ABSTRACT
Globally, dengue virus (DENV) is one of the most widespread vector-borne viruses. Dengue disease affects populations in endemic areas and, increasingly, tourists who travel to these countries, but there is currently no approved vaccine for dengue. A phase 3 efficacy trial with Sanofi-Pasteur's recombinant, live-attenuated, tetravalent dengue vaccine (CYD-TDV) conducted in South East Asia showed an overall efficacy of 56% against virologically confirmed dengue infections of any severity and any of the 4 serotypes, but the long-term protection of the vaccine has yet to be demonstrated. To address longevity of antibody titers and B cell memory, we recalled study participants from an earlier CYD immunogenicity study (Phase 2) conducted in Singapore that enrolled healthy volunteers in the year 2009. Depending on the age group, 57–84% of the participants initially generated a neutralizing antibody titer ≥ 10 to all 4 DENV serotypes 28 d after the third and final dose. We observed very low antibody titers in blood samples collected from 23 vaccinees 5 y after the first dose, particularly titers of antibodies binding to virus particles compared with those binding to recombinant E protein. The in vivo efficacy of plasma antibodies against DENV-2 challenge was also tested in a mouse model, which found that only 2 out of 23 samples were able to reduce viremia. Although the sample size is too small for general conclusions, dengue immune memory after vaccination with CYD-TDV appears relatively low.
ABBREVIATIONS
| DENV | = | dengue virus |
Introduction
Globally, an estimated 390 million new infections of dengue virus (DENV) are thought to occur each year, with approximately a third of infected individuals manifesting clinical symptoms.Citation1 A chimeric tetravalent DENV vaccine (CYD-TDV) developed by Sanofi-Pasteur was the first dengue vaccine candidate to be tested in large efficacy trials in Asia and Latin America. CYD-TDV comprises 4 yellow fever (YF) vaccine constructs in which the YF pre-membrane protein (prM) and E glycoprotein are individually replaced by the prM-E of the 4 DENV serotypes. Three trials have been conducted to date, all with the same immunization schedule that included 3 doses, 6 months apart, and study participants were followed for 25 months after the first dose. The initial Phase 2b trial (2,669 children in the vaccine test group and 1,333 in the control group), conducted in Thailand in 4–11-year-old children, showed that the primary estimate of efficacy was only 30.2% (95% CI −13.4 to 56.6) and was not significant.Citation2 A multi-center Phase 3 clinical trial was performed in 5 countries in South East Asia, involving 10,257 children aged 2–14 y (6,851 children in the vaccine test group and 3,424 children in the control group).Citation3 The Latin American Phase 3 clinical trial involved 20,869 children aged 9–16 y (12,574 in the vaccine test group and 6,261 in the control group).Citation4
The analysis of the pooled data from the 2 Phase 3 trials showed an overall efficacy of 60.3% (54.7% for DENV-1, 43% for DENV-2, 71.6% for DENV-3 and 76.3% for DENV-4), and also highlighted that efficacy results were higher in children aged 9 and above compared with children below the age of 9(5) Across all age groups, the level of protection in baseline seronegative subjects was clearly lower than in baseline seropositive subjects, but was overall higher in older children. In subjects 9 and above, the efficacy in baseline seropositive individuals was 81.9%, and in baseline seronegatives 52.5%. In those below the age of 9, the efficacy was 70.1% in seropositives, but only 14.4% (non-significant) in seronegatives.
In addition to providing protection against each individual serotype, safety and persistence of protective immune memory over prolonged periods are key parameters that define the value of a vaccine. The findings from a 3 y follow-up study addressing the long-term safety of CYD-TDV of the 2 Phase 3 trials showed that the relative risk of hospitalization across all age groups was 1.04 in the Asia Pacific trial and 0.53 in the Latin America trial.Citation5 A higher relative risk of hospitalization for vaccine recipients below the age of 9 was evident (1.58), and for those aged 2–5 it was as high as 7.45.Citation5 There were no safety signals in older children: In children aged 9 and above, the relative risk was 0.57 in the Asian trial and 0.53 in the Latin American trial. In addition to this large observation-based follow-up study, a longitudinal study by Capeding et al. monitored neutralizing titers in children and adults vaccinated with CYD-TDV over a 5-year period following the last immunization.Citation6 A marked decline in neutralizing titers was observed over time in vaccinees that did not experience a natural dengue or flavivirus infection within the follow-up period. While neutralizing titers measured in the blood are a useful readout of DENV immunity, the correlation between neutralizing titers and protection is still not clear.Citation7
Epidemiological studies have shown that natural infection confers long-term protection against re-infection with the same (homologous) DENV serotype but not against heterologous serotypes.Citation8 During a repeat infection with a heterologous serotype, serotype cross-reactive DENV-specific B and T cells are rapidly activated, producing large quantities of high affinity antibodies within a few days of infection.Citation9-11 Antibody titers reach a nadir 3 to 4 weeks after fever onset and subside to near baseline within one year.Citation12,13 These rapidly declining titers represent cross-reactive antibodies, produced by short-lived B cells after infection, which gradually disappear due to their restricted biological half-life.Citation12 In the context of immune memory, the CYD-TDV vaccine proved to be more effecacious in individuals with pre-existing immunity to DENV or Japanese encephalitis virus (JEV).Citation3-5 This suggests that re-activation of specific B and T cells was an important mechanism contributing to the protective capacity of the vaccine. It is possible that protection after vaccination depends largely on cross-reactive antibodies circulating in the blood at the time of infection. In this scenario, protection would be limited to the window of time in which the titers of these antibodies remain above a protective threshold.Citation14-16
The aim of this study was to assess the longevity of DENV-specific antibodies titers and to quantify memory B cells in the blood of vaccinees 5 y after the final immunization with the CYD-TDV. Plasma samples of 23 vaccinees aged 27-50 y previously enrolled in the phase 2 immunogenicity study in Singapore were compared with positive control samples from individuals with previous natural infections. The data show substantially lower antibody titers in vaccinees than in individuals with a history of natural infection, suggesting that immunity after vaccination with CYD-TDV is low after 5 y.
Results
Low antibody titers 5 y after vaccination with CYD-TDV
A CYD-TDY safety and immunogenicity double-blind randomized trial was initially conducted in Singapore in 2009, in which 300 adults (aged >21) were enrolled at the National University of Singapore.Citation17 After official unblinding of the trial, we contacted 50 participants who had received the CYD-TDV vaccine and whose contact addresses were still available. Of these 50, 23 participants consented to have blood taken for this study. In August 2014, e.g., approximately 5 y after the first vaccination, we obtained blood samples to assess DENV-specific humoral immunity. Pre-existing immunity (if known) and age of the participants are summarized in . We have previously established 3 ELISAs to separately quantify plasma antibodies binding to recombinant DENV E protein, to recombinant EDIII, and to UV-inactivated virus particles (UV-DENV) for each serotype.Citation12 We employed these 3 assays to determine endpoint titers in the 23 vaccinee samples. As a positive control, we used a pool of 3 plasma samples from healthy donors seropositive for DENV-specific IgG. Compared to the positive control, endpoint titers in the vaccinee samples were 10- to >200 -fold lower in the UV-DENV ELISA and in the E protein ELISA (). High antibody titers, which were specific for E protein, particularly for E protein of DENV-2, were only detected in samples from DV-19 and DV-20 (). Antibody titers to EDIII were comparable between the vaccine samples and positive control ().
Figure 1. Vaccinees show ELISA titers comparable to dengue-naive individuals. End-point titers of DV-1 to DV-23, a positive control (2 readings), and a negative control (2 readings) were determined for A) UV-DENV ELISA, B) E protein ELISA, and C) EDIII ELISA of all 4 serotypes. The same data are also represented as OD450 (sample) divided by the OD450 value of the positive control on the same plate for D) UV-DENV, E) E protein, and F) EDIII. The dotted lines indicate the ratio for the negative control for each serotype and provide a cut-off for the respective readouts. The color of the dotted line corresponds to the color of the respective serotype.
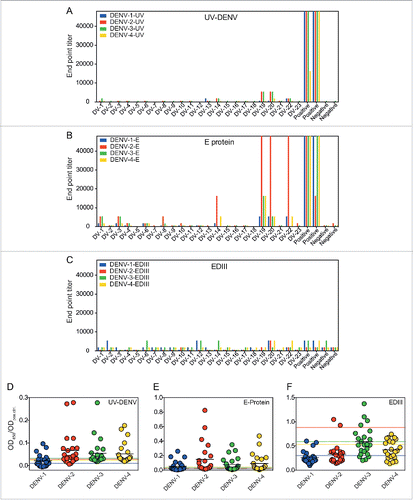
Table 1. List of vaccinees analyzed in the study.
We also performed a second analysis of antibody titers, based on the ratio of the optical density (OD) of vaccine samples divided by the OD of the positive control (). This analysis allowed a direct comparison of antibody titers in the vaccinees with titers in patients with natural DENV infection, which we had studied previously.Citation12 After natural secondary infections, an OD ratio >1 (i.e., an OD value similar or higher than the positive control) was observed within the first 3 months of infection, before a gradual reduction to a ratio of approximately 0.5 after one year. However, in the majority of the vaccine samples studied here, the OD ratio was below 0.1 and not higher than the negative control ().
We hypothesized that the higher titers of antibodies to UV-DENV and E protein observed in some participants () may be related to pre-existing dengue immunity at the time of vaccination. Screening was only performed in a subset of study participants prior to the start of the original clinical trial; therefore, this information was only available for 9 out of 23 participants (). Unexpectedly, antibody titers did not correlate with immune status at vaccination (), but a larger sample number would be required to confirm this finding. Unfortunately, pre-existing titers were not known for participants DV-19 and DV-20, both of which showed high antibody titers in this study. In summary, five years after vaccination, 21 out of 23 CYD-TDV vaccinees had low antibody titers to all four DENV serotypes
Figure 2. Pre-existing immunity at the time of vaccination does not result in markedly increased titers. Samples tested in the ELISAs described in were grouped according to immune status at the time of vaccination. DENV-immune (2) and DENV–naïve individuals (7) are shown as individual symbols for A) UV-DENV ELSIA, B) E protein ELISA, and C) EDIII ELISA. The pre-immune status was unknown (undefined) for 14 samples, represented in gray bar graphs for each ELISA readout. Box and whisker plots indicate the min to max values.
Plasma antibodies in vaccinees show limited in vivo efficacy against DENV-2
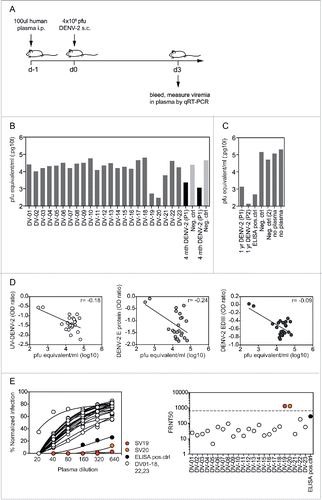
Although the readouts from the ELISAs provide information about the relative amounts of antibodies present against different epitopes, the protective efficacy of those antibodies is unknown. Similarly, poor correlations between neutralizing titers, determined by neutralization assay, and in vivo efficacy have been reported previously.Citation7,18 Mouse models offer a valuable method to determine the neutralizing capacity of human plasma antibodies in vivo.Citation19,20 We tested the neutralizing capacity of DENV-specific antibodies from the vaccinees by transferring plasma (100 µl) into interferon α/β and gamma-receptor (IFNAGR)-deficient mice, and challenging with DENV-2 after 24 hours (). IFNAGR mice were selected for this experiment because wild-type mice are not susceptible to dengue infection.Citation21 The DENV-2 serotype was selected because it results in a high and reproducible viremia in mice, compared with the other serotypes. Viral loads were measured in the blood of infected mice at the peak of viremia 3 d after infection. Plasma from a recovered patient who had experienced secondary DENV-2 infection 4 months earlier was used as a positive control Citation12 (). In a separate experiment, we also tested the positive control plasma used in the ELISAs as well as samples from 2 patients with DENV-2 infection one year earlier.Citation12 All control samples reduced viremia in mice more than 100-fold (). In contrast, only 2 out of 23 vaccinee plasma samples reduced viremia by more than 10 times (). These two samples, DV-19 and DV-20, were the same as those that showed high titers in both the UV-DENV and the E protein ELISA. However, we did not observe a significant correlation between viremia and either DENV-2 UV-DENV or E titers, most likely because of the comparatively low titers of the remaining samples (). To address a potential correlation of in vivo efficacy with neutralizing titers, we determined the 50% focus reduction neutralization test (FRNT50) titers for all samples and the ELISA positive control (). In line with the ELISA data, samples DV-19 and DV-20 had substantially higher titers than all other samples and the positive control. The in vivo experiments suggested that relatively high antibody titers were required to achieve significant antibody-mediated reduction of viremia. Samples DV-19 and DV-20 showed titers of > 48,000 for DENV-2 in the E protein ELISA, titers of 5,400 for DENV-2 in the UV-DENV ELISA, and titers > 640 in the FRNT assay. Interestingly, samples DV-19 and DV-20 were also the only 2 samples with DENV-2 EDIII titers higher than the negative control (). With the limited number of samples analyzed here, it was not possible to conclude whether E protein-specific, EDIII-specific, and/or virus particle-specific antibodies were responsible for the in vivo efficacy.
Figure 3. Low in vivo efficacy of vaccine plasma antibodies. A) Experiment setup. Plasma (100 μl) was transferred intraperitoneally into IFNAGR mice 24 h before subcutaneous infection with DENV-2. Blood was collected at day 3 after infection and virus was quantified by qRT-PCR. B) Pfu equivalents/ml plasma for samples DV-1 to DV-23. The positive control was a plasma sample from an individual with known DENV-2 infection 4 months earlier (P1). Plasma from a DENV-naïve donor was used as a negative control. The protective effect of plasma DV-19 and DV-20 was confirmed in an independent experiment. C) To further validate the in vivo model the following were tested: plasma from P1 and from an additional individual P2, both collected one year after DENV-2 infection, positive control used in the ELISA readouts, negative control used in (B) and a second negative control. D) No significant correlation was observed between viremia in mice following plasma transfer and antibody titers to UV-DENV, E protein, and EDIII. The Spearman r for the individual correlations is indicated. E) DENV-2 neutralization curves for all samples and the ELISA positive control, with corresponding FRNT50 values illustrated on the right. The FRNT50 values for DV-19 and −20 were not exact and were arbitrarily set above the highest plasma dilution tested, which is indicated by the dashed line.
DENV-specific memory B cells are scarce in the blood and secrete low amounts of antibodies when stimulated
While pre-existing antibodies in the plasma, as detected in the previous assays, are crucial for early virus neutralization, the rapid activation of pre-existing immune memory cells also contributes to protection after secondary infection.Citation22 We performed ELISPOT assays to quantify the numbers of specific memory B cells present in vaccinee samples by re-stimulating PBMCs with Protein A, CpG, and IL-21 on ELISPOT plates coated with virus particles Citation23 or tetanus toxoid.Citation24 (). The efficiency of re-stimulation varied between samples (), but the number of DENV-specific memory B cells per total IgG-secreting antibody-secreting cells (ASC) was typically below 0.5% (). Tetanus-specific immunity could be detected in 17 out of 20 samples tested and, in one particular sample, represented > 2% of total memory B cells (). Interestingly, DENV-specific B cell spots were generally small, compared to tetanus-specific B cells, which generated larger spots (), indicating that a low amount of antibodies was secreted per DENV-specific B cell. Percentages of specific cells for DV-20 to DV-23 could not be calculated because total IgG ASC numbers were not available for these samples, but DENV-specific memory B cells were only detected in DV-22.
Figure 4. B cell ELISPOT for the quantification of DENV-specific memory. PBMCs from vaccinees DV-1 to DV-19 were stimulated with non-specific B cell stimuli (protein A, CpG, and IL-21) for 6 d for the quantification of A) the total number of IgG-secreting cells (ASCs) per million PBMCs, B) DENV-specific ASCs as a percentage of total IgG-secreting ASCs, and C) tetanus-specific ASCs as a percentage of total IgG-secreting ASCs. There were insufficient cells for the tetanus-specific assay for donors DV-11,-12 and -13 (nd = not done). D) Representative pictures of the ASC spots for samples DV-1, DV-14, and DV-15. The coating of the respective wells is indicated on the right.
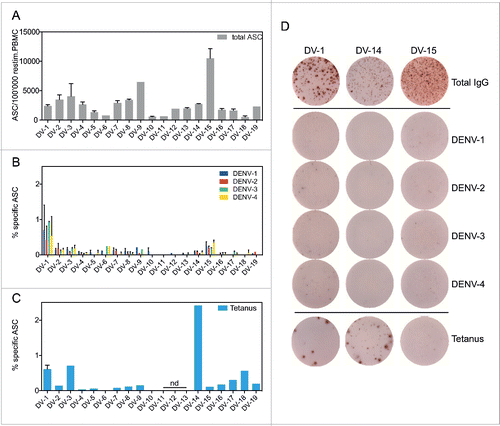
The low frequencies of DENV-specific memory B cells detected in the vaccinees, including in those with known previous natural dengue infections (DV-6 and DV-22), suggest that DENV-specific memory B cell numbers are generally low, even after natural dengue infection.
Discussion
The CYD-TDV live-attenuated dengue vaccine candidate has been tested recently in large efficacy trials.Citation2-5 While the efficacy of the vaccine is now established, few data are available on the long-term immune memory generated by this vaccine.Citation6 Such data are essential to decide if and when re-vaccination might be required. Because the vaccine is more efficacious in individuals with pre-existing DENV immunity (plaque reduction neutralizing titers (PRNT50) of > 10) from previous DENV or related flavivirus infections, it can be assumed that protection relies on the expansion of pre-existing cross-reactive immune memory.Citation3,4 It is well established that such cross-reactive protection is temporary, lasting 2 y at best.Citation14-16 Re-vaccination with the CYD-TDV vaccine might therefore be required to maintain sufficient levels of protective immunity.
The in vivo protection model used here provides a functional readout to assess the efficacy of antibodies generated after vaccination. Pre-existing antibodies in the blood at the time of infection are crucial to reduce the initial viral load and can be sufficient for protection.Citation25 Within a few days of infection, memory B and T cells, which are activated, expanded, and differentiated, begin to produce high amounts of antibodies and “kill” infected cells, respectively, further decreasing the viral load.Citation10,23,26-30 The plasma transfer model specifically addresses the role of pre-existing antibodies, and not memory lymphocytes. Our findings suggest that relatively high pre-existing antibody titers are required for a significant reduction in viral load (> 16,200 in the E protein ELISA, > 1,800 in the UV-DENV ELISA, > 1,800 in the EDIII ELISA, and > 640 in the FRNT50). While several samples reached these titers in one or 2 of the readouts, only DV-19 and DV-20 showed high titers in all 4 readouts (), suggesting that a combination of high titers to different epitopes is most efficacious for protection. Owing to exceptionally high titers and the serotype cross-reactive nature of antibodies in DV-19 and DV-20 It is very likely that these 2 participants experienced a natural infection in the 5 y after vaccination.
While the functional relevance of memory B cells for protection in dengue has not been formally demonstrated, memory B cells were found to be a correlate of protection in a JEV mouse model.Citation31 The DENV-specific memory B cell numbers detected in vaccinees in this study was low (< 0.5%). However, we have previously quantified DENV particle-specific memory B cells in healthy donors with DENV infection of an unknown time point and found similarly low numbers.Citation23 Mathew et al. reported a wide range of 1–40% specific memory B cells in patients with acute natural DENV infection, which were relatively stable over the first 6 months.Citation32 Later time points were not analyzed in that study and it is possible that memory B cells remain elevated for a few months before declining in numbers. For comparison, one year after smallpox vaccination, which provides life-long protection, the number of specific memory B cells stabilizes at approximately 0.1% and remains constant for decades.Citation33 Interestingly, the correlation of memory B cells and serum antibody titers to viral antigens over time depends on the nature of the vaccine and appears to be less significant for persisting viruses or in the case of booster immunizations, indicating that long-lived plasma cells and memory B cells are regulated independently.Citation34
The low antibody titers and limited in vivo efficacy of plasma transfer suggest that 5 y after vaccination with CYD-TDV, immunity to DENV mediated by pre-existing antibodies is largely lost. While the in vivo protection model presented here needs to be further validated and compared to ELISA and neutralization readouts, the data suggest that to be protective, immunity to dengue needs to be sustained over time with antibody titers above a certain level at the time point of infection.
Methods
Human samples
This study was conducted according to the principles expressed in the Declaration of Helsinki. It was approved by the Domain Specific Review Board of Singapore's National Healthcare Group and patients gave written informed consent (DSRB Refs. 2014/00380). The study involving samples from patients with previous natural infection was approved by the Domain Specific Review Board of Singapore's National Healthcare Group (Domain E) and patients gave written informed consent (DSRB Refs. 2010/00227). The study involving samples from healthy donors was approved by the National University of Singapore Institutional review board (NUS-IRB:09-256) and donors gave written informed consent.
ELISA
ELISAs were conducted as described previously.Citation12 In brief, half-area plates (Greiner) were coated with PEG-precipitated virus particle, recombinant E protein, or recombinant EDIII of DENV serotypes 1–4. The following DENV strains isolated from patients were used for virus particle ELISAs: DENV1-08K3126 (unpublished genotype I strain isolated by the Environmental Health Institute, Singapore), DENV2-TSV01 (AY037116.1), DENV3-VN32/96 (EU482459), and DENV4-2641Y08 (HQ875339.1). The soluble domains of E proteins and the EDIII of DENV1-4 were produced in S2 cells with a His-tag and were purified using Ni-beads. The following strains were used for the construction of E and EDIII: DENV1-05K2916 (EU081234), DENV2-TSV01 (AY037116.1), DENV-3 05K4141 (EU081214.1), and DENV4-2641Y08 (HQ875339.1). Recombinant proteins were coated at 75 ng/well. For all ELISAs, plates were blocked with PBS, 0.05% Tween 20 (PBST) containing 3% skimmed milk. Plasma samples and the positive control were diluted in blocking buffer before being transferred to the coated plates. For the positive control, we pooled 3 plasma samples out of 8 tested based on a high OD value against all 4 serotypes in the UV-DENV ELISA. For the negative control, 3 plasma samples without dengue immunity were pooled. Anti-human IgG-HRP (Sigma) was added at a concentration of 1:2000. Three,3,5,5-tetramethylbenzidine HRP substrate solution (Sigma) was used for color development and the reactions were stopped with 1M HCl. The endpoint titer was defined as the lowest OD450 value that was higher than 2 times the background. For OD ratios, sample ODs at a 1:600 dilution were divided by the OD of the positive control at a 1:600 dilution on the same plate.
ELISPOT
PBMC from 9 ml whole blood were isolated using BD Vacutainer CPT Sodium Citrate tubes. Plasma (top layer) was collected for ELISA. The PBMC layer was collected and washed twice with 1× PBS before resuspending cells in RPMI containing 10% FCS and Penicillin/Streptomycin. PBMCs (2.5 × 106 in 2.5 ml medium) were added to 6-well plates (Thermo Scientific). The B cell stimulation cocktail (final concentration of 6 µg/ml CpG, 1/10,000 dilution of Staphylococcus Aureus Cowan (Sigma), 50 ng/ml IL-21 (Immunotools)) was added to each well. For the non-stimulated control, PBMCs (0.5 × 106 in 0.5 ml) were incubated in a 48-well plate (Thermo Scientific). Supernatant and cells were collected on day 6. ELISPOT plates were coated overnight with anti-human Ig, UV-(Merck) inactivated PEG-precipitated DENV1-4, or tetanus toxoid (0.5 μg/well; Merck), and blocked with RPMI, 10% FCS. Cultured cells were washed twice with 1× PBS before transfer to the ELISPOT plates for overnight incubation at 37°C (5 × 105 cells per well coated with antigen; 1 × 104, 0.2 × 104, and 0.04 × 104 per well coated with anti-Ig). Plates were washed and incubated with anti-human IgG HRP (Sigma), and spots were developed using AEC substrate. Spots were counted using an Immuno-Spot analyzer (Cellular Technology Ltd). For the calculation of the total number of IgG-secreting cells, spots in non-stimulated controls (if any) were deducted from total IgG-secreting cells.
In vivo protection assay
Female or male 6- to 8-week-old IFN-α/β/γ receptor-deficient mice (AG129) were purchased from B&K Universal Limited. Mice were intraperitoneally (i.p.) administered 100 μl filtered (0.22 μm) human plasma. Decrease of viremia after transfer of 100 or 200 μl of plasma, as tested in n = 3 mice/group (data not shown), was not significantly different; therefore we chose the smaller amount (100 μl) for our experiments. After 24 h, mice were subcutaneously infected with 200 µl of 2.7 × 107 PFU/ml DENV-2 TSV01. After a further 72 h, blood was collected by retro-orbital bleeding and plasma was kept at −80°C until analysis. Viremia was quantified by RT-PCR as described previously with slight modification in primers.Citation35 RNA was isolated from 70 µl of plasma and a standard virus sample with known pfu/ml was used in all assays for quantification.Citation36 IFN-α/β/γ receptor-deficient AG129 mice backcrossed to the C57BL/6 background were used to test the samples shown in . All mice were bred and kept under specific-pathogen-free conditions at the Biomedical Resource Center, Singapore.
The mouse experiments were conducted according to the rules and guidelines of the Agri-Food and Veterinary Authority (AVA) and the National Advisory Committee for Laboratory Animal Research (NACLAR), Singapore. The experiments were reviewed and approved by the Institutional Review Board of the Biological Resource Center, Singapore.
Neutralization assay
BHK-21 cells (1 × 105/well) were seeded 24-well plates and cultured overnight at 37°C. Plasma samples, diluted 1:10 to 1:320 in serum-free medium, were mixed with an equal volume of DENV-2 and incubated at room temperature for 2 h (final plasma dilution 1:20 to 1:640). Virus-antibody mixtures were transferred to the 24-well plates and incubated for 1 h at 37°C before adding an equal volume of RPMI containing 10% FCS and 0.8% methylcellulose. After five days, cells were fixed with 3.7% formalin. Cells were permeabilized with 1% Triton X-100 and incubated with antibody 4G2, followed by goat anti-mouse IgG-HRP. Foci were visualized with TRUE BLUE substrate. FRNT50 values were calculated using Prism software.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature 2013; 496(7446):504-7. Epub 2013/04/09; PMID:23563266; http://dx.doi.org/10.1038/nature12060
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 2012; 380(9853):1559-67. Epub 2012/09/15; PMID:22975340; http://dx.doi.org/10.1016/S0140-6736(12)61428-7
- Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384(9951):1358-65; PMID:25018116; http://dx.doi.org/10.1016/S0140-6736(14)61060-6
- Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO, Carrasquilla G, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Eng J Med 2015; 372(2):113-23; http://dx.doi.org/10.1056/NEJMoa1411037
- Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N Eng J Med 2015; 373(13):1195-206; http://dx.doi.org/10.1056/NEJMoa1506223
- Capeding MR, Laot T, Boaz M, Wartel-Tram A, Crevat D. Immunogenicity and safety of a tetravalent dengue vaccine during a five-year follow-up period. Trials Vaccinol 2015; 4:19-23; http://dx.doi.org/10.1016/j.trivac.2015.03.002
- Thomas SJ, Endy TP. Current issues in dengue vaccination. Curr Opin Infect Dis 2013; 26(5):429-34; PMID:23963259; http://dx.doi.org/10.1097/01.qco.0000433310.28771.cc
- Bhoomiboonchoo P, Nisalak A, Chansatiporn N, Yoon IK, Kalayanarooj S, Thipayamongkolgul M, Endy T, Rothman AL, Green S, Srikiatkhachorn A, et al. Sequential dengue virus infections detected in active and passive surveillance programs in Thailand, 1994-2010. BMC Public Health 2015; 15:250
- Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinski A, Jumnainsong A, Edwards C, Quyen NT, Duangchinda T, et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol 2015; 16(2):170-7; PMID:25501631; http://dx.doi.org/10.1038/ni.3058
- Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A, Kwissa M, Pulendran B, Wilson PC, Wittawatmongkol O, et al. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J Virol 2012; 86(6):2911-8; PMID:22238318; http://dx.doi.org/10.1128/JVI.06075-11
- Xu M, Hadinoto V, Appanna R, Joensson K, Toh YX, Balakrishnan T, Ong SH, Warter L, Leo YS, Wang CI, et al. Plasmablasts generated during repeated dengue infection are virus glycoprotein-specific and bind to multiple virus serotypes. J Immunol 2012; 189(12):5877-85: Epub 2012/11/16; PMID:23152560; http://dx.doi.org/10.4049/jimmunol.1201688
- Toh YX, Gan V, Balakrishnan T, Zuest R, Poidinger M, Wilson S, Appanna R, Thein TL, Ong AK, Ng LC, et al. Dengue serotype cross-reactive, anti-e protein antibodies confound specific immune memory for 1 year after infection. Front Immunol 2014; 5:388; PMID:25177321; http://dx.doi.org/10.3389/fimmu.2014.00388
- Biswas HH, Ortega O, Gordon A, Standish K, Balmaseda A, Kuan G, Harris E. Early clinical features of dengue virus infection in nicaraguan children: a longitudinal analysis. PLoS Negl Trop Dis 2012; 6(3):e1562; PMID:22413033; http://dx.doi.org/10.1371/journal.pntd.0001562
- Montoya M, Gresh L, Mercado JC, Williams KL, Vargas MJ, Gutierrez G, Kuan G, Gordon A, Balmaseda A, Harris E. Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl Trop Dis 2013; 7(8):e2357. Epub 2013/08/21; http://dx.doi.org/10.1371/journal.pntd.0002357
- Olkowski S, Forshey BM, Morrison AC, Rocha C, Vilcarromero S, Halsey ES, Kochel TJ, Scott TW, Stoddard ST. Reduced risk of disease during postsecondary dengue virus infections. J Infect Dis 2013; 208(6):1026-33. Epub 2013/06/19; PMID:23776195; http://dx.doi.org/10.1093/infdis/jit273
- Anderson KB, Gibbons RV, Cummings DA, Nisalak A, Green S, Libraty DH, Jarman RG, Srikiatkhachorn A, Mammen MP, Darunee B, et al. A shorter time interval between first and second dengue infections is associated with protection from clinical illness in a school-based cohort in Thailand. J Infect Dis 2014; 209(3):360-8. Epub 2013/08/22; PMID:23964110; http://dx.doi.org/10.1093/infdis/jit436
- Leo YS, Wilder-Smith A, Archuleta S, Shek LP, Chong CY, Leong HN, Low CY, Oh ML, Bouckenooghe A, Wartel TA, et al. Immunogenicity and safety of recombinant tetravalent dengue vaccine (CYD-TDV) in individuals aged 2-45 y: Phase II randomized controlled trial in Singapore. Human Vaccines Immunotherapeutics 2012; 8(9):1259-71; PMID:22894958; http://dx.doi.org/10.4161/hv.21224
- Halstead SB. Identifying protective dengue vaccines: guide to mastering an empirical process. Vaccine 2013; 31(41):4501-7. Epub 2013/07/31; PMID:23896423; http://dx.doi.org/10.1016/j.vaccine.2013.06.079
- Kreil TR, Burger I, Bachmann M, Fraiss S, Eibl MM. Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clin Exp Immunol 1997; 110(3):358-61; PMID:9409636; http://dx.doi.org/10.1046/j.1365-2249.1997.4311446.x
- Saeland E, Jakobsen H, Ingolfsdottir G, Sigurdardottir ST, Jonsdottir I. Serum samples from infants vaccinated with a pneumococcal conjugate vaccine, PncT, protect mice against invasive infection caused by Streptococcus pneumoniae serotypes 6A and 6B. J Infect Dis 2001; 183(2):253-60; PMID:11110649; http://dx.doi.org/10.1086/317934
- Zellweger RM, Shresta S. Mouse models to study dengue virus immunology and pathogenesis. Front Immunol 2014; 5:151; PMID:24782859; http://dx.doi.org/10.3389/fimmu.2014.00151
- Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol 2015; 15(3):149-59; PMID:25677494; http://dx.doi.org/10.1038/nri3802
- Balakrishnan T, Bela-Ong DB, Toh YX, Flamand M, Devi S, Koh MB, Hibberd ML, Ooi EE, Low JG, Leo YS, et al. Dengue virus activates polyreactive, natural IgG B cells after primary and secondary infection. PLoS One 2011; 6(12):e29430. Epub 2012/01/05; PMID:22216280; http://dx.doi.org/10.1371/journal.pone.0029430
- Jahnmatz M, Kesa G, Netterlid E, Buisman AM, Thorstensson R, Ahlborg N. Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses. J Immunol Methods 2013; 391(1-2):50-9; PMID:23454005; http://dx.doi.org/10.1016/j.jim.2013.02.009
- Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol 2006; 6(3):231-43; PMID:16498452; http://dx.doi.org/10.1038/nri1783
- Garcia-Bates TM, Cordeiro MT, Nascimento EJ, Smith AP, Soares de Melo KM, McBurney SP, Evans JD, Marques ET, Jr, Barratt-Boyes SM. Association between magnitude of the virus-specific plasmablast response and disease severity in dengue patients. J Immunol 2013; 190(1):80-7. Epub 2012/12/04; PMID:23203929; http://dx.doi.org/10.4049/jimmunol.1103350
- Kwissa M, Nakaya HI, Onlamoon N, Wrammert J, Villinger F, Perng GC, Yoksan S, Pattanapanyasat K, Chokephaibulkit K, Ahmed R, et al. Dengue virus infection induces expansion of a CD14(+)CD16(+) monocyte population that stimulates plasmablast differentiation. Cell Host Microbe 2014; 16(1):115-27; PMID:24981333; http://dx.doi.org/10.1016/j.chom.2014.06.001
- Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, Broadwater A, Kolla RV, De Silva AD, de Silva AM, et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A 2013; 110(22):E2046-53. Epub 2013/04/13
- Dung NT, Duyen HT, Thuy NT, Ngoc TV, Chau NV, Hien TT, Rowland-Jones SL, Dong T, Farrar J, Wills B, et al. Timing of CD8+ T cell responses in relation to commencement of capillary leakage in children with dengue. J Immunol 2010; 184(12):7281-7. Epub 2010/05/21; PMID:20483770; http://dx.doi.org/10.4049/jimmunol.0903262
- Rivino L, Kumaran EA, Jovanovic V, Nadua K, Teo EW, Pang SW, Teo GH, Gan VC, Lye DC, Leo YS, et al. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J Virol 2013; 87(5):2693-706. Epub 2012/12/21
- Konishi E. Memory B cells: a proposed new immunological correlate for protective efficacy of Japanese encephalitis vaccine. Expert Rev Vaccines 2013; 12(8):871-3; PMID:23944374; http://dx.doi.org/10.1586/14760584.2013.814828
- Mathew A, West K, Kalayanarooj S, Gibbons RV, Srikiatkhachorn A, Green S, Libraty D, Jaiswal S, Rothman AL. B-cell responses during primary and secondary dengue virus infections in humans. J Infect Dis 2011; 204(10):1514-22. Epub 2011/09/21; PMID:21930609; http://dx.doi.org/10.1093/infdis/jir607
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol 2003; 171(10):4969-73; PMID:14607890; http://dx.doi.org/10.4049/jimmunol.171.10.4969
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Eng J Med 2007; 357(19):1903-15; http://dx.doi.org/10.1056/NEJMoa066092
- Ito M, Takasaki T, Yamada K, Nerome R, Tajima S, Kurane I. Development and evaluation of fluorogenic TaqMan reverse transcriptase PCR assays for detection of dengue virus types 1 to 4. J Clin Microbiol 2004; 42(12):5935-7; PMID:15583346; http://dx.doi.org/10.1128/JCM.42.12.5935-5937.2004
- Zust R, Toh YX, Valdes I, Cerny D, Heinrich J, Hermida L, Marcos E, Guillén G, Kalinke U, Shi PY, et al. Type I interferon signals in macrophages and dendritic cells control dengue virus infection: implications for a new mouse model to test Dengue vaccines. J Virol 2014; 88(13):7276-85; PMID:24741106; http://dx.doi.org/10.1128/JVI.03827-13
