number of doses, the date of the last dose, and the outcome (oral medication, intravenous rehydration to correct dehydration, admission), was usually obtained from their medical records in the hospital in cooperation with their pediatrician. If the vaccination status was not available in their medical record, it was obtained by telephone interview with their guardian. Children administered the last dose of vaccine within 14 d before the onset of gastroenteritis were excluded because of development of a protective immune response.
Vaccine coverage of the general population
Because there is no register for national vaccination in Japan, data regarding rotavirus vaccine coverage in the general population are not readily available. We therefore obtained the number of rotavirus vaccines shipped monthly by pharmaceutical companies (GlaxoSmithKline and Merck & Co.) into Saga Prefecture and divided this by the number of doses (RV1: 2, RV5: 3). Thus, we estimated the number of people that received the vaccine in each month. This was then divided by the number of monthly births taken from the demographic data for Saga Prefecture and the cities of Saga and Ogi. In this way, we estimated the monthly vaccine coverage, as shown in . Additionally, we obtained information for Ogi city regarding financial assistance for the vaccine.
Statistical analysis
The SAS statistical software package (Ver. 9.3 for Windows; SAS Institute, Cary, NC, USA) and Microsoft Excel (version 2010, Microsoft Japan) were used for statistical analysis. To compare the characteristics of gastroenteritis cases between rotavirus-positive cases and those negative cases, we used the χ2 test for categorical variables and the Wilcoxon rank sum test for continuous variables. The trend of monthly increase of vaccine coverage was tested by a logistic regression model, using PROC LOGISTIC in SAS.
The effectiveness of the rotavirus vaccine was estimated by the screening method using the Farrington algorithm, according to the following formula.Citation30
PCV is the proportion of cases vaccinated, which means the proportion of vaccinated among rotavirus-positive cases. The exact 95% confidence interval (CI) for the PCV was constructed by using the BINOMIAL option in the EXACT statement of PROC FREQ in SAS. PPV is the proportion of the population vaccinated, which means vaccination coverage of the general population. We employed the person-time method to derive PPV (i.e., person-time during the investigation period in the vaccinated divided by that in both the vaccinated and unvaccinated) because the target population aged over time. Person-months accumulated in each stratum by vaccination status (and age category [2–11 or 12–21 months] in age-stratified analysis) in the target population was calculated for the 8 month period in the 2012/13 season. For example, in age-stratified analysis (), a child who was born in May 2012 contributed 6 person-months to the age category of 2–11 months and 2 person-months to that of 12–21 months, for the above 8 month period. A P value of less than 0.05 was considered statistically significant, and for vaccine effectiveness, its estimate was regarded as significant if its 95% CI did not include the null value (0).
Abbreviations
| RVGE | = | rotavirus gastroenteritis |
| RV1 | = | Rotarix® |
| RV5 | = | RotaTeq® |
| PCV | = | the proportion of cases vaccinated |
| PPV | = | the proportion of the population vaccinated |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Tomohiro Ichimaru (Saga-Ken Medical Center Kouseikan), Toshimitu Takayanagi (Saga National Hospital), Noriko Rikitake (Saga Chubu Hospital), Tadashi Sato (Ureshino Medical Center), and Miyoko Imayoshi (Higashisaga Hospital) for their significant contributions.
Funding
This study was supported by Research on Emerging and Reemerging Infectious Diseases, Health and Labor Sciences Research Grants from the Ministry of Health, Labour and Welfare, Japan.
References
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, the WHO-coordinated Global Rotavirus Surveilance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12: 136-41; PMID:22030330; http://dx.doi.org/10.1016/S1473-3099(11)70253-5
- Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis 2006; 12: 304-6; PMID:16494759; http://dx.doi.org/10.3201/eid1202.050006
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9: 565-72; PMID:12737740; http://dx.doi.org/10.3201/eid0905.020562
- Kawamura Y, Ohashi M, Ihira M, Hashimoto S, Taniguchi K, Yoshikawa T. Nationwide survey of rotavirus-associated encephalopathy and sudden unexpected death in Japan. Brain Dev 2014; 36: 601-7; PMID:23972382; http://dx.doi.org/10.1016/j.braindev.2013.07.013
- Grech V, Calvagna V, Falzon A, Mifsud A. Fatal, rotavirus-associated myocarditis and pneumonitis in a 2-year-old boy. Ann Trop Paediatr 2001; 21: 147-8; PMID:11471258; http://dx.doi.org/10.1080/02724930120058214
- Nakano I, Taniguchi K, Ishibashi-Ueda H, Maeno Y, Yamamoto N, Yui A, Komoto S, Wakata Y, Matsubara T, Ozaki N. Sudden death from systemic rotavirus infection and detection of nonstructural rotavirus proteins. J Clin Microbiol 2011; 49: 4382-5; PMID:21998424; http://dx.doi.org/10.1128/JCM.01303-11
- Yokoo M, Arisawa K, Nakagomi O. Estimation of annual incidence, age-specific incidence rate, and cumulative risk of rotavirus gastroenteritis among children in Japan. Jpn J Infect Dis 2004; 57: 166-71; PMID:15329449
- Nakagomi T, Nakagomi O, Takahashi Y, Enoki M, Suzuki T, Kilgore PE. Incidence and burden of rotavirus gastroenteritis in Japan, as estimated from a prospective sentinel hospital study. J Infect Dis 2005; 192 Suppl 1: S106-10; PMID:16088792; http://dx.doi.org/10.1086/431503
- Kamiya H, Nakano T, Inoue M, Abd TT, Patel M, Orenstein WA, Parashar UD. A retrospective evaluation of hospitalizations for acute gastroenteritis at 2 sentinel hospitals in central Japan to estimate the health burden of rotavirus. J Infect Dis 2009; 200 Suppl 1: S140-6; PMID:19817592; http://dx.doi.org/10.1086/605028
- Ito H, Otabe O, Katsumi Y, Matsui F, Kidowaki S, Mibayashi A, Nakagomi T, Nakagomi O. The incidence and direct medical cost of hospitalization due to rotavirus gastroenteritis in Kyoto, Japan, as estimated from a retrospective hospital study. Vaccine 2011; 29: 7807-10; PMID:21821087; http://dx.doi.org/10.1016/j.vaccine.2011.07.105
- Yen C, Tate JE, Hyde TB, Cortese MM, Lopman BA, Jiang B, Glass RI, Parashar UD. Rotavirus vaccines: current status and future considerations. Hum Vaccin Immunother 2014; 10: 1436-48; PMID:24755452; http://dx.doi.org/10.4161/hv.28857
- Leshem E, Lopman B, Glass R, Gentsch J, Bányai K, Parashar U, Patel M. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14: 847-56; PMID:25082561; http://dx.doi.org/10.1016/S1473-3099(14)70832-1
- Kawamura N, Tokoeda Y, Oshima M, Okahata H, Tsutsumi H, Van Doorn LJ, Muto H, Smolenov I, Suryakiran P.V., Han HH. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine 2011; 29: 6335-41; PMID:21640780; http://dx.doi.org/10.1016/j.vaccine.2011.05.017
- Iwata S, Nakata S, Ukae S, Koizumi Y, Morita Y, Kuroki H, Tanaka Y, Shizuya T, Schidel F, Brown MV, Lawrence J. Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double-blind, placebo-controlled, multicenter trial. Hum Vaccin Immunother 2013; 9: 1626-33; PMID:23732903; http://dx.doi.org/10.4161/hv.24846
- Oishi T, Taguchi T, Nakano T, Sudo S, Kuwajima H, Group SRS. The occurrence of severe rotavirus gastroenteritis in children under 3 years of age before and after the introduction of rotavirus vaccine: a prospective observational study in three pediatric clinics in Shibata City, Niigata Prefecture, Japan. Jpn J Infect Dis 2014; 67: 304-6; PMID:25056079; http://dx.doi.org/10.7883/yoken.67.304
- National Institute of Ingectious Diseases and Tuberculosis asnd Infectious Diseases Control Division MoH, Labour and Welfare. Rotavirus,2010-2013,Japan. Japan: Infectious Agents Surveillance Report, 2014.
- Castilla J, Beristain X, Martínez-Artola V, Navascués A, García Cenoz M, Alvarez N, Polo I, Mazon A, Gil-setas A, Barricarte A. Effectiveness of rotavirus vaccines in preventing cases and hospitalizations due to rotavirus gastroenteritis in Navarre, Spain. Vaccine 2012; 30: 539-43; PMID:22122860; http://dx.doi.org/10.1016/j.vaccine.2011.11.071
- Bellido-Blasco JB, Sabater-Vidal S, Salvador-Ribera MeM, Arnedo-Pena A, Tirado-Balaguer MD, Meseguer-Ferrer N, Silvestre-Silvestre E, Romeu-Garcia MA, Herrero-Carot C, Moreno-Munoz M-R. Rotavirus vaccination effectiveness: a case-case study in the EDICS project, Castellón (Spain). Vaccine 2012; 30: 7536-40; PMID:23103196; http://dx.doi.org/10.1016/j.vaccine.2012.10.049
- Adlhoch C, Hoehne M, Littmann M, Marques AM, Lerche A, Dehnert M, Eckmanns T, Wichimann O, Koch J. Rotavirus vaccine effectiveness and case-control study on risk factors for breakthrough infections in Germany, 2010-2011. Pediatr Infect Dis J 2013; 32: e82-9; PMID:23334342; http://dx.doi.org/10.1097/INF.0b013e3182720b71
- Chang WC, Yen C, Wu FT, Huang YC, Lin JS, Huang FC, Yu HT, Chi CL, Lin HY, Tate JE, Parashar UD, Wu HS, Hsiung CA. Effectiveness of 2 rotavirus vaccines against rotavirus disease in Taiwanese infants. Pediatr Infect Dis J 2014; 33: e81-6; PMID:24569388; http://dx.doi.org/10.1097/INF.0000000000000105
- Marlow R, Ferreira M, Cordeiro E, Trotter C, Januário L, Finn A, Rodrigues F. Case control study of rotavirus vaccine effectiveness in portugal during six years of private market use. Pediatr Infect Dis J 2014
- Cortese MM, Immergluck LC, Held M, Jain S, Chan T, Grizas AP, Knizew S, Barrett C, Quaye O, Mijatovic-Rustempasic S, et al. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics 2013; 132: e25-33; PMID:23776114; http://dx.doi.org/10.1542/peds.2012-3804
- Braeckman T, Van Herck K, Meyer N, Pirçon JY, Soriano-Gabarró M, Heylen E, Zeller M, Azou M, Capiau H, Koster JD, et al. Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: case-control study. BMJ 2012; 345: e4752; PMID:22875947
- Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, Gonzalez A, Malespin O, Amador J J, Umana J, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA 2009; 301: 2243-51; PMID:19491186; http://dx.doi.org/10.1001/jama.2009.756
- Mast TC, Khawaja S, Espinoza F, Paniagua M, Del Carmen LP, Cardellino A, Sanchez E. Case-control study of the effectiveness of vaccination with pentavalent rotavirus vaccine in Nicaragua. Pediatr Infect Dis J 2011; 30: e209-15; PMID:21768920; http://dx.doi.org/10.1097/INF.0b013e31822a8527
- Cardellino A, Khawaja S, Sánchez Cruz E, Mast TC. Effectiveness of vaccination with the pentavalent rotavirus vaccine in Nicaragua as determined using the screening method. Hum Vaccin Immunother 2013; 9: 1449-53; PMID:23571175; http://dx.doi.org/10.4161/hv.24338
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, Lee BW, Doorn LJ, Teoh YL, Teoh YL, et al. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: a randomized clinical trial in an Asian population. Vaccine 2012; 30: 4552-7; PMID:22497874; http://dx.doi.org/10.1016/j.vaccine.2012.03.030
- Shono A, Kondo M. Factors that affect voluntary vaccination of children in Japan. Vaccine 2014; 33: 1406-11; PMID:25529291; http://dx.doi.org/10.1016/j.vaccine.2014.12.014
- Chen RT, Orenstein WA. Epidemiologic methods in immunization programs. Epidemiol Rev 1996; 18: 99-117; PMID:9021306; http://dx.doi.org/10.1093/oxfordjournals.epirev.a017931
- Farrington CP. Estimation of vaccine effectiveness using the screening method. Int J Epidemiol 1993; 22: 742-6; PMID:8225751; http://dx.doi.org/10.1093/ije/22.4.742
