ABSTRACT
Immunization with pertussis vaccine during pregnancy is recommended in a number of countries to prevent newborn deaths from whooping cough. In some jurisdictions, vaccine uptake during pregnancy is low. We undertook a survey of the knowledge, attitudes, beliefs, and behaviors of pregnant women who had been approached to participate in a randomized, controlled trial of tetanus-diphtheria-acellular pertussis (Tdap) vaccine during pregnancy. A total of 346 women completed the survey. Knowledge about pertussis and pertussis vaccine was generally low; the mean number of correct answers was 10.65 out of 19 questions. Attitudes toward maternal immunization were generally favorable; 51.7%–94.7% of women had positive responses to 10 attitudinal statements. Substantial uncertainty was shown in responses to a number of the attitudinal statements related to vaccination during pregnancy; 22.3%–45.7% neither agreed nor disagreed with the statements. Importantly, 89% of women reported that they would get immunized with pertussis vaccine during pregnancy if their physician recommended it. We conclude that a national recommendation to be immunized with pertussis vaccine during pregnancy supported by their physicians' recommendation would be well received by Canadian women.
Introduction
Widespread use of pertussis vaccine led to dramatic decreases in the incidence of pertussis in the mid to late 20th century; however, recently, a resurgence of pertussis has been observed in a number of countries, despite high rates of vaccine coverage.Citation1 Deaths from pertussis occur mostly in young infants who are too young to have initiated or completed their primary series with pertussis vaccine;Citation2,3 infant deaths from pertussis are reported whether or not a resurgence of pertussis is reported in other age groups.Citation4 A number of strategies have been proposed to prevent pertussis in infants and the resultant morbidity and mortality. Adding an infant dose of pertussis vaccine (neonatal immunization),Citation5 immunizing all close contacts of the young infant (cocooning),Citation6 and immunization of women during pregnancy (maternal immunization)Citation7 have all been proposed as potential interventions. The immunogenicity of a neonatal dose of pertussis vaccine has been variable, perhaps related to whether the combined diphtheria-tetanus-acellular pertussis vaccine or an acellular pertussis vaccine alone is used.Citation8,9 While a cocooning strategy makes sense and there is some evidence for its effectiveness,Citation10 logistically, it has been difficult to implement on a large scaleCitation11 and is expensive.
Maternal immunization has been demonstrated to be the most cost-effective of the currently available strategies and has been implemented in the United States and in the United Kingdom in response to outbreaks of pertussis and increased infant deaths.Citation12 The effectiveness of the intervention has been demonstrated in several epidemiological and case-control studies in the UK, where high levels of uptake (approximately 70%) were achieved. Citation13,14 In the US, uptake of pertussis vaccine during pregnancy has been estimated to be < 5 %, despite being recommended for more than 5 y.Citation12 In Canada, pertussis immunization during pregnancy is recommended as a response to pertussis outbreaks but is not given routinely in any jurisdiction. In this observational study, we surveyed pregnant women to determine their knowledge, attitudes, beliefs, and behaviors regarding vaccination during pregnancy in general and more specifically about the use of pertussis vaccine during pregnancy.
Results
Demographics
There were 346 respondents to the survey: 171 of 325 participants in the clinical trial completed the survey; 77 women who declined participation in the clinical trial completed the survey (the number of nonparticipants approached was not collected); and 98 women with unknown clinical trial status completed the survey. Most women (72.5%) were between 25 and 35 y of age (). The respondents to the survey were predominantly white (84.1%), having their first (53.5%) or second (33.8%) baby, and were highly educated (75.4% had a university degree or higher, with 26.9% holding an advanced degree).
Table 1. Characteristics of survey respondents.
Knowledge and awareness
Nearly all (90.2%) participants had heard of pertussis, although only 5.8% reported having had it themselves (). Most participants knew that pertussis was a greater risk to their infant than to themselves; 68.2% thought that pertussis was a very high or moderately high risk to the newborn, and 67.6% thought it was a moderate or moderately low risk to adults. The mean number of correct answers to the 19 knowledge questions was 10.65 (95% confidence interval 10.28–11.01).
Attitudes
The majority of participants had attitudes that supported immunization against pertussis during pregnancy (). Most (89.9%) either disagreed or strongly disagreed that pertussis was rare and that immunization was no longer required. Most participants (84.1%) also disagreed or strongly disagreed that getting the infection was better than being immunized. Although 72.3% of participants agreed or strongly agreed that it was important for children to be immunized against pertussis by 6 months of age, 20.5% neither agreed nor disagreed with this statement. A total of 71.4% of participants agreed or strongly agreed that close contacts of their infant should be immunized against pertussis but, again, more than one-fifth (22.3%) neither agreed nor disagreed. Nearly all (94.7%) participants who responded to the question agreed or strongly agreed that pertussis poses a serious threat to young infants, and 87.1% who responded to the question agreed or strongly agreed that pertussis vaccine is effective in preventing pertussis.
Table 2. Attitudes and beliefs regarding pertussis, pertussis vaccine and vaccination during pregnancy.
While attitudes about receiving pertussis vaccine during pregnancy were generally favorable, many women neither agreed nor disagreed with these attitudinal statements (). The proportion of responses that could be interpreted as being against vaccination during pregnancy was <10% for most of the attitudinal statements. A total of 62.1% of women agreed or strongly agreed that giving pertussis vaccine to pregnant women would protect the infants, although 35.8% neither agreed nor disagreed; 70.3% of women who responded to the question agreed or strongly agreed that it was safe to give pertussis vaccine during pregnancy, although 27.8% of the women who responded to the statement neither agreed nor disagreed. In response to the statement that it is best to avoid vaccines during pregnancy, 52.9% disagreed or strongly disagreed, while 34.4% neither agreed nor disagreed. Only 37.0% of respondents agreed or strongly agreed that it was best to avoid vaccination during the first 3 months of pregnancy, while 45.7% neither agreed nor disagreed. A total of 40.8% of women disagreed or strongly disagreed that it was safer to wait until after delivery to receive a vaccine while 48.8% neither agreed nor disagreed with this statement. The majority of respondents (63.9%) agreed or strongly agreed that it is better to be immunized during pregnancy than to risk pertussis disease in the newborn; 30.9% neither agreed nor disagreed with this statement.
Maternal concerns and response to recommendations
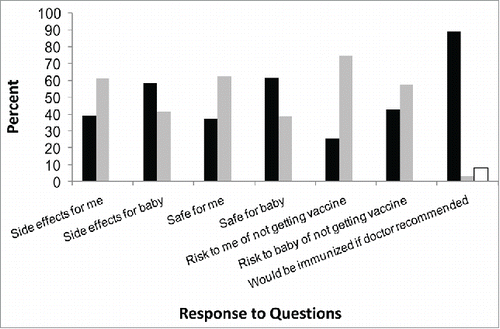
Most of the women who participated in the study were more concerned about the safety and adverse effects of the vaccine for their fetus/infant than for themselves (). A total of 61.6% were concerned about the safety of the vaccine and 58.4% concerned about adverse effects for the baby, compared to only 37.3% and 39%, respectively, for themselves. Only 25.4% were concerned about the risk of not getting the vaccine for themselves, while 42.8% were concerned about the risk of not getting the vaccine for their infant. Importantly, fully 89% indicated that they would get immunized with pertussis vaccine during pregnancy if it was recommended by their doctor; only 2.9% said they would not, and 8.1% were undecided.
Figure 1. Responses to the questions “To feel more comfortable about receiving the tetanus-diphtheria-acellular pertussis vaccine during pregnancy I need more information about…” and the question “If my doctor recommended it, I would be immunized with pertussis vaccine during pregnancy.” Black bars equal yes, gray bars no, white bar I don't know.
In the univariate analysis, increased knowledge score correlated with increased level of education, having heard of pertussis prior to participating in the study, and being aware of an adult pertussis vaccine (all p < 0.05). Respondents who participated in the clinical trial also had higher knowledge scores and differed in their attitudes regarding maternal immunization (p < 0.05). Univariate analysis was also undertaken to assess the effect of demographic variables, awareness of pertussis and pertussis vaccine, and knowledge score on uncertainty in response to the attitudinal statements (those who responded “neither agree nor disagree” compared to those who either agreed/strongly agreed and disagreed/strongly disagreed). Lower knowledge scores were significantly correlated with less certainty regarding the attitudinal statements (p < 0.05). Uncertainty regarding the attitudinal questions also varied significantly over time; early respondents who completed the survey were more uncertain about the attitudinal statements than those who completed the survey toward the end of the study (p < 0.05).
Discussion
Immunizing pregnant women is increasingly being advocated to protect themselves, their fetuses, and their newborns.Citation7,15 Influenza vaccine has been recommended for pregnant women since the 1960s and is now the World Health Organizations' highest priority for influenza vaccination.Citation16 Vaccines are being developed specifically for maternal immunization to protect neonates and young infants from group B StreptococcusCitation17 and Respiratory Syncytial Virus.Citation18 The combined, adult-formulation tetanus-diphtheria-acellular pertussis vaccine (Tdap) is recommended in the UK in response to an increase in infant deaths from pertussis and is recommended to be given during every pregnancy in the US.Citation12 Uptake, however, in the US is low.Citation12
The reasons for low Tdap utilization in pregnant women are likely multifactorial, but knowledge and attitudes of women regarding vaccination during pregnancy is likely an important factor.Citation19 As have others,Citation20-24 we previously found that knowledge about pertussis and pertussis vaccine is low in the general population,Citation25 similar to what we found in this study in pregnant women who completed our survey. Pregnancy, however, is a time when women have frequent contact with the healthcare system so there are numerous opportunities to intervene with education and specific recommendations.
In this study, attitudes toward immunization during pregnancy were most favorable. Only a small proportion of respondents had attitudes that would be categorized as negative; most participants had either favorable attitudes or neutral attitudes (“neither agree nor disagree”). We cannot discern from our data whether participants who chose “neither agree nor disagree” were indifferent to the statement or were undecided. Lack of knowledge regarding the issue might be one reason to take a neutral stance, and lower knowledge scores did correlate with greater proportions neither agreeing nor disagreeing with the attitudinal statements. It is revealing that a remarkable 89% indicated that they would be immunized during pregnancy if their physician recommended it; this suggests that the neutral responses were more a reflection of indecision than indifference. Others have also found that a physician recommendation is the most important predictor of acceptance of Tdap vaccination during pregnancy,Citation20,23,24 although the intention to be immunized if their physician recommended it was higher in our study than others have reported.Citation21,23 Healthcare providers are more likely to recommend Tdap vaccination during pregnancy if there are strong national recommendations.Citation22
Our study has a number of strengths and weaknesses. The study was undertaken with a rigorously validated survey instrument and participants were pregnant at the time, making the issues in the survey timely and relevant. Survey responses were collected over 6 y during the clinical trial, and attitudes about maternal immunization changed with time as recommendations for influenza vaccination during pregnancy became more firmly established and pertussis recommendations were implemented in other jurisdictions. This resulted in a change in attitudes over time, with less respondents being uncertain about their attitude toward maternal immunization. In addition, both women who were participating in the clinical trial and those who declined participation were invited to complete the survey. All of the survey respondents had been approached about participating in the clinical trial of maternal immunization which may have biased the responses toward a more positive attitude, even in those who ultimately decided not to enroll in the clinical trial. Therefore, these results may not be generalized to the entire population of pregnant women. We did detect differences in both knowledge and attitudes depending on whether the respondents were participants in the clinical trial. As well, our participants were predominantly white and highly educated. In Canada, 19.1% of the population self-identifies as a visible minority,Citation26 not very different from the 15.9% of our participants who described themselves as nonwhite. In Canada, 16.5% of the population has completed an undergraduate university degree and 6.7% an advanced degree, substantially lower than the 48.6% and 26.9%, respectively, for our participants.Citation27
In summary, in this survey of pregnant women, immunization with pertussis vaccine during pregnancy was generally well received. Knowledge of pertussis and pertussis vaccine, however, was generally low, suggesting that some of the women who were undecided might be more favorably inclined toward immunization if they were provided with additional information. In keeping with the evidence in the literature relating to the general population, this study provides data specific to pregnant women that a physician recommendation is an important factor in the decision-making process, This suggests that, with strong support from healthcare providers and an appropriately targeted information campaign, maternal immunization with pertussis vaccine could be a successful strategy in Canada.
Methods
Study setting and population
The study took place in Halifax, Nova Scotia; Calgary and Edmonton, Alberta; Montreal, Quebec; Vancouver, British Columbia; and Ottawa, Ontario, between January 2008 and April 2014; during the study period, no outbreaks of pertussis occurred in the study areas. Healthy women with uncomplicated pregnancies who were approached to participate in a randomized, controlled trial of Tdap vaccination during pregnancy were asked to complete the survey. Both women who participated in the clinical trial and those who declined participation were eligible to complete the survey. Women were recruited through family physicians' and obstetricians' offices and in hospital prenatal clinics.
Study design
Surveys were distributed to pregnant women at the time of their physician or clinic visit after they were approached to participate in the randomized, controlled trial of Tdap during pregnancy. Surveys were completed in the waiting rooms and collected prior to leaving or were returned by mail in postage-paid envelopes. Completing and returning the questionnaire denoted informed consent. The study received Research Ethics Board approval at all participating sites.
Survey instrument development
The survey contained 52 questions, including demographics, information and awareness (11 items), knowledge (19 items), attitudes and beliefs (14 items), and information sources and intentions (8 items). Knowledge questions covered general immunization information as well as immunization information specific to pertussis. Attitudinal statements testing opinions about maternal immunization were structured with a 5-point Likert response scale ranging from “strongly disagree” to “strongly agree.” In the development of the survey instrument, content validity was assessed by presenting the questions to a panel of 5 infectious disease specialists who have a focus in vaccine research. Each question as well as the survey overall was evaluated by the experts using a rating worksheet with a 4-point ordinal rating scale. Items that received a high content rating (3 or 4) were retained; those with low ratings were eliminated or modified and re-evaluated. In addition to this quantitative assessment of content validity, a qualitative assessment was obtained through use of a focus group of 5 target participants to provide feedback on clarity, wording, and relevance of the survey items. Test–retest validity was also assessed by having 3 individuals complete the survey twice at an interval of 1 month. A correlation coefficient > 0.7 was used to denote reasonable consistency over time. Prior to implementation of the survey, the instrument was validated with participants in a clinical trial of the kinetics of the antibody response to Tdap vaccine in women of child-bearing age and further modified and validated with participants in a second study of the kinetics of the antibody response to Tdap in pregnant women.Citation28
Data management and statistical analysis
Paper-based surveys were double-entered by 2 people working independently via Remark Web Survey® Professional software (Versions 4 and 5). The data were downloaded, transferred to a secure server and loaded into SAS® (versions 8 and 9) datasets. SAS® was used to compare the 2 entries and correct data entry errors.
The first level of analysis comprised a review of the descriptive, summative statistics for trends in the data. The second level of analysis involved tests of association. Continuous variables were presented by summary statistics (i.e., mean and standard error) and the categorical variables by frequency distributions (i.e., frequency counts, percentages, and their 2-sided 95% exact binomial confidence intervals). Differences in survey responses were assessed using Fisher's exact tests or Pearson Chi-square tests. For continuous variables, logistic regression was used. Overall knowledge scores were compared using t-tests. Associations between attitude questions, behavioral responses, and demographics were estimated using ordinal logistic regression or Fisher's exact tests. P-values < 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
Drs. Halperin and Langley have served as consultants on ad hoc advisory boards organized by Sanofi Pasteur, which provided funding for this study.
Supplemental_Material.zip
Download Zip (90.5 KB)Funding
The study was funded by a grant from Sanofi Pasteur, which played no role in the design, performance, or analysis of the study.
References
- Edwards KM. Overview of pertussis: focus on epidemiology, sources of infection, and long term protection after infant vaccination. Pediatr Infect Dis J. 2005; 24(6 Suppl):S104-8; PMID:15931137; http://dx.doi.org/10.1097/01.inf.0000166154.47013.47
- Halperin SA, Wang EE, Law B, Mills E, Morris R, Déry P, Lebel M, MacDonald N, Jadavji T, Vaudry W, et al. Epidemiological features of pertussis in hospitalized patients in Canada, 1991–1997: report of the Immunization Monitoring Program–Active (IMPACT). Clin Infect Dis. 1999;28:1238-43; PMID:10451159; http://dx.doi.org/10.1086/514792
- Haberling DL, Holman RC, Paddock CD, Murphy TV. Infant and maternal risk factors for pertussis-related infant mortality in the United States, 1999 to 2004. Pediatr Infect Dis J. 2009;28:194-8; PMID:19209089; http://dx.doi.org/10.1097/INF.0b013e31818c9032
- van Hoek AJ, Campbell H, Amirthalingam G, Andrews N, Miller E. The number of deaths among infants under one year of age in England with pertussis: results of a capture/recapture analysis for the period 2001 to 2011. Euro Surveill. 2013;18(9). pii:20414; PMID:23470020
- Wood N, Siegrist CA. Neonatal immunization: where do we stand? Curr Opin Infect Dis. 2011;24:190-5; PMID:21415741; http://dx.doi.org/10.1097/QCO.0b013e328345d563
- Wong CY, Thomas NJ, Clarke M, Boros C, Tuckerman J, Marshall HS. Maternal uptake of pertussis cocooning strategy and other pregnancy related recommended immunizations. Hum Vaccin Immunother 2015;11:1165-72; PMID:25874807; http://dx.doi.org/10.1080/21645515.2015.1019188
- Englund JA. Maternal immunization–Promises and concerns. Vaccine. 2015;33:63723; PMID:26263199; doi: 10.1016/j.vaccine.2015.07.084.
- Wood N, McIntyre P, Marshall H, Roberton D. Acellular pertussis vaccine at birth and one month induces antibody responses by two months of age. Pediatr Infect Dis J. 2010;29:209-15; PMID:20009964; http://dx.doi.org/10.1097/INF.0b013e3181bc98d5
- Halasa NB, O'Shea A, Shi JR, LaFleur BJ, Edwards KM. Poor immune responses to a birth dose of diphtheria, tetanus, and acellular pertussis vaccine. J Pediatr. 2008;153:327-32; PMID:18534242; http://dx.doi.org/10.1016/j.jpeds.2008.03.011
- Quinn HE, Snelling TL, Habig A, Chiu C, Spokes PJ, McIntyre PB. Parental Tdap boosters and infant pertussis: a case-control study. Pediatrics. 2014;134:713-20; PMID:25225136; http://dx.doi.org/10.1542/peds.2014-1105
- Healy CM, Rench MA, Baker CJ. Implementation of cocooning against pertussis in a high-risk population. Clin Infect Dis. 2011;52:157-62; PMID:21288837; http://dx.doi.org/10.1093/cid/ciq001
- Advisory Committee on Immunization Practices. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women–Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62(7):131-5; PMID:23425962
- Dabrera G, Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Fry NK, Ramsay M. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clin Infect Dis. 2015; 60:333-7; PMID:25332078; http://dx.doi.org/10.1093/cid/ciu821
- Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, Fry NK, Miller E, Ramsay M. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014; 384(9953):1521-8; PMID:25037990; http://dx.doi.org/10.1016/S0140-6736(14)60686-3
- National Vaccine Advisory Committee. The National Vaccine Advisory Committee: Reducing patient and provider barriers to maternal immunizations. Public Health Reports. 2015; 130(1), 10-42; PMID:25552752
- World Health Organization. Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec. 2012;87:461-76; PMID:23210147
- Madhi SA, Dangor Z, Heath PT, Schrag S, Izu A, Sobanjo-Ter Meulen A, Dull PM. Considerations for a phase-III trial to evaluate a group B Streptococcus polysaccharide-protein conjugate vaccine in pregnant women for the prevention of early- and late-onset invasive disease in young-infants. Vaccine. 2013;31(Suppl 4):D52-7; PMID:23973347; http://dx.doi.org/10.1016/j.vaccine.2013.02.029
- Graham BS, Anderson LJ. Challenges and opportunities for respiratory syncytial virus vaccines. Curr Top Microbiol Immunol. 2013;372:391-404; PMID:24362701; http://dx.doi.org/10.1007/978-3-642-38919-1_20
- MacDougall DM, Halperin SA. Improving rates of maternal immunization: challenges and opportunities. Hum Vaccin Immunother. 2015 Nov 9 [Epub ahead of print]; PMID:26552807; http://dx.doi.org/10.1080/21645515.2015.1101524
- Wiley, K. E., Massey, P. D., Cooper, S. C., Wood, N., Quinn, H. E., Leask, J. Pregnant women's intention to take up a post-partum pertussis vaccine, and their willingness to take up the vaccine while pregnant: A cross sectional survey. Vaccine. 2013;31(37):3972-8; PMID:23777954; http://dx.doi.org/10.1016/j.vaccine.2013.06.015
- Chamberlain AT, Seib K, Ault KA, Orenstein WA, Frew PM, Malik F, Cortés M, Cota P, Whitney EA, Flowers LC, et al. Factors associated with intention to receive influenza and tetanus, diphtheria, and acellular pertussis (Tdap) vaccines during pregnancy: A focus on vaccine hesitancy and perceptions of disease severity and vaccine safety. PLoS Curr 2015;7. pii: ecurrents.outbreaks.d37b61bceebae5a7a06d40a301cfa819; PMID:25789203; http://dx.doi.org/10.1371/currents.outbreaks.d37b61bceebae5a7a06d40a301cfa819
- Clark SJ, Adolphe S, Davis MM, Cowan AE, Kretsinger K. Attitudes of US obstetricians toward a combined tetanus-diphtheria-acellular pertussis vaccine for adults. Infect Dis Obstet Gynecol. 2006; 2006:87040 (1-5); PMID:17485814; http://dx.doi.org/10.1155/IDOG/2006/87040
- Varan AK, Esteves-Jaramillo A, Richardson V, Esparza-Aguilar M, Cervantes-Powell P, Omer SB. Intention to accept Bordetella pertussis booster vaccine during pregnancy in Mexico City. Vaccine. 2014; 32:785-92; PMID:24394441; http://dx.doi.org/10.1016/j.vaccine.2013.12.054
- Beel ER, Rench MA, Montesinos DP, Mayes B, Healy CM. Knowledge and attitudes of postpartum women toward immunization during pregnancy and the peripartum period. Hum Vaccin Immunother 2013; 9:1926-31; PMID:23782490; http://dx.doi.org/10.4161/hv.25096
- Halperin BA, MacDougall D, MacKinnon-Cameron D, Li L, McNeil SA, Langley JM, Halperin SA. Universal tetanus, diphtheria, acellular pertussis (Tdap) vaccination of adults: what the Canadian public knows and wants to know. Vaccine. 2015; 33:6840-8; PMID:26392011; http://dx.doi.rog/10.1016/j.vaccine.2015.09.012
- Statistics Canada. Immigration and ethnocultural diversity in Canada. Available from: http://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-010-x/99-010-x2011001-eng.cfm#a3. Accessed 22 August 2015.
- Statistics Canada. Number and proportion of the population aged 25 to 64 by highest level of educational attainment, Canada, 2011. Available from: http://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-012-x/2011001/tbl/tbl01-eng.cfm. Accessed 22 August 2015.
- Halperin BA, Morris A, MacKinnon-Cameron D, Mutch J, Langley JM, McNeil SA, MacDougall D, Halperin SA. Kinetics of the antibody response to tetanus-diphtheria-acellular pertussis vaccine in women of childbearing age and postpartum women. Clin Infect Dis. 2011; 53:885-92; PMID:21946190; http://dx.doi.org/10.1093/cid/cir538
