ABSTRACT
We examined the coverage and timing of rotavirus vaccination and the impact of rotavirus vaccine introduction on coverage and timing of the pentavalent vaccine. We used data from the Demographic and Health Surveys in Honduras (2011/2012) and Peru (2012). The samples were divided into 2 subcohorts: children born before and after the introduction of rotavirus vaccine. We compared coverage and timing of the pentavalent vaccine in the aforementioned subcohorts. Coverage with the first and second doses of rotavirus vaccination was 95% (95% confidence intervals: 93–97%) and 91% (89–95%) in Honduras and 79% (77–82%) and 72% (69–75%) in Peru, respectively. Coverage increased in both countries over the years. The proportion of children vaccinated according to age-appropriate vaccination schedules varied between 67% (second dose of rotavirus vaccinations in Peru) and 89% (first dose of rotavirus vaccination in Honduras). Coverage with the first and second doses of pentavalent vaccination remained constant over the years in Honduras, while in Peru there was a significant increase in coverage over the years (p for trend, <0.0001). In both countries, timing of pentavalent vaccination was better in post-rota-cohorts than in pre-rota-cohorts. Since its introduction, coverage of rotavirus vaccination has improved over time in both countries. An introduction of rotavirus vaccination in both countries appears to have improved the coverage and timing of other similarly scheduled vaccinations.
ABBREVIATIONS
| DHS | = | Demographic and Health Survey |
| NIP | = | National Immunization Programs |
| PAHO | = | Pan American Health Organization |
| Penta1 | = | first dose of pentavalent vaccine |
| Penta2 | = | second dose of pentavalent vaccine |
| Rota1 | = | first dose of rotavirus vaccine |
| Rota2 | = | second dose of rotavirus vaccine |
| SAGE | = | WHO's Strategic Advisory Group of Experts |
| WHO | = | World Health Organization |
Introduction
Rotavirus is one of the leading causes of high morbidity and mortality among children under the age of 5 years, particularly in low-income countries.Citation1,2 Worldwide, an estimated 453,000 deaths occurred due to rotavirus among children under the age of 5 y in 2008, i.e. prior to the introduction of the rotavirus vaccine.Citation3,4 The burden of rotavirus disease is considerable in Latin America. According to Gutierrez, rotavirus was responsible for around 15,000 deaths, 75,000 hospitalizations and 2 million outpatient clinic visits every year in Latin American countries and the Caribbean.Citation5 In around 50% of children hospitalized due to acute gastroenteritis, rotavirus was the causative agent.Citation6 In Peru, gastroenteritis caused by rotavirus is responsible for approximately 810 deaths per year in children under 5 y of age.Citation7 Solórzano Girón et al. reported that between 2000 and 2004 in Honduras, an estimated 66,600 outpatient visits, 1888 hospitalizations, and 70 in-hospital deaths among children under the age of 5 y could be attributed to rotavirus.Citation8 These estimates likely underestimate the true burden of diarrheal disease attributable to rotavirus since, in Honduras, approximately 50% of children with acute diarrhea do not receive formal care for the illness, and 80% of diarrheal deaths occur outside of hospitals.Citation8
Currently, 2 oral, live attenuated, effective rotavirus vaccines are internationally available. The World Health Organization (WHO) recommends including rotavirus vaccines in all national immunization programmes and reiterates the use of rotavirus vaccines to be part of a comprehensive strategy to control diarrheal diseases.Citation3 Brazil, El Salvador and Panama were among the first countries worldwide that introduced rotavirus vaccines into their national immunization programmes. As of January 2015, 17 Latin American countries have introduced rotavirus vaccines into their national immunization programmes.Citation9 Most of these countries introduced the Rotarix® vaccine, a monovalent, live attenuated, oral vaccination (GlaxoSmithKline Biologicals, Rixensart, Belgium) with 2 doses. Chang et al. reported a decrease in rotavirus prevalence after the introduction of the rotavirus vaccine in a study in a peri-urban community in Lima, Peru.Citation10
In contrast to other childhood vaccinations (e.g. the pentavalent vaccination that protects against diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae type B), which can be administered at later ages than recommended, rotavirus vaccination has a strict administration schedule.Citation11 The vaccine should not be administered before the age of 6 weeks. The interval between 2 vaccine doses should be at least 4 weeks.Citation3 The WHO's Strategic Advisory Group of Experts (SAGE) on immunization in its April 2012 session recognized that employing more flexible immunization schedules was necessary in order to increase coverage of rotavirus vaccination.Citation3 Furthermore, SAGE further recognized that the burden of disease attributable to rotavirus in countries is disparate and hence country-specific plans were necessary to best assess how the removal of age restrictions on vaccine administration could be introduced in a manner that supports existing programs.Citation3 In the Region of the Americas, the PAHO's Technical Advisory Group on Vaccine-preventable Diseases recommends further compliance with the age recommendations of rotavirus vaccine. However, a later administration of the rotavirus vaccine among children up to one year of age can be considered in regions with high mortality due to rotavirus infections.Citation12
The correctly scheduled administration of rotavirus vaccination may have an impact on other childhood vaccinations administered at the same age (e.g., pentavalent or diphtheria, tetanus and pertussis [DTP] vaccination). Thus far, only a few studies have examined the impact of rotavirus vaccination on coverage and timing of other childhood vaccinations. In two Australian studies, the introduction of rotavirus vaccination actually improved the timing of DTP vaccination.Citation13,14
Considering the burden of rotavirus-associated diarrhea in Latin America, we aimed to investigate the coverage and timing of rotavirus vaccination and the impact of rotavirus vaccination on the timing of other similarly scheduled vaccinations such as the pentavalent vaccination in countries for which Demographic and Health Survey (DHS) data were available. DHS data on rotavirus vaccination were available for 2 Latin American countries, Honduras and Peru.
Results
Coverage estimates for rotavirus vaccination
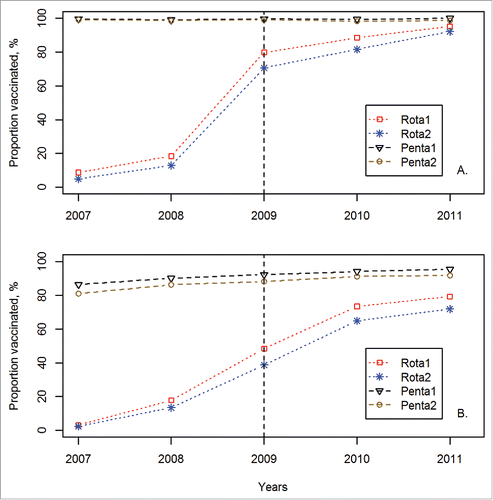
Coverage with rotavirus vaccination increased constantly in both countries over the years (). The increase was more prominent in Honduras, reaching a coverage with the first and second doses of rotavirus vaccination (referred to as rota1 and rota2 henceforth) of 95.3% (95% confidence intervals, 92.6–97.1%) and 92.1% (95% CI: 88.7–94.5%), respectively, in the year 2011 (). In Peru the coverage in the same year was 79.3% (95% CI: 76.6–81.7%) and 71.9% (95% CI: 69.0–74.7%) ().
Figure 1. Coverage of 2 doses of rotavirus and 2 doses of pentavalent vaccination in Honduras (A) and Peru (B). A vertical dash line represents the year of introduction of rotavirus vaccine. The vaccination coverage was estimated among children between 12 and 60 months of age. Information on coverage was based on vaccination records and maternal reports.
Timing of rotavirus vaccination
The proportion of children vaccinated with rotavirus vaccination according to age recommendations as per national immunization schedules varied between 67% (rota2 in Peru) and 89% (rota1 in Honduras) (, columns 2–6). Only a few children received rotavirus vaccine too early (less than 2% in both countries). The proportion of children who received rota1 unacceptably late, i.e., after the upper age limit of 15 weeks, was low in both countries. However, about 13% of children in Peru received rota2 after the age limit of 25 weeks. This proportion decreased by year of birth in both countries (). The proportion of children vaccinated with an interval of less than 4 weeks between 2 rotavirus vaccine doses was very low in both countries (0.25% in Honduras and 0.78% in Peru). We analyzed the correct timing of rotavirus vaccine over time and found that it improved slightly in both countries (). Of the 5094 children in Honduras who received both rota1 and the first dose of pentavalent vaccine (referred to as penta1 henceforth), 83% were administered on the same day and 17% on different days. Of 3616 children who were only vaccinated with penta1 and not with rota1, 84% were vaccinated within the recommended age window for rotavirus vaccination (i.e. missed opportunities for rota1). Of the 4821 children in Peru who were vaccinated with both rota1 and penta1, 83% were vaccinated on the same day and 17% on different days. Of the 1989 children who only received penta1, 71% were administered within the recommended age window (i.e., missed opportunities).
Figure 2. Timing of administration of the first dose of rotavirus vaccination in Honduras and (A) and Peru (B) by year of birth.
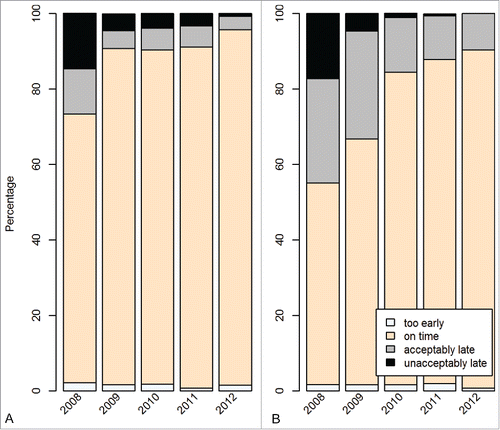
Table 1. Timing of administration of rotavirus vaccination and proportion of children vaccinated on time with pentavalent vaccination by country.
Impact of rotavirus vaccination on coverage and timing of pentavalent vaccination
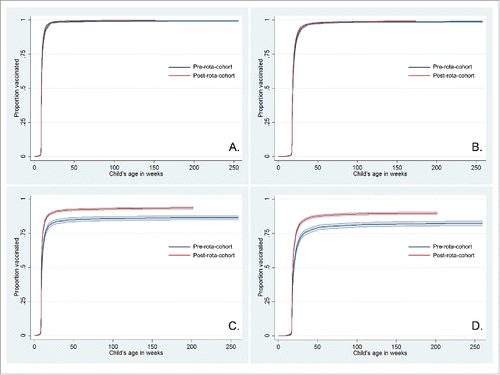
The coverage with penta1 and penta2 over the years remained constant in Honduras; there was a marginal decrease in penta2 coverage (p for trend, 0.38) (). The coverage with penta1 and penta2 doses increased significantly over the years in Peru (p for trend for both doses, <0.0001) (). There was a gap in coverage with rotavirus vaccination compared to pentavalent vaccination, which decreased over the years in both countries. The coverage of rota1 and rota2 was slightly lower than 10 percentage points compared to penta1 and penta2 in Honduras in 2011, respectively. There was a coverage gap of nearly 20 percentage points between rota2 and penta2 in Peru in the same year. Timing of penta1 and penta2 was slightly better in the post-rota-cohort as compared to the pre-rota-cohort in Honduras (penta1: log-rank test, n = 8707, p < 0.0001; penta2: log-rank test, n = 8249, p < 0.0001) ( and ). In Peru, timing of both doses of pentavalent vaccination was visibly better in the post-rota-cohort as compared to the pre-rota-cohort (penta1: log-rank test, n = 7824, p < 0.0001; penta2: log-rank test, n = 8040, p < 0.0001) ( and ).
Figure 3. Cumulative vaccination coverage with pentavalent vaccine by subcohort in Honduras (A-B) and Peru (C-D).* Curves show inverse Kaplan-Meier estimates and 95% confidence intervals. A. Penta1 vaccination in Honduras; B. Penta2 vaccination in Honduras; C. Penta1 vaccination in Peru; D. Penta2 vaccination in Peru.
Discussion
The present study is a large-scale analysis of rotavirus vaccination in Honduras and Peru. We used DHS data to assess the coverage and timing of this vaccination as well as to examine the impact of rotavirus vaccine introduction on the coverage and timing of pentavalent vaccination.
The results suggest that rotavirus vaccination coverage has increased since its introduction in 2009 in both countries. The situation was better in Honduras; vaccination coverage was over 90% for both vaccine doses compared to 72% (rota2) and 79% (rota1) in Peru. Our findings are consistent with a report from the CDC that used administrative data and reported vaccination coverage rates similar to ours for both countries.Citation15 A previous analysis using the 2011–12 DHS survey in Honduras also reported similar rotavirus vaccination coverage and timeliness results. The proportion of children vaccinated according to the age recommendations for rotavirus vaccination was higher in Honduras than in Peru. In Honduras, not only is rotavirus vaccination coverage high, additionally nearly all administered doses are valid and generally timely. On the other hand, in Peru, despite steady improvements in vaccination coverage, timing of rotavirus vaccine administration remains an issue.
According to the Regional Immunization and Vision strategy 2007–2015 of the Pan American Health Organization (PAHO), National Immunization Programs (NIPs) in the Americas have reached approximately 90% vaccination coverage for all childhood vaccines, and the goal for national vaccination coverage is to achieve greater than or equal to 95% coverage. Based on our analysis for rotavirus vaccination, Honduras has achieved the 95% target at least for the first dose of the vaccine and is close to achieving this target for the second dose as well. However, Peru is lagging behind in terms of rotavirus vaccination coverage and has still not achieved coverage greater than or equal to 90%. Further efforts are needed to improve rotavirus vaccination coverage, in particular in Peru. It is likely that the stringent age restrictions for initiating and completing the rotavirus vaccination series contribute to a lower coverage with rotavirus vaccination.Citation16 One option to improve rotavirus vaccination coverage would thus be to broaden age restrictions. According to Patel et al., lives saved in low- and middle-income countries by removing age restrictions for rotavirus vaccination would far outnumber the potential excess of vaccine-associated intussusception deaths.Citation17 Without age restrictions, a rotavirus vaccination program in these countries would prevent 45% or 203,000 deaths of all rotavirus deaths (102,000–281,500), which would represent 47,200 more deaths prevented (18,700–63,700) than with an age-restricted schedule. Removal of age restrictions should particularly be given consideration in settings where mortality benefits outweigh the risks of rotavirus vaccine associated morbidity and mortality. Immunization programs will hence be able to access children who are currently excluded from the benefits of rotavirus vaccination.Citation17
Although rotavirus vaccination coverage increased over the years in both countries, it is still lower than the pentavalent vaccination. The gap in vaccination coverage between rotavirus and pentavalent vaccinations was greater in Peru with nearly 20 percentage points between rota2 and penta2 in 2011. Flannery et al. reported similar findings for Brazil where rotavirus vaccination coverage (83%) was lower compared to coverage for other recommended childhood immunizations (≥94%).Citation16 Similar findings were also observed in other Latin American countries, including Peru.Citation15 Factors that might explain this coverage gap include differences in how countries implement WHO's recommendations to initiate rotavirus vaccination at age 6–15 weeks, vaccine shortages, or logistical challenges resulting from the relatively large rotavirus vaccine cold chain volume and the need for additional vaccine carriers to deliver rotavirus vaccines.Citation18 Evaluating the reasons for the coverage gap between pentavalent and rotavirus vaccinations and addressing them will be important to gain the full benefit of rotavirus vaccination. Possible strategies for narrowing this gap in vaccination coverage could include improvements in the correct timing of vaccination, tracking infants who miss vaccinations, and assessing the benefits and risks of the WHO age restriction policy.Citation19
The results of our analysis suggest that the introduction of the rotavirus vaccine might have had a favorable impact upon coverage and timing of other similarly scheduled vaccinations, as has been observed in previous publications.Citation13,14 An improvement was particularly visible in Peru where vaccination coverage and correct timing of pentavalent vaccination increased in the cohort of children born after the introduction of rotavirus vaccination as compared to the cohort of children born before introduction of rotavirus vaccination. In Peru, pneumococcal vaccines were also introduced in 2009 and it is quite possible that the introduction of 2 new vaccines actually directed significant resources and attention to the national immunization program. This might account for the observed improvement in pentavalent vaccination coverage and timing in the country.Citation20 Consistent with previous observations, timeliness of childhood vaccinations typically decreases with subsequent doses and the greatest delays typically occur for the last dose.Citation14 In earlier studies, however, improvements were seen in all doses of the DTP vaccine, a definite trend toward increased rates for timely uptake were observed specifically for the third dose of DTP vaccine.Citation14 In the current analysis only 2 doses of the pentavalent vaccine were analyzed, however, improvements in timing were particularly prominent for the second dose of the vaccine.
Limitations
A major limitation of analyzing vaccination data from DHS surveys is the availability of vaccination cards. Vaccination data in our analysis were mostly obtained from child health cards available from the mother or at local health care facilities. Vaccination records were available for 85% and 75% of children in the samples in Honduras and Peru, respectively. For children without vaccination records, we took account of mothers' reports to generate vaccination coverage estimates. Therefore, the likelihood of recall bias and incompleteness of data must be taken into account when interpreting our results. We restricted ourselves to vaccination card reports to examine vaccination timing in order to minimize biased estimates, i.e. overestimating correctly timed vaccines. Considering the proportion of children in the samples in Honduras and Peru with vaccination cards, our results on vaccination timing cannot be considered to be nationally representative.
Conclusions
Our study shows that since its introduction, rotavirus vaccination coverage has improved in both countries. To maximize benefits of rotavirus vaccination, strategies are needed to further improve its coverage and timing. In both countries, an introduction of rotavirus vaccination seems to have contributed to an improvement in coverage and timing of other similarly scheduled vaccinations. These findings may have implications for other countries where delays in other vaccinations are common.
Material and methods
Study design and sampling
We used data from DHS conducted in Honduras (2011/2012) and Peru (2012). DHS are nationally representative household surveys conducted worldwide in low- and middle-income countries with the purpose to collect data on a wide range of monitoring and impact evaluation indicators in the areas of population, health and nutrition (www.dhsprogram.com). In brief, DHS respondents are selected using a multi-stage sampling process, and most DHS are stratified by urban and rural locations and/or by geographical regions. All women between 15 and 49 y of age living in households included in the survey were eligible to participate. Data were collected during face-to-face interviews. DHS methodology is described in detail elsewhere.Citation21
Vaccination data
In the DHS surveys included in the current analysis, information on childhood vaccinations was collected on all children between 0 and 59 months of age living in the households surveyed (n = 10,888 in Honduras and n = 9620 in Peru). Respondents were asked to show the interviewer the vaccination card of children born in the past 5 y. If the card was available (85% in Honduras and 75% in Peru), the interviewer copied the vaccination data (whether the child received the vaccine and the precise date of vaccination) into the questionnaire. If the card was not available (15% in Honduras and 25% in Peru), respondents were asked to recall vaccinations given to the child. In the latter case, the exact date of vaccination was not recorded.
Statistical analysis
First, we estimated the proportion of children vaccinated with rota1, rota2, penta1 and penta2 doses among children between 12 and 59 months of age. Sample weights provided in the surveys were used to obtain country representative estimates. This analysis is based on data from vaccination cards and maternal reports. We estimated the proportion of children vaccinated before the age of 8 weeks (too early rota1) and with an interval between the first and second doses less than 4 weeks (interval-invalid rota2). Furthermore, we estimated the proportion of children vaccinated after the upper age limits (15 weeks for rota1 and 25 weeks for rota2). We then divided the sample into 2 subcohorts: children born prior to and after the introduction of rotavirus vaccination (referred to as pre- and post-rota-cohort). In both countries, rotavirus vaccination was introduced in January 2009 (). We compared coverage and timing of penta1 and penta2 vaccine doses in the 2 aforementioned subcohorts. The recommended schedules for rotavirus and pentavalent vaccine administration are the same in both countries (). Finally, we applied the Kaplan-Meier method to estimate cumulative vaccination coverage of pentavalent vaccination in the 2 sub-cohorts. In this analysis, we included all children between 0 and 59 months of age. This analysis was only based on data from vaccination cards. If vaccination had not been received by the day of the interview, the case was classified as censored. The Kaplan-Meier method was performed with the statistical program Stata for Windows, version 12.1 (StataCorp LP, Texas, United States). and were made with the software tool R, version 3.1.3 (The R Foundation for Statistical Computing, https://www.r-project.org).
Table 2. Introduction of rotavirus vaccination, age at vaccine administration and selected socio-demographic characteristics of the study population.
Ethical approval
Our analysis is based on existing survey data collected by DHS (The DHS Programme, www.dhsprogram.com). As we performed secondary data analysis of anonymized data, ethical approval was not required. The Institutional Review Board of ICF International in Calverton, MD, USA, approved both surveys. All study participants provided informed consent before participating.
Contributors
AS interpreted the data and co-wrote the manuscript. FP provided intellectual input and critically reviewed and edited the manuscript. MKA conceived and oversaw the study, performed the statistical analyses and was primarily responsible for writing the manuscript. He had access to all data and takes full responsibility for their integrity. All authors reviewed and approved the submitted version of the manuscript.
Disclosure of potential conflicts of interest
The authors declare that they do not have conflicts of interest relating to this study.
Acknowledgments
The authors thank the DHS Programme for providing the datasets. The authors also thank Ms. Doris Maribel Rivera Medina from the Organización para el Desarrollo y la Investigación Salud en Honduras (ODISH), for sharing her valuable in-country insights on rotavirus vaccination. The work was supported by intramural funding from the Helmholtz Association (Programme Infection Research), and by iMed – the Helmholtz Association's Initiative on Personalized Medicine.
References
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9 (5):565-72; PMID:12737740; http://dx.doi.org/10.3201/eid0905.020562
- Bernstein DI. Rotavirus overview. Pediatr Infect Dis J 2009; 28 (3 Suppl):S50-S53; PMID:19252423; http://dx.doi.org/10.1097/INF.0b013e3181967bee
- WHO. Rotavirus vaccines. WHO position paper - January 2013. Geneva, Switzerland: World Health Organization; 2013. Report No.: 88.
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12 (2):136-41; PMID:22030330; http://dx.doi.org/10.1016/S1473-3099(11)70253-5
- Ulloa-Gutierrez R, Avila-Aguero ML. Rotavirus vaccination in Central American children. Expert Rev Vaccines 2014; 13 (6):687-90; PMID:24702467; http://dx.doi.org/10.1586/14760584.2014.905747
- De Oliveira LH, Danovaro-Holliday MC, Andrus JK, de Fillipis AM, Gentsch J, Matus CR, Widdowson MA; Rotavirus Surveillance Network. Sentinel hospital surveillance for rotavirus in latin american and Caribbean countries. J Infect Dis 2009; 200 Suppl 1:S131-S139; PMID:19821710; http://dx.doi.org/10.1086/605060
- Espejo PW, Peralta FO, Pacheres HC, del Valle LJ, Tapia AC, Mayra JB, Ruiz J, Mendoza Jdel V. Diarrhoea caused by rotavirus in a regional Peruvian hospital: determination of circulating genotypes. Trans R Soc Trop Med Hyg 2014; 108 (7):425-30; PMID:24778205; http://dx.doi.org/10.1093/trstmh/tru059
- Solorzano Giron JO, Molina IB, Turcios-Ruiz RM, Quiroz Mejia CE, Amendola LM, De Oliveira LH, Andrus JK, Stupp PW, Bresee JS, Glass RI. Burden of diarrhea among children in Honduras, 2000–2004: estimates of the role of rotavirus. Rev Panam Salud Publica 2006; 20 (6):377-84; PMID:17341328
- PATH. Rotavirus vaccine access and delivery. PATH 2015 Available from: http://sites.path.org/rotavirusvaccine/country-introduction-maps-and-spreadsheet/
- Chang MR, Velapatino G, Campos M, Chea-Woo E, Baiocchi N, Cleary TG, Ochoa TJ. Rotavirus seasonal distribution and prevalence before and after the introduction of rotavirus vaccine in a peri-urban community of Lima, Peru. Am J Trop Med Hyg 2015; 92 (5):986-8; PMID:25778507; http://dx.doi.org/10.4269/ajtmh.14-0431
- World Health Organization. WHO Package Insert. Rotarix WHO leaflet - tube. Version number: GDS03/WHO Insert01. Date of issue: 04.06.2009. GlaxoSmithKline Group of Companies.
- PAHO. Final report of XX Meeting of the Technical Advisory Group on Vaccine-preventable Diseases (TAG). Available from: http://www.paho.org/immunization/toolkit/resources/tech-recommendations/TAG-2012.pdf
- Hull BP, Menzies R, Macartney K, McIntyre PB. Impact of the introduction of rotavirus vaccine on the timeliness of other scheduled vaccines: the Australian experience. Vaccine 2013; 31 (15):1964-9; PMID:23422140; http://dx.doi.org/10.1016/j.vaccine.2013.02.007
- Wendy B. Vaccination with 3-dose paediatric rotavirus vaccine (RotaTeq(R)): impact on the timeliness of uptake of the primary course of DTPa vaccine. Vaccine 2012; 30 (35):5293-7; PMID:22575163; http://dx.doi.org/10.1016/j.vaccine.2012.04.071
- CDC. Progress in the introduction of rotavirus vaccine - Latin American and the Caribbean, 2006–2010. Centers for Disease Control and Prevention 2011; 60(47):1611-1614. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6047a2.htm
- Flannery B, Samad S, de Moraes JC, Tate JE, Danovaro-Holliday MC, De Oliveira LH, Rainey JJ. Uptake of oral rotavirus vaccine and timeliness of routine immunization in Brazil's National Immunization Program. Vaccine 2013; 31 (11):1523-8; PMID:23313652; http://dx.doi.org/10.1016/j.vaccine.2013.01.004
- Patel MM, Clark AD, Sanderson CF, Tate J, Parashar UD. Removing the age restrictions for rotavirus vaccination: a benefit-risk modeling analysis. PLoS Med 2012; 9 (10):e1001330; PMID:23109915; http://dx.doi.org/10.1371/journal.pmed.1001330
- De Oliveira LH, Danovaro-Holliday MC, Sanwogou NJ, Ruiz-Matus C, Tambini G, Andrus JK. Progress in the introduction of the rotavirus vaccine in Latin America and the Caribbean: four years of accumulated experience. Pediatr Infect Dis J 2011; 30(1 Suppl):S61-S66; PMID:21183843; http://dx.doi.org/10.1097/INF.0b013e3181fefdd6
- Patel MM, Clark AD, Glass RI, Greenberg H, Tate J, Santosham M, Sanderson CF, Steele D, Cortese M, Parashar UD. Broadening the age restriction for initiating rotavirus vaccination in regions with high rotavirus mortality: benefits of mortality reduction versus risk of fatal intussusception. Vaccine 2009; 27 (22):2916-22; PMID:AMBIGUOUS; http://dx.doi.org/10.1016/j.vaccine.2009.03.016
- Ochoa TJ, Egoavil M, Castillo ME, Reyes I, Chaparro E, Silva W, Campos F, Sáenz A. Invasive pneumococcal diseases among hospitalized children in Lima, Peru. Rev Panam Salud Publica 2010; 28 (2):121-7; PMID:20963279; http://dx.doi.org/10.1590/S1020-49892010000800008
- The DHS Program. Demographic and Health Surveys. DHS Overview. Available from: http://dhsprogram.com/What-We-Do/Survey-Types/DHS.cfm
