ABSTRACT
In Chronic Myeloid Leukemia (CML), standard treatment consists of modern tyrosine-kinase inhibitors (TKI). Nevertheless, there is evidence that immune responses against leukemia-associated antigens (LAA) may play an important role in disease control. Dendritic cell (DC)- based immunotherapy is able to induce T cell responses against LAA and might therefore pose an interesting therapeutic option in CML, especially in the setting of minimal residual disease (MRD). GMP production of DC for clinical vaccination remains a time- and cost- intensive procedure and standardized DC generation is warranted. We asked whether maturation-induction with IFN-γ and IFN-α has an influence on functional properties of DC derived from peripheral blood mononuclear cells (PBMC) in CML patients. Monocyte-derived DC from healthy donors and from patients with CML were analyzed after maturation-induction with our TNF-α-containing standard cytokine cocktail with or without addition of IFN-α and/or IFN-γ. Our results confirm that the addition of IFN-γ leads to enhanced IL-12 secretion in healthy donors. In contrast, in CML patients, IFN-γ was not able to increase IL-12 secretion, possibly due to a higher degree of cell adherence and lower cell yield during the cell culture. Our data suggest, that- in contrast to healthy donors-, additional interferons are not beneficial for maturation induction during large-scale DC production in patients with CML.
Introduction
Chronic myeloid leukemia is a clonal myeloproliferative disorder in which the characteristic reciprocal translocation, t(9;22)(q34;q11) forms the Philadelphia chromosome and leads to the fusion gene BCR-ABL.Citation1 In CML, the predominant gene product of this reciprocal translocation is the BCR/ABL fusion protein p210, which shows abnormal tyrosine-kinase activity that is crucial for the pathogenesis of the disease.Citation2,3 The introduction of tyrosine kinase inhibitors (TKI) has revolutionized CML treatment and has become a paradigm of “targeted therapy” since the late 1990ies.Citation4,5 TKI treatment with Imatinib and in particular the second generation TKI Nilotinib and Dasatinib has substantially increased the proportion of patients achieving deep molecular responses. This improvement of the quality of remission has translated into a substantially longer progression-free and promising overall survival.Citation5 However, most CML patients– despite achieving deep molecular remissions- remain BCR/ABL-positive, suggesting disease persistence at least at a very low level. In this situation of minimal residual disease (MRD) on a molecular level, additional immunotherapeutic strategies such as interferon therapy or vaccination against leukemia- associated antigens seems particularly promising with regard to a possible eradication of residual leukemia cells. In fact, there is a large body of evidence showing that CML is an “immunoresponsive” disease: 1) interferon therapy is able to induce complete cytogenetic responses in up to 30% of CML patients; Citation6 2) induction of T cell responses against proteinase-3 correlates with ongoing remissions under interferon therapy and after allogeneic stem cell transplantation (SCT); Citation7 3) donor lymphocyte infusions (DLI) are able to induce long-lasting remissions in relapsed CML patients after allogeneic SCT Citation8 and 4) immune responses against CML-specific and CML-associated antigens such as BCR/ABL, proteinase-3 and WT-1 can be detected in CML patients and vaccination is able to enhance these immunological responses and can also induce clinical responses in some CML patients.Citation9-19 Untreated CML patients show severe immunosuppression caused by multiple mechanisms: induction of myeloid-derived suppressor cells, regulatory T cells, secretion of soluble factors such as VEGF, PIGF and TGF-ß and the expression of immunosuppressive molecules such as PD-1 or NOX/NOX2. Additionally, function and maturation of dendritic cells may be impaired.Citation20-24 Therefore, the deep molecular responses observed under TKI therapy may also reverse immunosuppression that is induced by a large tumor burden. DC are key regulators of MHC-restricted T cell responses in humans and are considered as “nature's adjuvant.”Citation25 The feasibility of DC- based immunization strategies in CML patients has been demonstrated by several groups including ours, although most of these studies were performed in CML patients, who had not achieved a major molecular remission.Citation15,26-29 Ex- vivo generation and maturation of DC is a crucial step for these strategies and different cytokine cocktails have been described which are mainly based on GM-CSF, IL-4 and TNF-α.Citation15,16,30,31,32 Kalinski et al. reported that interferons are able to induce DC that combine several important features with regard to their TH1-polarizing capacity: mature phenotype, responsiveness to secondary lymphoid organ chemokines and high IL-12 producing ability.Citation33,34 Therefore, the aim of our present study was to assess the influence of interferons during ex vivo DC generation on the functional properties of DC in CML patients.Citation15
Results
DC generation in healthy donors and CML patients: Influence of interferons on phenotype and cell yield
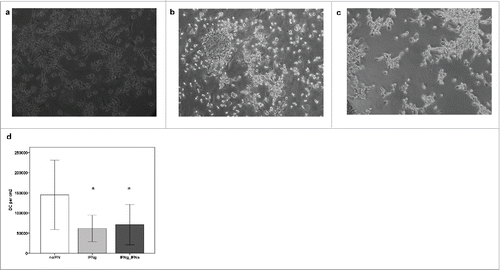
After 8 d of cell culture, peripheral blood monocytes had acquired a typical DC morphology. Viability was always > 80%. The immunophenotype of DC at the end of the culture was consistent with mature DC showing lineage- negative cells with high expression of CD80, CD83, CD86, and HLA- DR.Citation15,16 The addition of IFN-γ (or IFN-γ plus IFN-α in CML patients) did not significantly affect the TNF-α− induced mature phenotype as measured by flow cytometry (data not shown). However, cell culture in CML patients revealed substantially more cell adherence after addition of interferon-γ (). In fact, addition of IFN-γ led to 7.1% adherent cells in the cell culture as compared to 0.9% without IFN-γ. However, this effect of IFN-γ on cell adherence was reversed when IFN-α was added (adherent cells 0.8%) (data not shown). The influence of interferons on DC yield in CML patients after 8 d of cell culture was even more pronounced: mean DC yield without interferons was 105 667 cells per cm2, whereas it decreased to 59 133 per cm2 (only 50% of the cell yield with TNF-α alone) after addition of IFN-γ during maturation induction. Possibly due to the small number of patients, this difference was not statistically significant (p = 0.2). Combined use of IFN-γ and IFN-α was not able to reverse this negative impact on DC yield (DC with IFN-γ and IFN-α: 54 333 per cm2) ().
Figure 1. Cell adherence and cell yield of DC from CML patients after maturation induction with IFN-γ. Using our standard cytokine cocktail, DC generation from PBMC of CML patients and healthy individuals gives comparable results (a and b). In contrast, addition of interferons leads to an increase of cell adherence and a lower DC yield (c and d). a: DC culture from a healthy donor, b: DC culture from a patient with CML in chronic phase cultured with our standard cytokine cocktail (GM-CSF, IL-4, TNF-α) without interferons, c: Increased DC adherence in a DC culture containing IFN-γ in a chronic phase CML patient, d: Decreased DC yield (in cells per cm2) in cell cultures from CML patients containing IFN- γ or IFN-γ + IFN-α. *=Difference (as compared to “no IFN”) not statistically significant (p=0.2), possibly due to high variation in a low number of samples (no IFN: n = 3, IFN-γ: n = 3, IFN-γ + IFN-α: n = 3).
Influence of interferons on the cytokine secretion profile of DC after CD40 ligation in healthy donors
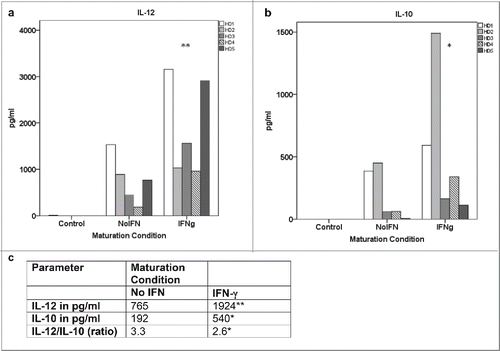
TH1-polarizing capacity of DC from healthy donors as measured by IL-12 secretion was studied by using xenogeneic CD40L-transfected 3T3 cells in order to mimic DC-T cell-interactions. 3T3 WT cells served as control. In DC from healthy donors, inclusion of IFN-γ into the maturation cocktail indeed led to an increase of IL-12 secretion after CD40-ligation by 3T3-CD40L cells (mean IL-12 secretion without IFN versus IFN-γ: 765 vs. 1924 pg/ml, p = 0.043, ). However, there was also an increase of IL-10 production resulting in an overall unchanged or even decreased ratio of IL-12 to IL-10 (mean IL-10 secretion without IFN vs. IFN-γ: 192 vs. 540 pg/ml, p=0.068; IL-12/IL-10 ratio without IFN versus IFN-γ: 3.3 vs. 2.6, ). The production of IL-6 and IL-8 was not changed (data not shown).
Figure 2. Influence of IFN-γ on the cytokine secretion profile of DC after CD40 ligation in healthy donors. DC from healthy donors were generated under different maturation conditions (standard cytokine cocktail without vs. with IFN-γ). The cytokine profile was measured by CBA (or ELISA for IL-12) after CD40 ligation. a: IL-12 secretion into the culture supernatant without vs. with IFN-γ (p=0.043), b: IL-10 secretion into the culture supernatant without vs. with IFN-γ (p = 0.068),c: IL-12/IL-10 ratio without vs. with IFN-γ.HD = healthy donor **= p<0 .05 as compared to “no IFN;” * = p = not significant as compared to “no IFN.”.
Cytokine secretion profile of DC after CD40 ligation in chronic phase CML patients: influence of interferons
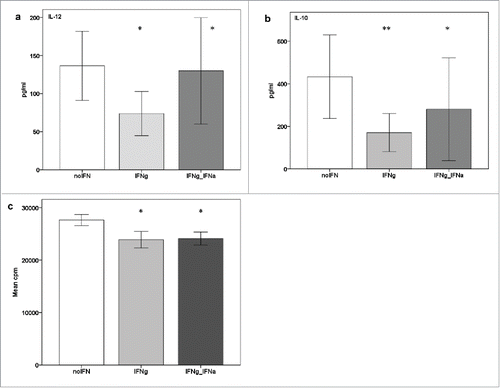
Subsequently, the influence of IFN-γ was also studied in DC from chronic phase BCR/ABL+ CML patients. At the end of the cell culture, DC were stimulated by xenogeneic, CD40L-transfected 3T3 cells. The cytokine profile was measured thereafter. Surprisingly, IL-12-production was not increased after the addition of IFN-γ. On the contrary, a tendency toward lower IL-12-production was observed (mean IL-12 secretion without IFN vs. IFN-γ: 136.6 vs. 73.8 pg/ml, p= 0.055, ) when IFN-γ was added into the DC culture. Since combined use of IFN−γ and IFN-α has been previously reported to increase IL-12 secretion in DC from healthy donors, we also used a combination of IFN-γ and IFN-α in our DC cultures from CML patients to exclude that these results were caused by a lack of IFN-α during the DC culture. However, even the combined use of IFN-γ and IFN-α was not able to consistently increase IL-12 production of CML-DC after CD40-ligation (mean IL-12 in pg/ml: without IFN: 136.6; with IFN-γ 73,8; with IFN-γ plus IFN-α: 130 (). Additionally, IL-10 production was also observed to be lower after addition of IFN-γ into the DC culture (mean IL-10 in pg/ml: without IFN: 433.5; with IFN-γ: 170.6; with IFN-γ plus IFN-α: 280.5, ). With regard to production of IL-4, the cytokine profile remained largely unchanged after addition of interferons and CD40 ligation (data not shown).
Figure 3. Influence of IFN-γ on the cytokine secretion profile of DC after CD40 ligation in chronic phase CML patients. a: IL-12 secretion of DC from patients with CML after maturation-induction with our standard cytokine cocktail without vs. with interferons (without IFN vs. IFN-γ: p = 0.055; without IFN vs. IFN-γ plus IFN-α: p = 0.91), b: IL-10 secretion of DC from patients with CML after maturation-induction with our standard cytokine cocktail without vs. with interferons (without IFN vs. IFN-γ: p = 0.28; without IFN vs. IFN-γ plus IFN-α: p = 0.9), c: T cell stimulatory capacity of DC generated with our standard cytokine cocktail without interferons as measured by a 3H-thymidine proliferation assay (mean CPM of cells after maturation-induction without IFN: 27342 + 712; with IFN-γ: 24029 + 727); with IFN-γ plus IFN-α: 24005 +168). IL-12 was determined by ELISA, all other cytokines by CBA. *= not statistically significant.
T cell stimulatory capacity of DC in chronic phase CML patients: Influence of interferons
To assess the T cell stimulatory capacity of DC matured under these different conditions in patients with chronic phase CML, an allogeneic MLR was used: In line with our previous results regarding the cytokine profile, no increase of the stimulatory ability of CML-DC was observed after addition of IFN-γ or IFN-γ plus IFN-α during cell culture (mean CPM of cells after maturation-induction without IFN: 27342 + 712; with IFN-γ: 24029 + 727; with IFN-γ plus IFN-α: 24005 +168), ).
Discussion
IL-12 secretion by DC is a very helpful surrogate parameter for the TH1-polarizing capacity of in vitro generated DC.Citation35 Previous reports in healthy individuals have described multiple different cytokine cocktails for maturation induction in DC. IL-12 production was commonly used as a measure for the TH1-polarizing capacity. IL-1ß/TNF-α and IFN-γ are among the cytokines which have been used for ex vivo DC generation.Citation33,36 Subsequently, it was demonstrated that combined use of IFN-γ and IFN-α was able to induce a mature DC phenotype with superior IL-12 production in DC from healthy individuals.Citation34,37 During the interaction of DC with T cells, CD40- ligation is a crucial step leading to IL-12 production and consequently TH1-polarized T cells.Citation35,38 Additionally, it has recently been shown, that CD40 ligation leads to “reticulation” of DC, promoting intercellular transfer between DC with consecutive improved antigen-presenting capacity.Citation39 Therefore, our approach was to mimic CD40-CD40L interaction by CD40L-transfected 3T3 cells and thus establish an in vitro method to quantify the TH1-polarizing capacity of in vitro generated DC by IL-12 production after CD40-ligation.
In this study, we show that – in contrast to DC from healthy individuals- interferons are not sufficiently able to increase the TH1-polarizing capacity of DC from CML patients as compared to our maturation protocol including TNF-α alone. Since phenotype and cytokine profiles of DC have been extensively characterized,Citation40 our study focused on IL-12- and IL-10-production, which is assumed to reflect the ability of in vitro generated DC to induce TH1 polarization. Therefore, we used IL-12/IL-10 ratios as a measure for the TH1- polarizing capacity of DC.Citation32 Essentially we asked whether IL-12 production can be increased by IFN-α and/or IFN-γ in DC from CML patients in chronic phase. Our results with DC from healthy donors generated in the presence of IFN-γ confirm that IL-12 production can be increased by IFN-γ after CD40 ligation, however, this effect was not observed with DC from chronic phase CML patients, neither with IFN-γ alone nor with a combination of IFN-γ and IFN-α. Since enhancement of IL-12 secretion was only observed with CD40L-transfected cells and not with 3T3 wild type cells, we were able to exclude that the effect is simply mediated by xenoreactivity. In line with the cytokine profile observed after maturation in the presence of interferons (with an overall unchanged IL-12/IL-10 ratio), an improvement of the T cell stimulatory capacity by interferons was not observed. Regarding possible causes for these results, an increased cell adherence during cell culture and a substantially reduced DC yield were the most prominent findings after addition of interferons into the maturation cocktail. Increased cell adherence has previously been described to impair DC maturation and cytokine secretion.Citation32 However, in our case this would neither explain the difference between healthy donors and CML patients nor the substantial reduction of the DC yield that was observed after inclusion of interferons into the maturation cocktail. Therefore, it has to be assumed that – besides inducing a higher cell adherence - interferons also reduce the percentage of surviving cells during DC culture. While increased cell adherence was a common feature both in DC from healthy donors and from CML patients, the lack of IL-12 production upon maturation in the presence of interferons was only found in DC from CML patients and thus seems to be CML-related. CML-related features leading to this difference might be: 1) direct cytotoxic effects of interferons on “leukemic” (= BCR/ABL+) PBMC and/or “leukemic” DC. This seems at least very likely since interferons were the standard of care for a long period; Citation41-44 2) an impairment of DC function itself which has been previously reported; Citation24 or 3) a treatment-related functional impairment in DC from CML patients since DC were generated from patients under therapy with tyrosine kinase inhibitors. Impaired immune responses have been described under TKI therapy by several groups.Citation45
Interestingly, Bocchia et al. reported on possible synergistic effects of IFN-α and a BCR/ABL multipeptide vaccine in a small observational study.Citation46 This is not necessarily in contrast to our observation, since 1) the effects of IFN-α_on (leukemic) DC during in vitro culture may not reflect the situation in vivo and 2) additional immunostimulatory effects of IFN-α on immunological effector cells such as T cells are well established.
In conclusion, our results are in line with previous publications showing that functional DC can be generated ex vivo in CML patients, both in the chronic phase of the disease (giving rise to BCR/ABL+ “leukemic” DC) and in cytogenetic/molecular remission.Citation15,26,47,48,49 With regard to an interferon-induced “DC1 phenotype” that has been previously described (reviewed in Citation40), we could confirm higher IL-12 secretion in DC from healthy donors as compared to our standard cocktail containing GM-CSF, IL-4 and TNF-α alone. However, this interferon-induced increase in IL-12 production could not be found in DC from CML patients.
Due to higher cell adherence and a lower DC yield observed after the use of interferons, we currently do not recommend their use under the culture conditions used in our GMP facility.
Immunotherapeutic perspectives in CML in the MRD setting under TKI therapy include vaccination strategies with DC + additional immune checkpoint inhibitors. Consequently, improved large- scale DC generation from CML patients is clearly needed. Therefore, refined protocols for DC generation, which address the challenges of cell adherence/ cell yield and - at the same time - support an appropriate DC1- phenotype, are warranted.
Material and methods
Generation of DC from PBMCs
DC were generated from eight healthy donors and three patients with chronic phase BCR/ABL+ CML as previously described and in accordance with local ethical guidelines.Citation15,16,26 PBMCs were enriched by density gradient centrifugation on Lymphoprep (Nycomed Pharma, Oslo, Norway) and then stored on liquid nitrogen in 42.5% human serum albumin (HSA, 20% solution, Octapharma, Langenfeld, Germany), 10% DMSO (Sigma, St. Louis, USA), and 47.5% RPMI (BioWhittaker, Walkersville, USA). Thawing of PBMCs was performed by the addition of HBSS w/o Ca2+/Mg++ (BioWhittaker) containing 5% HSA. PBMCs were washed twice and then exposed to plastic adherence for monocytic enrichment. Briefly, PBMCs were seeded at a density of 1.4 –1.6 ×106 cells/cm2 in 75 –cm2 culture flasks (Corning Inc., Nagog Park, Acton, MA), and allowed to adhere for 1.5 h at 37°C in RPMI 1640 containing 5% HSA and 1% sodium pyruvate (BioWhittaker). Non-adherent cells were removed by washing 4 times. Adherent cells were cultured in RPMI 1640 supplemented with100 ng GM-CSF (Essex Pharma GmbH, Munich, Germany), 1000 U/ml clinical grade IL-4 (kindly provided by Essex Pharma), 10% FCS and 1% sodium pyruvate for 5 d As the standard procedure, 50 ng/ml clinical grade TNF-α (Boehringer Ingelheim, Ingelheim, Germany) was added to the culture medium on day 5 for another 3–4 d
To assess the influence of interferons, IFN-γ or IFN-γ plus IFN-α (Strathmann Biotec AG, Hamburg, Germany) was added at a concentration of 1000 U/ml during the last 3–4 d of the cell culture. DC were then stimulated with 3T3 wild type cells or 3T3 cells that had been transfected with human CD40L (3T3-CD40L) for 24 - 48 hours. Xenogeneic transfected and non-transfected 3T3 cells were kindly provided by C.A. Schmitt, Berlin. On days 8–9, DC were harvested and washed three times and were then frozen and stored on liquid nitrogen as previously described.Citation16 The phenotype of DC was characterized by flow cytometry as previously described.Citation15 The purity of the DC culture was always > 70%, T cell contamination was <10 %.
Proliferation assays
Proliferation assays were performed as previously described.Citation50 Briefly, allogeneic PBMCs were used as responder cells. Triplicates of 105 PBMCs per well were cultured in RPMI 1640 medium supplemented with 4 mM L-Glutamine, 25 mM HEPES (Lonza, Verviers, Belgium) and 10–15 % heat-inactivated FCS (HyClone, Thermo Fisher Scientific, Utah, USA), further referred to as complete medium. 105 DC cultured with our standard cytokine cocktail with or without additional IFN-α or IFN-α plus IFN-γ were used as stimulator cells. After radiation with 30Gy (irradiation source: 137Cs, OB29, STS, Braunschweig, Germany), proliferative capacity of the responder cells was measured in a standard 4- day 3H-thymidine proliferation assay. During the last 16 h of the 4 d culture, 1 mCi/ml 3H-thymidine (Amersham Life Science, Buckingham, UK) was added. Cells were harvested and radioactivity measured in a scintillation counter. Maximum proliferation rate was determined by cultivation of 105 PBMCs in the presence of PHA. Minimum proliferation rate was determined by cultivation of PBMCs alone. Mean values were calculated.
Detection of cytokine secretion in the culture supernatant
The cytokine secretion in the culture supernatant was measured by Cytometric bead array (CBA) or ELISA. The cytokine pattern measured by the CBAs used in this study (Becton Dickinson, Franklin Lakes, NJ, USA) consisted of IFN-γ, TNF-α, IL-4, IL-6, IL-8, and IL-10. CBA were performed as previously described.Citation50 Briefly, 105 PBMCs were stimulated for 4 d with 105 xenogeneic 3T3 WT - or 3T3-CD40L-transfected cells. Culture supernatant was frozen at -20°C until cytokine analysis was performed according to the manufacturers instructions. IL-12p70 production in the cell culture supernatant was assessed by ELISA on a VersaMax microplate reader (Molecular Devices, Biberach, Germany) and analyzed by Softmax Pro software 6.2.2. All other cytokines were assessed by CBA and analyzed on a FACS Navios (Beckman Coulter, Pasadena, CA, USA) by FCAP-Software (Soft Flow Hungary Ltd., Pecs, Hungary).
Statistical analysis
Statistical analysis was done descriptively. The differences in parameters between two subsets (without IFN versus with IFN-γ and without IFN vs. with IFN-γ plus IFN-α) were calculated and the Wilcoxon test was applied in order to determine statistical significance. Differences with p values < 0.05 were considered to be statistically significant. Analyses were done by means of SPSS software (version 22).
Disclosure of potential conflicts of interest
The authors declare that they do not have any affiliations that would lead to a conflict of interest with respect to this work.
Acknowledgments
We thank I. Panzer and K. Neuhaus for their excellent technical assistance.
References
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med 1999; 340:1330-40; PMID:10219069; http://dx.doi.org/10.1056/NEJM199904293401706
- Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 1990; 247:1079-82; PMID:2408149; http://dx.doi.org/10.1126/science.2408149
- Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 1990; 247:824-30; PMID:2406902; http://dx.doi.org/10.1126/science.2406902
- Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006; 355:2408-17; PMID:17151364; http://dx.doi.org/10.1056/NEJMoa062867
- Rosti G, Castagnetti F, Gugliotta G, Palandri F, Baccarani M. Second-generation BCR-ABL inhibitors for frontline treatment of chronic myeloid leukemia in chronic phase. Crit Rev Oncol Hematol 2012; 82:159-70; PMID:21565522; http://dx.doi.org/10.1016/j.critrevonc.2011.04.002
- Talpaz M, Hehlmann R, Quintas-Cardama A, Mercer J, Cortes J. Re-emergence of interferon-α in the treatment of chronic myeloid leukemia. Leukemia 2013; 27:803-12; PMID:23238589; http://dx.doi.org/10.1038/leu.2012.313
- Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med 2000; 6:1018-23; PMID:10973322; http://dx.doi.org/10.1038/79526
- Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995; 86:2041-50; PMID:7655033
- Oji Y, Oka Y, Nishida S, Tsuboi A, Kawakami M, Shirakata T, Takahashi K, Murao A, Nakajima H, Narita M, et al. WT1 peptide vaccine induces reduction in minimal residual disease in an Imatinib-treated CML patient. Eur J Haematol 2010; 85:358-60; PMID:20633041; http://dx.doi.org/10.1111/j.1600-0609.2010.01497.x
- Bocchia M, Korontsvit T, Xu Q, Mackinnon S, Yang SY, Sette A, Scheinberg DA. Specific human cellular immunity to bcr-abl oncogene-derived peptides. Blood 1996; 87:3587-92; PMID:8611681
- Pinilla-Ibarz J, Cathcart K, Korontsvit T, Soignet S, Bocchia M, Caggiano J, Lai L, Jimenez J, Kolitz J, Scheinberg DA. Vaccination of patients with chronic myelogenous leukemia with bcr-abl oncogene breakpoint fusion peptides generates specific immune responses. Blood 2000; 95:1781-7; PMID:10688838
- Cathcart K, Pinilla-Ibarz J, Korontsvit T, Schwartz J, Zakhaleva V, Papadopoulos EB, Scheinberg DA. A multivalent bcr-abl fusion peptide vaccination trial in patients with chronic myeloid leukemia. Blood 2004; 103:1037-42; PMID:14504104; http://dx.doi.org/10.1182/blood-2003-03-0954
- Rojas JM, Knight K, Wang L, Clark RE. Clinical evaluation of BCR-ABL peptide immunisation in chronic myeloid leukaemia: results of the EPIC study. Leukemia 2007; 21:2287-95; PMID:17637811; http://dx.doi.org/10.1038/sj.leu.2404858
- Rezvani K. PR1 vaccination in myeloid malignancies. Expert Rev Vaccines 2008; 7:867-75; PMID:18767937; http://dx.doi.org/10.1586/14760584.7.7.867
- Westermann J, Kopp J, van Lessen A, Hecker AC, Baskaynak G, le Coutre P, Dohner K, Dohner H, Dorken B, Pezzutto A. Vaccination with autologous non-irradiated dendritic cells in patients with bcr/abl+ chronic myeloid leukaemia. Br J Haematol 2007; 137:297-306; PMID:17408402; http://dx.doi.org/10.1111/j.1365-2141.2007.06547.x
- Westermann J, Korner IJ, Kopp J, Kurz S, Zenke M, Dorken B, Pezzutto A. Cryopreservation of mature monocyte-derived human dendritic cells for vaccination: influence on phenotype and functional properties. Cancer Immunol Immunother 2003; 52:194-8; PMID:12649749
- Westermann J, Lessen A, Schlimper C, Baskaynak G, le Coutre P, Dorken B, Pezzutto A. Simultaneous cytokine analysis by cytometric bead array for the detection of leukaemia-reactive T cells in patients with chronic myeloid leukaemia. Br J Haematol 2006; 132:32-5; PMID:16371017; http://dx.doi.org/10.1111/j.1365-2141.2005.05844.x
- Westermann J, Schlimper C, Richter G, Mohm J, Dorken B, Pezzutto A. T cell recognition of bcr/abl in healthy donors and in patients with chronic myeloid leukaemia. Br J Haematol 2004; 125:213-6; PMID:15059144; http://dx.doi.org/10.1111/j.1365-2141.2004.04873.x
- Bocchia M, Defina M, Aprile L, Ippoliti M, Crupi R, Rondoni M, Gozzetti A, Lauria F. Complete molecular response in CML after p210 BCR-ABL1-derived peptide vaccination. Nat Rev Clin Oncol 2010; 7:600-3; PMID:20808301; http://dx.doi.org/10.1038/nrclinonc.2010.141
- Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood 2009; 114:1528-36; PMID:19420358; http://dx.doi.org/10.1182/blood-2008-09-179697
- Aurelius J, Martner A, Riise RE, Romero AI, Palmqvist L, Brune M, Hellstrand K, Thoren FB. Chronic myeloid leukemic cells trigger poly(ADP-ribose) polymerase-dependent inactivation and cell death in lymphocytes. J Leukoc Biol 2013; 93:155-60; PMID:23072905; http://dx.doi.org/10.1189/jlb.0512257
- Miyazono K. Tumour promoting functions of TGF-β in CML-initiating cells. J Biochem 2012; 152:383-5; PMID:22989931; http://dx.doi.org/10.1093/jb/mvs106
- Christiansson L, Soderlund S, Svensson E, Mustjoki S, Bengtsson M, Simonsson B, Olsson-Stromberg U, Loskog AS. Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in Sokal high risk chronic myeloid leukemia. PLoS One 2013; 8:e55818; PMID:23383287; http://dx.doi.org/10.1371/journal.pone.0055818
- Mumprecht S, Claus C, Schurch C, Pavelic V, Matter MS, Ochsenbein AF. Defective homing and impaired induction of cytotoxic T cells by BCR/ABL-expressing dendritic cells. Blood 2009; 113:4681-9; PMID:19252140; http://dx.doi.org/10.1182/blood-2008-05-156471
- Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med 1990; 172:631-40; PMID:2373994; http://dx.doi.org/10.1084/jem.172.2.631
- Westermann J, Kopp J, Korner I, Richter G, Qin Z, Blankenstein T, Dorken B, Pezzutto A. Bcr/abl+ autologous dendritic cells for vaccination in chronic myeloid leukemia. Bone Marrow Transplant 2000; 25 Suppl 2:S46-9; PMID:10933188; http://dx.doi.org/10.1038/sj.bmt.1702354
- Ossenkoppele GJ, Stam AG, Westers TM, de Gruijl TD, Janssen JJ, van de Loosdrecht AA, Scheper RJ. Vaccination of chronic myeloid leukemia patients with autologous in vitro cultured leukemic dendritic cells. Leukemia 2003; 17:1424-6; PMID:12835739; http://dx.doi.org/10.1038/sj.leu.2402979
- Takahashi T, Tanaka Y, Nieda M, Azuma T, Chiba S, Juji T, Shibata Y, Hirai H. Dendritic cell vaccination for patients with chronic myelogenous leukemia. Leuk Res 2003; 27:795-802; PMID:12804637; http://dx.doi.org/10.1016/S0145-2126(03)00011-0
- Litzow MR, Dietz AB, Bulur PA, Butler GW, Gastineau DA, Hoering A, Fink SR, Letendre L, Padley DJ, Paternoster SF, et al. Testing the safety of clinical-grade mature autologous myeloid DC in a phase I clinical immunotherapy trial of CML. Cytotherapy 2006; 8:290-8; PMID:16793737; http://dx.doi.org/10.1080/14653240600735743
- Li Y, Lin C, Schmidt CA. New insights into antigen specific immunotherapy for chronic myeloid leukemia. Cancer Cell Int 2012; 12:52; PMID:23241263; http://dx.doi.org/10.1186/1475-2867-12-52
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265-77; PMID:22437871; http://dx.doi.org/10.1038/nrc3258
- Zobywalski A, Javorovic M, Frankenberger B, Pohla H, Kremmer E, Bigalke I, Schendel DJ. Generation of clinical grade dendritic cells with capacity to produce biologically active IL-12p70. J Transl Med 2007; 5:18; PMID:17430585; http://dx.doi.org/10.1186/1479-5876-5-18
- Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol 2000; 164:4507-12; PMID:10779751; http://dx.doi.org/10.4049/jimmunol.164.9.4507
- Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. α-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res 2004; 64:5934-7; PMID:15342370; http://dx.doi.org/10.1158/0008-5472.CAN-04-1261
- Hilkens CM, Kalinski P, de Boer M, Kapsenberg ML. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood 1997; 90:1920-6; PMID:9292525
- Kalinski P, Schuitemaker JH, Hilkens CM, Wierenga EA, Kapsenberg ML. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-gamma and to bacterial IL-12 inducers: decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J Immunol 1999; 162:3231-6; PMID:10092774
- Kalinski P, Edington H, Zeh HJ, Okada H, Butterfield LH, Kirkwood JM, Bartlett DL. Dendritic cells in cancer immunotherapy: vaccines or autologous transplants? Immunol Res 2011; 50:235-47; PMID:21717071; http://dx.doi.org/10.1007/s12026-011-8224-z
- Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med 1996; 184:747-52; PMID:8760829; http://dx.doi.org/10.1084/jem.184.2.747
- Zaccard CR, Watkins SC, Kalinski P, Fecek RJ, Yates AL, Salter RD, Ayyavoo V, Rinaldo CR, Mailliard RB. CD40L induces functional tunneling nanotube networks exclusively in dendritic cells programmed by mediators of type 1 immunity. J Immunol 2015; 194:1047-56; PMID:25548234; http://dx.doi.org/10.4049/jimmunol.1401832
- Kalinski P. Dendritic cells in immunotherapy of established cancer: Roles of signals 1, 2, 3 and 4. Curr Opin Investig Drugs 2009; 10:526-35; PMID:19513941
- Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 2003; 8:237-49; PMID:12766484; http://dx.doi.org/10.1023/A:1023668705040
- Selleri C, Sato T, Del Vecchio L, Luciano L, Barrett AJ, Rotoli B, Young NS, Maciejewski JP. Involvement of Fas-mediated apoptosis in the inhibitory effects of interferon-α in chronic myelogenous leukemia. Blood 1997; 89:957-64; PMID:9028327
- Bhatia R, Verfaillie CM. The effect of interferon-α on β-1 integrin mediated adhesion and growth regulation in chronic myelogenous leukemia. Leuk Lymphoma 1998; 28:241-54; PMID:9517496
- Kantarjian HM, O'Brien S, Cortes JE, Shan J, Giles FJ, Rios MB, Faderl SH, Wierda WG, Ferrajoli A, Verstovsek S, et al. Complete cytogenetic and molecular responses to interferon-α-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer 2003; 97:1033-41; PMID:12569603; http://dx.doi.org/10.1002/cncr.11223
- Krusch M, Salih HR. Effects of BCR-ABL inhibitors on anti-tumor immunity. Curr Med Chem 2011; 18:5174-84; PMID:22087818; http://dx.doi.org/10.2174/092986711798184271
- Bocchia M, Gentili S, Abruzzese E, Fanelli A, Iuliano F, Tabilio A, Amabile M, Forconi F, Gozzetti A, Raspadori D, et al. Effect of a p210 multipeptide vaccine associated with imatinib or interferon in patients with chronic myeloid leukaemia and persistent residual disease: a multicentre observational trial. Lancet 2005; 365:657-62; PMID:15721470; http://dx.doi.org/10.1016/S0140-6736(05)70931-4
- Eibl B, Ebner S, Duba C, Bock G, Romani N, Erdel M, Gachter A, Niederwieser D, Schuler G. Dendritic cells generated from blood precursors of chronic myelogenous leukemia patients carry the Philadelphia translocation and can induce a CML-specific primary cytotoxic T-cell response. Genes Chromosomes Cancer 1997; 20:215-23; PMID:9365828; http://dx.doi.org/10.1002/(SICI)1098-2264(199711)20:3%3c215::AID-GCC1%3e3.0.CO;2-5
- Choudhury A, Gajewski JL, Liang JC, Popat U, Claxton DF, Kliche KO, Andreeff M, Champlin RE. Use of leukemic dendritic cells for the generation of antileukemic cellular cytotoxicity against Philadelphia chromosome-positive chronic myelogenous leukemia. Blood 1997; 89:1133-42; PMID:9028934
- Tong XM, Yao HP, Qian WB, Zhu LF, Fu ZH, Huang ZL, Jin J. The biological characteristics of dendritic cells derived in vitro from myelogeneous leukemia cells and healthy donor cells. Int J Lab Hematol 2008; 30:372-81; PMID:18205840; http://dx.doi.org/10.1111/j.1751-553X.2007.00986.x
- Westermann J, Florcken A, Willimsky G, van Lessen A, Kopp J, Takvorian A, Johrens K, Lukowsky A, Schonemann C, Sawitzki B, et al. Allogeneic gene-modified tumor cells (RCC-26/IL-7/CD80) as a vaccine in patients with metastatic renal cell cancer: a clinical phase-I study. Gene Ther 2011; 18:354-63; PMID:21068778; http://dx.doi.org/10.1038/gt.2010.143
