ABSTRACT
Despite ACIP recommendation and cost-effectiveness established in those 19–59 y old diabetes patients the uptake of Hepatitis B vaccine in diabetes patients is low. There is need to highlight the impact of Hepatitis B virus (HBV) infection in diabetes patients in terms of healthcare utilization and costs to recognize the burden of HBV in this population.
This retrospective claims analysis included patients with diabetes and HBV (cases; n=1,236) and those with diabetes without HBV (controls; n=4,944), identified by ICD-9-CM diagnosis codes. Cases were matched with 4 controls using propensity score matching. Healthcare utilization and cost were compared; incremental effect of HBV infection was assessed using multivariate analysis.
In the adjusted analyses, the mean number of hospitalizations (0.6 vs 0.4), outpatient service visits (34.2 vs. 20.4), and office visits (10.9 vs. 9.8) were 41%, 68%, and 11% higher, respectively, in cases vs. controls (all p<0.05). Gastroenterologist visits (0.8 vs. 0.2) and infectious disease visits (0.1 vs. 0.0) were 80% and 18% higher in subset of case and controls with these events. Cases ($39,435) incurred $16,397 incremental total costs compared with controls ($23,038). Medical ($30,968 vs. $17,765) and pharmacy costs ($8,029 vs. $5,114) were both significantly higher for cases (p < 0.0001).
Healthcare utilization and costs were higher among patients with diabetes and HBV than in those with diabetes alone. These results provide evidence supporting the need for HBV vaccination among unvaccinated diabetes patients.
ABBREVIATIONS
| ACIP | = | Advisory Committee on Immunization Practices |
| aDCSI | = | adapted Diabetes Comorbidity Severity Index |
| ED | = | emergency department |
| HBV | = | hepatitis B virus |
| HIRDSM | = | HealthCore Integrated Research Database |
| ICD-9-CM | = | International Classification of Diseases, Ninth Revision, Clinical Modification |
| NQF | = | National Quality Forum; SD, standard deviation |
Introduction
As a risk for people with diabetes mellitus, hepatitis B virus (HBV) infection is under-recognized. Adults with diabetes have a 60% higher prevalence rate of HBV infectionCitation1 and a higher case-fatality rate than those without diabetes.Citation2 Rates of chronic liver disease and hepatocellular carcinoma are also higher in people with diabetes.Citation3 The annual incidence of reported cases of HBV infection among adults with diabetes is 1.8 per 100,000,Citation2 which is likely an underestimate when asymptomatic infection, underdiagnosis, and under-reporting are considered.Citation4 The increased risk of HBV infection in adults with diabetes holds for both genders, across ethnic and racial groups, and for those without traditional HBV risk behaviors, such as use of injected drugs or multiple sexual partners.Citation1
HBV is stable and remains viable on surfaces up to a week,Citation5,6 making the virus transmissible through contaminated equipment used for routine diabetes care and blood glucose monitoring.Citation1,2,4 Between 1995 and 2006, 86% of the HBV outbreaks in long-term care facilities occurred among patients with diabetes who received assisted blood glucose monitoring.Citation7 People with diabetes can be exposed to HBV infection outside of institutional settings, such as physician offices, hospitals, health fairs, and schools, if assisted glucose monitoring is offered.Citation1
After reviewing the HBV-related morbidity and mortality and the limitations of infection control measures, the Advisory Committee on Immunization Practices (ACIP) recommended in 2011 that all previously unvaccinated adults aged 19 through 59 y with diabetes mellitus be vaccinated against hepatitis B as soon as possible after a diagnosis of diabetes.Citation8 In 2013, vaccination coverage for persons with diabetes was 26.3% for those aged 19–59 y and 13.9% for those aged ≥60 years.Citation9
Although cost analyses have been conducted for HBV treatment in USACitation10-13 and for diabetes,Citation14-18 as well as for the cost-effectiveness of HBV vaccination in adults with diabetes,Citation4 research is lacking on the impact of both HBV in diabetes patients in terms of healthcare utilization and costs. Such research can help highlight the need to vaccinate diabetes patients eligible for vaccination. To fill this research gap, the primary objective of the current study was to measure healthcare utilization and costs for patients with both HBV infection and diabetes compared with patients with diabetes alone using a real-world population of adults enrolled in large commercial health plans.
Results
Patient characteristics
A total of 918,488 patients (1,240 patients with diabetes and HBV infection [cases]; 917,248 patients with diabetes but no HBV infection [controls]) met all inclusion criteria for the study (). After propensity score matching, the final study population was 6,180 patients (1,236 cases; 4,944 controls). Prior to matching, the 2 cohorts were statistically different on a number of categories, including age, gender, severity of diabetes, and comorbidities (). After matching, the two groups were similar in nearly all baseline covariates.
Figure 1. Patient Attrition. aAt least 2 medical claims for diabetes at least 30 d apart (250.xx); OR at least 1 medical claim for diabetes and at least 1 pharmacy claim for a diabetes medication (GPI 27xx or 39100016x). bHepatitis B identified by ICD-9-CM codes 070.2x or 070.3x. cHepatitis C identified by ICD-9-CM codes 070.44, 070.54, 070.70, 070.71, 070.41, 070.51, or V02.62.
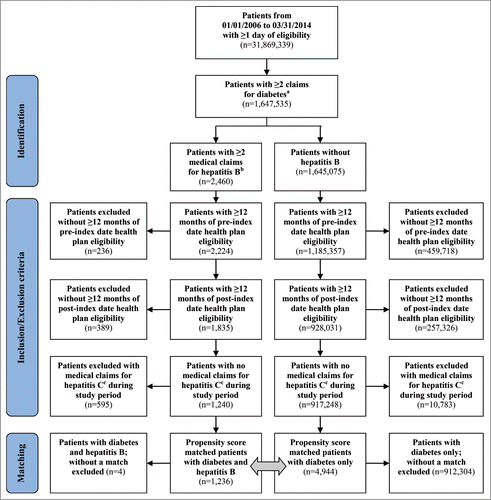
Table 1. Baseline Patient Clinical Characteristics Included in Propensity Score by Matched Status.Footnotea
Overall, patients in the matched cohorts had a mean age of 54 y and the majority was men. The majority of patients in both cohorts (60.3% cases and 56.9% controls) had mild diabetes; 32.2% of cases and 36.9% of controls had moderate diabetes. The most common comorbidities at baseline in cases and controls were hyperlipidemia (55.9% vs. 56.5%), hypertension (54.5% vs. 54.6%), and other liver disease (29.4% vs. 30.0%). Among patients with both diabetes and HBV infection, 282 patients (22.8%) had late-stage liver disease identified during the post-index period.
Healthcare utilization
Patients with diabetes plus HBV infection had greater healthcare resource utilization than patients with diabetes alone (). The mean adjusted number of hospitalizations [0.6 (95% CI 0.5–0.7) vs 0.4 (95% CI 0.4–0.5); p < 0.0001], office visits [10.9 (95% CI 10.4–11.4) vs 9.8 (95% CI 9.6–10.1); p < 0.0001], gastroenterologist visits [0.8 (95% CI 0.7–0.9) vs 0.2 (95% CI 0.2–0.2); p < 0.0001], infectious disease specialist visits [0.1 (95% CI 0.1–0.1) vs 0.0 (95% CI 0.0–0.1); p = 0.0001], and outpatient visits [34.2 (95% CI 32.0–36.5) vs 20.4 (95% CI 19.5–21.3); p < 0.0001] was higher for cases than controls. The number of ED visits was similar between the two groups [0.3 for both cases (95% CI 0.2–0.3) and controls (95% CI 0.3–0.4); p = 0.0124]. A similar pattern was observed among patients with at least one visit, with utilization higher for cases than controls for the mean adjusted number of hospitalizations [1.5 (95% CI 1.3–1.6 vs 1.0 (95% CI 1.0–1.1); p < 0.0001] and gastroenterologist visits [1.6 (95% CI 1.5–1.8) vs 0.9 (95% CI 0.8–1.0); p < 0.0001]. Infectious disease specialist visits [1.7 (95% CI 1.3–2.2) vs 1.4 (95% CI 1.2–1.8); p = 0.2527] and ED visits were similar between the two groups [0.8 visits for both cases (95% CI 0.7–0.9) and controls (95% CI 0.8–0.9); p=0.7478].
Table 2. Multivariate Analysis of Annualized Healthcare Utilizationa.Footnotea
Healthcare costs
Mean adjusted total costs for cases ($39,435) were 71% higher compared with controls ($23,038). Total medical costs were $30,968 (95% CI $28,311-$33,874) in cases compared with $17,765 (95% CI $16,788-$18,802) in controls, and pharmacy costs were also higher for cases than controls (). Incremental costs were highest for outpatient services ($7,039) and inpatient hospitalizations ($6,008) and lowest for gastroenterologist ($67), infectious disease specialist ($8), and general office visits ($118). Costs for ED visits were lower for cases than controls, with incremental costs of $99. Among patients with at least one healthcare utilization event, costs were significantly higher for all healthcare utilization events except ED visits. There was no significant difference in costs in subset of patients that had at least one ED visit.
Table 3. Multivariate Analysis of Annualized Costsa.Footnotea
Impact of late-stage liver disease
Among cases (that is, patients with diabetes and who had HBV infection), utilization varied according to the specific type of late-stage liver disease identified. In a multivariate analysis, patients with diabetes and decompensated cirrhosis were more likely to be hospitalized and visit the ED than those without an HBV-associated liver disease (data not shown).
Discussion
The results of this retrospective claims analysis demonstrated that HBV infection is associated with increased financial burden in patients with diabetes. Patients with diabetes plus HBV infection had higher healthcare utilization compared with those who had diabetes alone, in particular inpatient hospitalizations, office and specialist visits, and use of outpatient services. Patients with diabetes alone, however, were more likely to visit an ED than those with both diabetes and HBV infection, which is consistent with the greater number of office visits among patients with diabetes and HBV infection needed to manage their care. This finding did not hold when ED visits were compared among patients who had at least one ED visit. A possible explanation is that management of HBV infection in the outpatient setting resulted in fewer emergent care visits. Another explanation may be that patients with diabetes plus HBV may have been more likely to be admitted to the hospital, thus resulting in an underrepresentation of ED utilization among these patients. In fact, the mean number of hospitalizations was higher among patients with both diabetes and HBV infection, and their mean lengths of stay were 61% longer than those who had diabetes alone. The distribution for diseases unrelated to diabetes was not significantly different across cases and controls (as demonstrated in ), but the costs in the two groups may still have differed and confounded the results. This study did not break down the costs that are disease related or not but the confounding due to differing costs may not be significant as the cases were matched to controls with a similar clinical profile (as shown in ).
As expected, patients with diabetes and HBV infection also incurred higher annual medical and pharmacy costs compared with patients who had diabetes without HBV infection. Costs were also higher among the subset of patients with diabetes and HBV who had been diagnosed with late-stage liver disease during the follow-up period. These findings are consistent with previous research that demonstrated escalating costs associated with progressive liver disease among people with chronic HBV infection.Citation10 Pharmacy costs were higher among patients with diabetes plus HBV than in those with diabetes alone despite higher pharmacy utilization among patients with diabetes alone. A possible explanation for this finding may be higher cost per medication for patients with diabetes plus HBV. Prior economic comparisons of HBV treatments in hypothetical populations concluded that cost-effectiveness varied widely depending on patient response rates and drug resistance.Citation11,13 This study demonstrated higher costs among patients with diabetes and HBV in a real world environment using administrative claims.
A strength of this study was the large, geographically diverse population, and the ability to examine actual healthcare use and costs. However, the study had limitations. The data were extracted from administrative claims, which are designed for billing and reimbursement rather than research purposes. The claims may have contained incomplete information or undetected coding errors or omissions. Information on sociodemographic factors such as educational background, income, etc. that can be used for matching were not available in this administrative claims database. The ability to determine the severity of diabetes or HBV infection was limited by the information contained in the claims. Furthermore, some patients who were placed in the diabetes-only group may have had undiagnosed HBV infection. In cases where a visit to the ED resulted in hospitalization, that incident was counted as an inpatient hospitalization and not an ED visit, which may have under-represented the number of ED visits in this patient population. While the population was geographically diverse, all patients were members of a large commercial health plan. The results may not be generalizable to patients with other types of insurance or to those who are uninsured.
As this analysis illustrates, the financial burden associated with diabetes and HBV infection, particularly in the presence of late-stage liver disease, can be considerable. It provides evidence that there is a potential to reduce the economic impact of HBV by vaccinating patients with diabetes following their diagnosis. Typically, health plans reimburse hepatitis B vaccination for patients with diabetes if delivered by the physician. Healthcare quality organizations could also potentially have a role in improving vaccination coverage. For example, the National Quality Forum (NQF) recognized hepatitis B vaccination in diabetes as one of the gaps in adult immunization measures and measure development. Development of hepatitis B vaccination measure in diabetes and endorsement of such measure by NQF may raise the significance of delivery of hepatitis B vaccination in patients with diabetes.
HBV infection increased the financial burden of patients with diabetes, particularly in patients with late-stage liver disease. Healthcare utilization and costs were higher among patients with both diabetes and HBV infection than in those with diabetes alone. These results suggest providers should consider vaccination against HBV infection among patients with diabetes who have not previously been vaccinated or infected with HBV.
Materials and methods
Data source and patient identification
This retrospective, observational analysis used data contained in the HealthCore Integrated Research Database (HIRDSM). The HIRDSM contains medical and pharmacy claims data from 14 commercial health plans across the US. This claims analysis was conducted in compliance with state and federal laws, including the Health Insurance Portability and Accountability Act of 1996. As all claims data were from a limited dataset with de-identified patient information and no patients were identified, Institutional Review Board approval was not required.
Patients eligible for inclusion had at least one medical or pharmacy claim for diabetes (either type 1 or type 2) between January 1, 2006 and March 31, 2014 (the study period). Claims for HBV must have occurred during the intake period (between January 1, 2007 and March 31, 2013) to allow for 12-month pre- and post-index periods. The pre-index period was used to capture baseline characteristics. All patients were required to have 2 or more medical claims any time from January 2006 to March 2014 (at least 30 d apart) with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code (250.xx) suggesting diabetes or at least 1 medical claim with a diagnosis code for diabetes along with at least 1 pharmacy claim for a diabetes medication during the study period. Patients with a diagnosis code indicating the presence of hepatitis C (ICD-9-CM codes 070.44, 070.54, 070.70, 070.71, 070.41, 070.51, or V02.62) were excluded from the study to ensure utilization and cost results were attributable only to HBV infection.
Patients were then divided into one of 2 cohorts: the diabetes plus HBV infection cohort (cases) composed of diabetes patients who had 2 or more medical claims (at least 30 d apart) with diagnosis codes for HBV infection (ICD-9-CM code 070.2x or 070.3x); the diabetes-only cohort (controls) contained patients who had claims for diabetes during the intake period with no diagnosis codes for HBV infection at any point during the study period. The index date for cases was defined as the date of the first medical claim for HBV. The index date for controls was the date of the first medical or pharmacy claim for diabetes in the diabetes-only cohort observed after 12 months from the start of eligibility; this was to ensure all patients had at least 12 months of pre-index health plan eligibility. Patients were followed until they disenrolled or end of study period (March 31, 2014).
Propensity score matching
Propensity score matching was used to adjust for measured confounders between study cohorts.Citation19 Logistic regression propensity scores used observed patient demographics (eg, age, gender, US region, etc.) and baseline clinical characteristics (eg, comorbidities and use of healthcare resources not related to HBV infection). The logistic regression analysis weighed the predictor variables that best discriminated between the two groups. This formula was applied to each patient's values on all predictor variables to produce a predicted score, which was that patient's propensity score. Variables included in the final propensity score model (Appendix) were selected based on previous literature establishing their biologic rationale and confirmed by the balance achieved between cohorts after matching on propensity scores. Patients with diabetes plus HBV infection were matched with patients with diabetes only based on the eighth digit of the propensity score using a 1:4 greedy matching algorithm.Citation20,21
Disease severity
Adapted Diabetes Comorbidity Severity Index (aDCSI) was used in propensity score matching to adjust for severity of diabetes. Based on the presence of diabetes-related comorbidities, aDCSI produces scores of 0 (no abnormality), 1 (some abnormality), or 2 (severe abnormality) in 7 complication categories: retinopathy, nephropathy, neuropathy (which has only 2 levels: 0=not present; 1=abnormal), cerebrovascular complications, cardiovascular complications, peripheral vascular disease, and metabolic complications.Citation22,23 The total combined score may range from 0 to 13. For the purposes of this analysis and based on expert clinical opinion, an aDCSI score of 0 designated mild diabetes; 1 to 4 designated moderate diabetes; and a score of 5 to 13 designated severe diabetes.
Late-stage liver disease was identified based on the presence of ICD-9-CM diagnostic codes during the follow-up period associated with liver disease and were assigned to mutually exclusive categories in descending priority beginning with liver transplant, fulminant hepatic failure, liver cancer, decompensated cirrhosis, and cirrhosis.Citation24 That is, if a patient had 2 of these conditions, the patient was assigned to the condition higher in hierarchy, indicating more severe disease.
Outcome measures
Healthcare utilization and costs were assessed for inpatient hospitalizations; emergency department (ED) visits; office visits (all-cause, gastroenterologist, and infectious disease specialist); outpatient services (such as laboratory procedures); skilled nursing facility services; and pharmacy prescriptions. All-cause costs were calculated as plan-paid and patient-paid costs, which included all coinsurance, deductible, and co-payments. Costs were adjusted to 2014 dollars based on the Consumer Price IndexCitation25 and were annualized to account for different follow-up times among patients. Total medical costs were a sum of inpatient, ED, office visit, outpatient costs, and skilled nursing facility costs; total costs included both total medical plus pharmacy costs.
Statistical analysis
Descriptive statistics, such as means (standard deviation [SD]) and relative frequencies, were reported for continuous and categorical data, respectively. Patient characteristics, which were obtained from health plan enrollment data in HIRDSM, were compared statistically between the two groups using the diabetes-only group as the reference group. The χ2 test was used for dichotomous variables and t-test was used for continuous dependent variables. The χ2 test and t-test were used only for pre-index demographic and clinical characteristics. Statistical significance was set at p<0.05.
Incremental healthcare utilization and between-group differences in costs were calculated using multivariate models controlling for baseline insulin use and use of antidiabetic agents associated with hepatotoxicity (ie, sulfonylureas, α-glucosidase inhibitors, biguanides, and thiazolidinediones).Citation26 The negative binomial regression with log-link function was used to analyze healthcare utilization; between-group cost differences were analyzed using generalized linear models with a gamma distribution and log-link function. Estimated β coefficients obtained by the generalized linear models were exponentiated to calculate the incremental differences between groups. The distribution of incremental costs were converted to actual cost (in dollars) to provide meaningful results for interpretation.
Disclosure of potential conflicts of interest
GK and RS were employed by the GSK group of companies at the time of the study conduct and during the development of the manuscript. GK is currently employed by CSL and reports ownership of stock options/restricted shares from the GSK group of companies and CSL. RS is now an employee of AstraZeneca and has ownership of stocks in the GSK group of companies. JS, GD, and AJK are employees of HealthCore, Inc., a wholly owned subsidiary of Anthem, Inc. DFL was an employee of HealthCore, Inc., at the time of study design and execution. DFL is now an employee of Takeda Pharmaceuticals USA, Inc.. HealthCore, Inc. received funding by the GSK group of companies to conduct the study. DFE and JS are shareholders of Anthem, Inc.
Acknowledgments
The authors thank Cheryl Jones of HealthCore, Inc., USA, Jenny Andersson of CROMSOURCE Ltd., UK on behalf of GSK Vaccines and Marie Cloes of Business and Decision Life Sciences on behalf of GSK Vaccines for editorial support, and Ning Wu, former employee of GSK Vaccines, for study support.
Parts of this study were presented in abstract and poster form at the 75th Annual Scientific Sessions of the American Diabetes Association, Boston, Massachusetts, June 5–9, 2015.
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study (GSK study ID HO-14–14386). GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and the publishing of the present manuscript.
References
- Schillie SF, Xing J, Murphy TV, Hu DJ. Prevalence of hepatitis B virus infection among persons with diagnosed diabetes mellitus in the United States, 1999-2010. J Viral Hepatitis 2012; 19:674-676; http://dx.doi.org/10.1111/j.1365-2893.2012.01616.x
- Reilly ML, Schillie SF, Smith E, Poissant T, Vonderwahl CW, Gerard K, Baumgartner J, Mercedes L, Sweet K, Muleta D, et al. Increased risk of acute hepatitis B among adults with diagnosed diabetes mellitus. J Diabet Sci Tech 2012; 6:858-866; http://dx.doi.org/10.1177/193229681200600417
- El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004; 126(2):460-468; PMID:14762783; http://dx.doi.org/10.1053/j.gastro.2003.10.065
- Hoerger TJ, Schillie S, Wittenborn JS, Bradley CL, Zhou F, Byrd K, Murphy TV. Cost-effectiveness of hepatitis B vaccination in adults with diagnosed diabetes. Diabetes Care 2013; 36:63-69; PMID:22933435; http://dx.doi.org/10.2337/dc12-0759
- Bond WW, Favero MS, Peterson NJ, Gravelle CR, Ebert JW, Maynard JE. Survival of hepatitis B virus after drying and storage for one week. Lancet 1981; 9:550-551; http://dx.doi.org/10.1016/S0140-6736(81)92877-4
- Favero MS, Bond WW, Peterson NJ, Berquist KR, Maynard JE. Detection methods for study of the stability of hepatitis B antigen on surfaces. J Infect Dis 1974; 129:210-212; PMID:4810942; http://dx.doi.org/10.1093/infdis/129.2.210
- Sawyer MH, Hoerger TJ, Murphy TV, Schillie SF, Hu D, Spradling PR, Byrd KK, Xing J, Reilly ML, Tohme RA, et al. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60:1709-1711; PMID:22189894
- Centers for Disease Control and Prevention. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60(50):1709-1711; PMID:22189894
- Williams WW, Lu PJ, O'Halloran A, Bridges CB, Kim DK, Pilishvili T, Hales CM, Markowitz LE. Vaccination coverage among adults, excluding influenza vaccination—United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64(4):95-102; PMID:25654611
- Lee TA, Veenstra DL, Iloeje UH, Sullivan SD. Cost of chronic hepatitis B infection in the United States. J Clin Gastroenterol 2004; 38:S144-S147; PMID:15602162; http://dx.doi.org/10.1097/00004836-200411003-00005
- Spackman DE, Veenstra DL. A cost-effectiveness analysis of currently approved treatments for HBeAg-positive chronic hepatitis B. Pharmacoeconomics 2008; 26:937-949; PMID:18850763; http://dx.doi.org/10.2165/00019053-200826110-00006
- Robotin M, Patton Y, Kansil M, Penman A, George J. Cost of treating chronic hepatitis B: comparison of current treatment guidelines. World J Gasteroenterol 2012; 18:6106-6113; http://dx.doi.org/10.3748/wjg.v18.i42.6106
- Park JY, Heo J, Lee TJ, Yim HJ, Yeon JE, Lim Y-S, Seo MJ, Ahn SH, Lee MS. A novel estimation of the relative economic value in terms of different chronic hepatitis B treatment options. PloS One 2013; 8:e57900; PMID:23536775; http://dx.doi.org/10.1371/journal.pone.0057900
- Tunceli O, Wade R, Gu T, Bouchard JR, Aagren M, Luo W. Cost of diabetes: comparison of disease-attributable and matched cohort cost estimation methods. Curr Med Res Opin 2010; 26:1827-1834; PMID:20491613; http://dx.doi.org/10.1185/03007995.2010.488544
- Gandra SR, Lawrence LW, Parasuraman BM, Darin RM, Sherman JJ, Wall JL. Total and component health care costs in a non-Medicare HMO population of patients with and without type 2 diabetes and with and without macrovascular disease. J Manag Care Pharm 2006; 12:546-554; PMID:16981800
- Pelletier EM, Smith PJ, Boye KS, Misurski DA, Tunis SL, Minshall ME. Direct medical costs for type 2 diabetes mellitus complications in the US commercial payer setting: a resource for economic research. Appl Health Econ Health Policy 2008; 6:103-112; PMID:19231904; http://dx.doi.org/10.1007/BF03256126
- Laliberté F, Bookhart BK, Vekeman F, Corral M, Duh MS, Bailey RA, Piech CT, Lefebvre P. Direct all-cause health care costs associated with chronic kidney disease in patients with diabetes and hypertension: a managed care perspective. J Manag Care Pharm 2009; 15:312-322.
- Stephens JM, Botteman MF, Hay JW. Economic impact of antidiabetic medications and glycemic control on managed care organizations: a review of the literature. J Manag Care Pharm 2006; 12:130-142; PMID:16515371
- Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika 1983; 70(1):41-55; http://dx.doi.org/10.1093/biomet/70.1.41
- Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. In: Proceedings of the Twenty-Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute, Inc., 2001 ; cited 2014 Dec 2. Available from: http://www2.sas.com/proceedings/sugi26/p214-26.pdf.
- Parsons LS. Using SAS software to perform a case-control match on propensity score in an observational study. In: Proceedings of the Thirtieth Annual SAS Users Group International Conference. Cary, NC: SAS Institute, Inc., 2005 ; cited 2014 Dec 2. Available from: http://www2.sas.com/proceedings/sugi25/25/po/25p225.pdf.
- Chang HY, Weiner JP, Richards TM, Bliech SN, Segal JB. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care 2012; 18(11):721-726; PMID:23198714
- Chang HY, Weiner JP, Richards RM, Bliech SN, Segal JB. Predicting costs with Diabetes Complications Severity Index in claims data. Am J Manag Care 2012; 18(4):213-219; PMID:22554010
- McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J Manag Care Pharm 2011; 17(7):531-546; PMID:21870894
- United States Department of Labor, Bureau of Labor Statistics. Consumer price index; cited 2014 Sep 17. Available from http://www.bls.gov/cpi/home.htm.
- Vagula M, Devi SS. Hepatotoxicity of antidiabetic drugs. US Pharm 2008; 33(5 Diabetes Suppl):3-9
Appendix.
Variables Included in Propensity Score Model
| • | Age on index date | ||||
| • | Gender | ||||
| • | Geographic region on index date | ||||
| • | Index year | ||||
| • | Length of pre-index eligibility | ||||
| • | Presence of diabetes during pre-index period | ||||
| • | aDCSI score | ||||
| • | Comorbiditiesa: cerebrovascular disease, chronic obstructive pulmonary disease, congestive heart failure, coronary artery disease, dementia, hemiplegia or paraplegia, HIV/AIDS, hypertension, hyperlipidemia, malignancy, metastatic solid tumor, moderate or severe renal disease, other liver disease, peptic ulcer disease, peripheral vascular disease, rheumatological disease | ||||
| • | Frequency of office visits not related to HBV or its related complications | ||||
| • | Pre-index hospitalization not related to HBV or its related complications | ||||
aDCSI= adapted Diabetes Comorbidity Severity Index; HBV=hepatitis B virus; HIV=human immunodeficiency virus
aComorbidities identified in the pre-index period based on the presence of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes
