ABSTRACT
Varicella zoster virus (VZV) is primarily known for causing varicella in childhood, but can reactivate again as herpes zoster (HZ) after a period of latency, mainly in persons older than 50 years. Universal varicella vaccination was introduced in Germany in 2004, while HZ vaccination has not been recommended yet. We aimed to quantify the potential long-term effects of universal childhood varicella vaccination and HZ vaccination of the elderly on varicella and HZ incidence in Germany over a time horizon of 100 years, using a transmission model calibrated to pre-vaccination data and validated against early post-vaccination data. Using current vaccination coverage rates of 87% (64%) with one (two) varicella vaccine dose(s), the model predicts a decrease in varicella cases by 89% for the year 2015. In the long run, the incidence reduction will stabilize at about 70%. Under the assumption of the boosting hypothesis of improved HZ protection caused by exposure to VZV, the model predicts a temporary increase in HZ incidence of up to 20% for around 50 years. HZ vaccination of the elderly with an assumed coverage of 20% has only limited effects in counteracting this temporary increase in HZ incidence. However, HZ incidence is shown to decrease in the long-term by 58% as vaccinated individuals get older and finally reach age-classes with originally high HZ incidence. Despite substantial uncertainties around several key variables, the model's results provide valuable insights that support decision-making regarding national VZV vaccination strategies.
Introduction
Varicella zoster virus (VZV) is the causative agent of varicella and, following reactivation after a period of latency, also of herpes zoster (HZ). Varicella occurs primarily in children; however, complications such as varicella-associated pneumonia are considerably more frequent in adults.Citation1 Herpes zoster occurs mostly in older adults and leads, as a main complication, in 5–30% to post-herpetic neuralgia, a long-lasting and debilitating painful syndrome.Citation2
In Germany, universal varicella vaccination of children aged 11 to 14 months using a one-dose schedule was endorsed by the Standing Committee on Vaccination (STIKO) in 2004.Citation3 This recommendation was extended in 2009 to a second vaccine dose to be administered at the age of 15 to 23 months. A first vaccine against HZ has been licensed in 2006, but was not available on the European market until 2013.Citation4
A strong decline in varicella cases and varicella-associated hospitalizations was observed in Germany and other countries with universal childhood varicella vaccination soon after the introduction of the program.Citation5,6 However, the long-term population-level impact of the program, especially on HZ, remains uncertain. The boosting hypothesis suggests that repeated exogenous exposure to circulating VZV decreases an individual's risk of developing HZ through subclinical boosters that counteract the waning of VZV immunity.Citation7 Under these conditions, the introduction of universal varicella vaccination in a population (and the successive decrease in VZV circulation) might subsequently result in an increase of HZ incidence.Citation8,9 In addition, as some unvaccinated children may escape the disease in early childhood due to decreased virus circulation, an age-shift of varicella toward older age-groups seems possible. This is of particular concern, since the frequency of varicella-associated complications increases with age.Citation1 Both potential long-term effects – the possible HZ incidence increase and the age shift of varicella cases – were therefore addressed in several studies, all using the approach of mathematical modeling since these effects are expected to occur decades after initiation of routine universal varicella vaccination.Citation10-15
We took advantage of the ongoing universal childhood varicella vaccination in Germany and were able to use already existing data on the short-term effects of vaccination for model validation. We created a dynamic mathematical model that predicts potential long-term effects of varicella and/or HZ vaccination in Germany. Our objective was to assess incidence, number of hospitalizations and deaths due to varicella and HZ over a period of 100 years after starting universal varicella vaccination by modeling the complex interactions between vaccination and both diseases, including the potential impact of an additional routine HZ immunization among the elderly. Besides a base case scenario, we performed various sensitivity analyses to identify crucial factors that impact the future incidence of varicella and HZ.
Results
Base case analysis
Effects of varicella vaccination
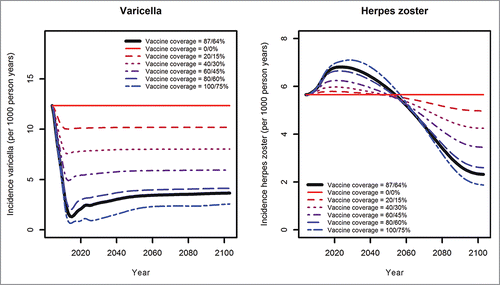
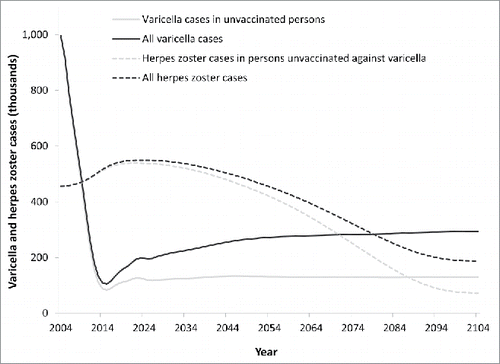
According to our model, varicella incidence will be reduced by about 90% within 10 years after initiation of universal varicella vaccination given the current nationwide vaccination coverage (first dose: 87%; second dose: 64%). However, the number of varicella cases will increase again after the maximum decrease and stabilize at about one third of the pre-vaccination era due to the rise of breakthrough cases (i.e. wild virus varicella infections occurring in vaccinated individuals due to incomplete or waned protection) (). HZ incidence is predicted to increase temporarily by up to 20% as compared to the pre-vaccination era for around 50 years, and then decrease by 58% in the long-term. Nearly 40% of all HZ cases after 100 years of vaccination will occur in individuals not vaccinated against varicella.
Figure 1. Varicella and herpes zoster cases over time in model population (see methods; no HZ vaccination).
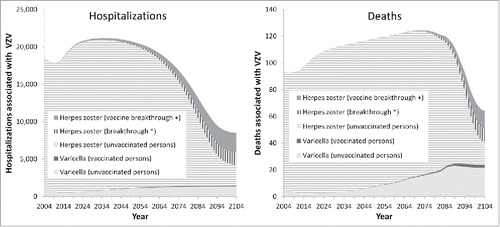
Similarly to varicella cases, varicella-associated hospitalizations and deaths will rapidly decrease after introduction of vaccination to a minimum in 2014 (−87% and −76%, respectively, compared to time before vaccination) (). However, after 100 years, the annual number of hospitalizations will have decreased by 29% compared to its level before start of the vaccination program, whereas the number of varicella-attributable deaths is predicted to increase from 5 to 24 per year (). When considering the boosting hypothesis, HZ-associated hospitalizations and deaths will increase in the short-term to a similar extent as HZ cases (by 24% and 25%, respectively, compared to time before vaccination). However, in the long-term, HZ-associated hospitalizations and deaths will decrease dramatically (by 58% and 56%, respectively) (). As hospitalizations and deaths due to HZ exceed by far those associated with varicella, the total number of hospitalizations and deaths due to both diseases combined will temporarily increase by about 20% and 33%, and will decrease by 54% and 31% in the long term, respectively. As HZ-associated burden of disease affects mainly the elderly, positive effects of universal childhood varicella vaccination on the number of HZ-related hospitalizations and deaths occur with a time delay of a life time (). Beyond the displayed 100 years, only very minor changes are predicted by the model: varicella associated hospitalizations still increase by about 3% and deaths by 7%, whereas HZ associated hospitalizations decrease by about 0.3% and deaths by 0.8%.
Figure 2. Hospitalizations and deaths associated with varicella zoster virus over time. + vaccine breakthrough = reactivation of vaccine virus after varicella vaccination. *breakthrough = reactivation of wild virus after varicella infection in vaccinated individuals.
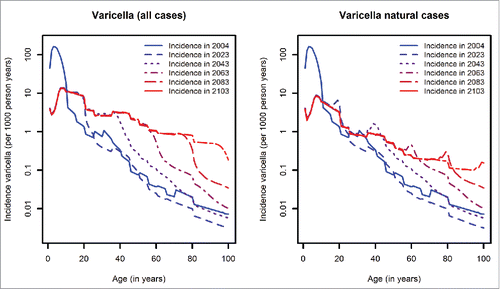
The effects of varicella vaccination on varicella and HZ will, however, differ considerably across age groups. There will be a strong decline in varicella cases in children younger than 10 years (in which age-group about 95% of all cases occurred before the vaccination era) (). In individuals older than 10 years, our model predicts an increase in varicella incidence in the long-term. This increase in teenagers and younger adults is mainly due to breakthrough varicella cases, while in individuals older than 40 years, the number of varicella cases in unvaccinated individuals will increase (). This causes an age-shift of varicella, which will be first noticeable 30 years after the introduction of varicella vaccination. In birth-cohorts following the introduction of vaccination, around 50% of the HZ cases among individuals older than 20 years will occur in the those individuals who received vaccination (at least one dose; 86.9% of the population).
Effects of HZ vaccination
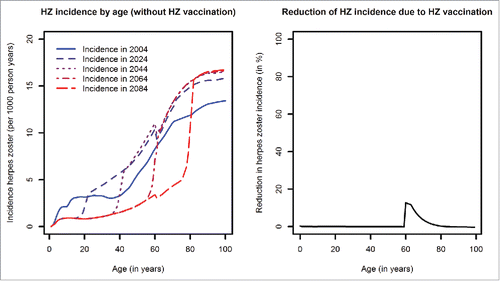
In our model, the addition of HZ vaccination in the elderly with an assumed coverage of 20% has very limited effects on HZ incidence. Moreover, this effect is only visible in a small age band closely following the age of vaccination (60 years) (). The temporary increase in HZ incidence in all age-groups and the dramatic decline in HZ incidence in birth-cohorts vaccinated against varicella will mark the epidemiology of HZ after introduction of universal childhood varicella vaccination, both in the presence and absence of a HZ vaccination program.
Figure 4. Left panel: Age-dependent HZ incidence at different time points (without HZ vaccination, introduction of varicella vaccination in 2004); Right panel: Relative reduction of HZ incidence by age predicted for 2103 when comparing scenarios with and without HZ vaccination (introduction of HZ vaccination in 2015, coverage 20%).
Sensitivity and scenario analyses
We performed various sensitivity and scenario analyses to complement the results of the base case analysis and to test their robustness.
Effects of varicella vaccination coverage
Scenario analyses were performed for different varicella vaccination coverage rates (ranging from 0 to 100% for the first and from 0 to 75% for the second dose) (). The higher the varicella vaccination coverage, the higher is the expected reduction in the number of varicella cases. With higher varicella vaccination coverage, the temporary increase in HZ incidence will be higher as will be the long-term reduction of HZ incidence when varicella-vaccinated cohorts enter age-classes with originally high HZ incidence. Independently from the level of varicella vaccination coverage, positive effects of varicella vaccination on HZ begin to compensate negative effects about 50 years after initiation of universal varicella vaccination ().
Effects of boosting and waning parameters
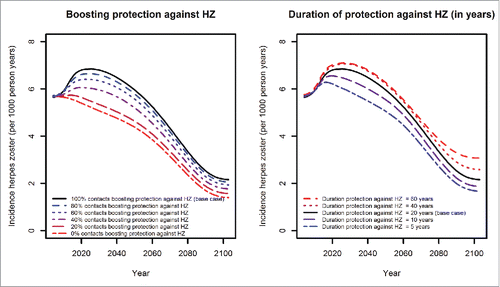
If not every exposure of a vaccinated individual to an infectious individual results in boosting of varicella-specific immunity in the vaccinated individual or if the protection provided by varicella vaccination is assumed to be short (on average 10 years for one dose and 20 years for 2 doses), the number of breakthrough varicella cases will increase compared to the base case analysis ().
Figure 6. Effects of different boosting (left) and waning scenarios (right, duration in years) on varicella incidence.
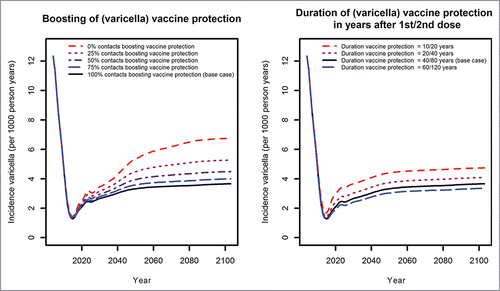
If only a small proportion of contacts to varicella patients boost protection against HZ or if the duration of protection acquired by boosting is considerably shorter than 20 years, there will be no or only a small temporary increase in HZ incidence ().
Effects of age at HZ vaccination
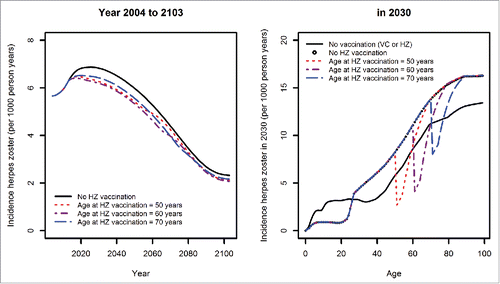
Even with vaccine coverage of 100% in the target age group, HZ vaccination with the currently available vaccine cannot completely prevent the temporal increase in HZ incidence (, left panel). The age of vaccination of 60 years seems to be most effective in reducing HZ incidence rates. Independently of the chosen age of vaccination, immunity induced by HZ vaccination lasts only for a relatively short period of time (, right panel).Citation16,17
Discussion
In our study, we provide predictions for short- and long-term effects of universal childhood varicella vaccination, with and without taking into account HZ vaccination for the elderly with the currently available vaccine. Our model is the first that uses for model validation data collected after the introduction of universal childhood varicella vaccination in Germany in 2004. The model correctly reflects the short-term effects (i.e. considerable reduction of varicella incidence among children younger than 10 years of age) as they were documented in the established sentinel surveillance system.Citation5,6
Under base case assumptions, we found a short-term reduction of 90% and a long-term reduction of 70% for varicella cases. Moreover, for HZ cases a temporary increase of 20% and a long-term reduction of 60% were predicted. These results are in line with previous studies suggesting similar effects in mathematical models calibrated for other countries.Citation10,15 We incorporated information on case severity and showed that HZ hospitalizations and deaths will show a temporal increase of about 25%, but a long-term reduction of more than 50% when compared to the pre-vaccination era. In contrast, the predicted changes in incidence of hospitalization and mortality of varicella due to an age-shift in varicella epidemiology are more complex. As hospitalization probability and case fatality increase substantially with age, the predicted age-shift could compromise the benefits of varicella vaccination. The majority of predicted varicella cases in older age-groups are breakthrough cases with unknown (but most likely milder) severity. However, infections in unprotected older (>40 years) individuals will increase as well. As varicella case fatality increases much stronger with age compared to varicella hospitalization probability, this would lead to an overall reduction of hospitalizations (by 29%) but to an increase in varicella associated deaths. However, the estimated number of 24 deaths per year due to varicella should be interpreted with caution. Deaths associated with varicella occur mostly in persons of older age and with severe pre-existing comorbidities; the cause of death statistics may not always be able to differentiate whether they died because of varicella, underlying primary, or following secondary diseases.
In our sensitivity analyses, we showed that predictions regarding natural varicella cases are robust across the studied boosting scenarios; in contrast, the estimated number of breakthrough cases was considerably affected by parameter variation especially regarding adults. If protection induced by varicella vaccination will not be boosted to lifelong duration by contact with varicella cases, varicella breakthrough cases will more than double when compared to the base case analysis, exceeding the number of natural cases and shifting the average age from children to adults. Breakthrough cases in adults will, however, only be of major concern, if the proposed relative lower case severity of breakthrough cases (as observed in children) does not apply for adults.
A major factor of uncertainty in the estimation of future HZ incidence (with respect to both, the temporary increase as well as the subsequent decline) is the assumed extent of boosting effects. The temporary increase of HZ cases observed in the base case analysis will be much smaller (or will even disappear) under the assumption that less than 50% of contacts with infectious individuals boost protection against HZ; the same is true if the acquired duration of protection is assumed to be rather short. Ogunjimi et al.Citation18 estimated the duration in their model based on individual immune response after HZ vaccination of boosting to be very short (1 to 2 years). In models without cumulative boosting events (like our model or the one used by Brisson et al.Citation10) this would lead to negligible effects of boosting. Due to the way multiple boosting events are modeled in Ogunjimi et al., HZ incidence still increased about 75% after the introduction of varicella vaccination in the best fitting model. Even more complex are the effects of different mixing matrixes. Apart from being low, the contact frequency in the POLYMOD studyCitation19 was also more homogenously distributed across age in Germany than in all other included countries. As the model is fitted to German sero-prevalence data, which are very similar to sero-prevalence data in other countries, the calibrated transmission probability for varicella is rather high. Since in our model only infectious contacts can contribute to exogenous boosting, individuals are boosted (when compared to other countries in the POLYMOD study) less frequently when they are teenagers and young adults, but more frequently when they are middle aged or elderly. Overall, this leads in the majority of cases to stronger predicted effects of boosting for Germany, at least when the focus is on the most recent event as in our model or in Brisson et al. or van Hoek et al.Citation10,15 In contrast, in models where multiple boosting is more important than one single recent boosting event,Citation18,20,21 the effects of boosting would be smaller in Germany compared to other countries. Up to now, there is no clear evidence of an increase of HZ incidence in Germany. The only available analysis of HZ incidence following introduction of varicella vaccination reported a small increase from 2005 to 2008 (standardized HZ incidence of 5.76, 5.68, 5.78, 5.92 per 1000 person years in 2005, 2006, 2007 and 2008, respectively).Citation22 This increase was not statistically significant and possibly attributable to other explanations rather than to effects of reduced boosting. While the US has a much longer history of varicella vaccination, the findings are inconsistent, with some studies reporting an increase in HZ incidence whereas others do not (review in Ogunjimi et al.Citation9). A modeling study investigating different mixing matrices in European countries suggested that the extent of increase in HZ incidence might differ across countries.Citation23
The extent of the predicted long-term decrease caused by the reduced HZ incidence in varicella vaccinated individuals is less clear. To date, there is only evidence for this effect in children,Citation24-29 whereas no data are available for adults. In addition, the observed protective effect of varicella vaccination in children varied considerably in observational studies ranging from 39% to 94%.Citation25,26,29 The overall effect of HZ vaccination on HZ incidence in our model is relatively small. On the one hand, the currently available HZ vaccine suffers from a lower vaccine efficacy when compared e.g. to varicella vaccine; on the other hand, protection induced by HZ vaccination is of shorter duration (7 to 12 years).Citation17,30-32 Moreover, vaccination modalities like the assumed vaccination coverage of 20% and the conservative way of modeling interactions of HZ vaccination and boosting of protection are also reducing its effects. However, even with very high vaccination coverage, HZ vaccination cannot completely compensate the potential temporary negative effect of varicella vaccination on HZ incidence under a wide range of assumptions.
Limitations
It needs to be pointed out that many of the parameters regarding varicella or HZ vaccination are unknown or at least not known in detail. For example, while there are 2 studies addressing short-term effectiveness of 2-dose varicella vaccination over 2–3 years,Citation33,34 there is only one study that focuses on long-term effectiveness,Citation35 and this study suffers from severe limitations, and vaccine effectiveness for one dose estimated in this study (94.4%) is in conflict with results observed in varicella outbreaks in nearly completely vaccinated groups.Citation36,37 In addition, there is only limited evidence available for many parameters regarding the boosting assumption for HZ and HZ vaccination. Neither the strong age-dependency observed in the registration trials, nor the short duration of HZ vaccine protection (as compared to varicella vaccination) are well understood. In particular, the dynamic interaction between varicella vaccination, natural boosting of vaccine induced protection, of HZ immunity, and HZ vaccination are still poorly understood. Moreover, some important assumptions of the model such as the milder course of varicella breakthrough cases or reduced HZ incidence in varicella vaccinated persons are based on observations in children, but for simplicity were assumed to be also true for adults.
Due to the complexity of VZV pathogenesis and immunobiology, analyses of the total effect of varicella vaccination on the VZV disease burden are complex. In contrast to most other vaccinations, the success of a routine varicella vaccination strategy does not only depend on vaccination effects on the primary outcome (in our case varicella incidence), but also on the effects on HZ incidence. As the HZ disease burden in terms of hospitalizations and deaths is much higher than that of varicella, the short- and long-term effects of varicella vaccination on HZ epidemiology play an important role in the evaluation of any varicella vaccination strategy. These effects are, however, unclear and highly variable in our model given the uncertainties regarding boosting of HZ protection and HZ incidence in individuals vaccinated against varicella. The evaluation is even further complicated by the “total effect on HZ” depending strongly on the time horizon, even given the same structure of the model.Citation17 In that regard, potential changes in demography and contact behavior are a primary source of uncertainty when making such long-term predictions.Citation38,39 While a stable population model facilitates comparison of outcomes across time, burden of disease can be different in the real population. Therefore, there is a trade-off between using a stable population model despite the reality of demographic changes and the uncertainty of predictions for the long-term development of the real population.
A new adjuvanted HZ vaccine that is currently still under development has demonstrated an efficacy of 97% in a phase 3 trial, i.e. considerably higher than the upper bound estimate we considered in the sensitivity analyses.Citation40 Therefore, conclusions drawn from our model do not necessarily apply to this new vaccine.
Conclusion
We observed potential positive and negative effects of VZV vaccination in our simulation study. The potential long-term negative effects, i.e. the age-shift of varicella disease toward older age-groups and the temporary increase in HZ incidence, are a result of rather conservative assumptions regarding highly uncertain aspects such as the boosting hypothesis, the duration of vaccine-induced varicella protection, and the degree of severity among corresponding breakthrough infections. But even if these assumptions hold true, over a long time horizon (i.e. more than 50 years) negative effects will be more than compensated by positive effects, namely the decrease in HZ incidence and associated hospitalizations and deaths. The potential negative effects 10 to 40 years after the introduction of universal childhood varicella vaccination can be only partially compensated by the introduction of HZ vaccination of the elderly with the currently available vaccine.
In our view, the results of this study have 3 major implications: (i) a sustained surveillance is necessary for monitoring changes in varicella and HZ epidemiology in countries that have introduced universal childhood varicella vaccination; (ii) the model should be updated once new evidence is available on e.g., the boosting dynamics, waning of vaccine-induced protection, or other factors with high impact such as the product profile of an improved HZ vaccine; (iii) VZV transmission models such as ours provide additional insights into the potential impact of both varicella and HZ vaccination. Due to the substantial uncertainty around several key variables, the model's results need to be interpreted with caution; if additional knowledge becomes available, it needs to be considered for decision-making regarding VZV vaccines.
Materials and methods
Model structure
A dynamic transmission model was developed with a model structure similar to the one proposed by van Hoek et al.Citation15 The model is described in detail in the appendix.
Calibration of the model
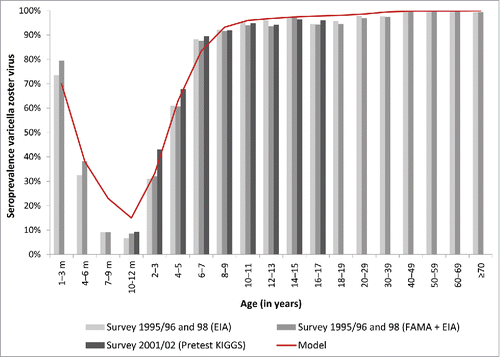
The model parameters “infectivity of varicella per contact,” and “age- and sex-specific probability to develop HZ” were calibrated to fit age-specific VZV seroprevalence data according to Wutzler et al.Citation41-43 and HZ incidence according to Ultsch et al.Citation22,44 Despite being after the introduction of varicella vaccination, we used data from Ultsch et al. 2011Citation44 (data from 2007/2008) and Ultsch et al. 2012Citation22 (data from 2005–2008) as calibration target. There is no significant difference compared to Schiffner-RoheCitation45 (data 2004), which has no data from persons under 50 years, uses larger (10 years) age-classes and does not differentiate between genders. Parameters were selected based on the lowest mean square error when fitted to the observed data. Calibration results for the sero-prevalence of varicella are shown in ().
Figure 9. Seroprevalence of varicella in Germany, by age.Citation41-43 EIA: Enzyme immunoassay (Enzygnost Anti-VZV-IgG (DADE Behring); FAMA: Fluorescent antibody to membrane antigen assay (FAMA); KiGGS: Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland.
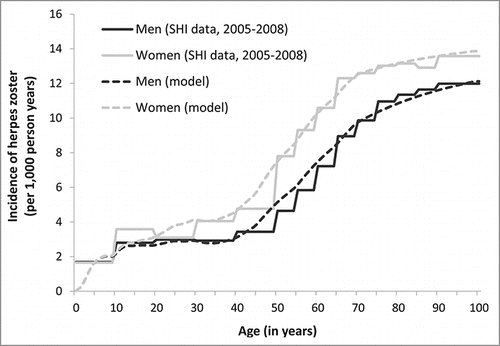
Calibration results regarding the age- and sex-specific incidence of HZ are shown in ().
Figure 10. Incidence of herpes zoster in Germany, by sex and age (reported dataCitation22,44 and model predictions; SHI = statutory health insurance).
Calculation of hospitalization probability and case fatality associated with varicella and HZ
Number of HZ cases is based on the HZ incidence estimated for Germany by Ultsch et al.Citation22,44 multiplied by the age-specific population according to the Federal Statistical Office in Germany.Citation46 As there is no data on age-specific incidence for varicella before the introduction of routine varicella vaccination in Germany, incidence of varicella was estimated by the model fitted to seroprevalence data for Germany.Citation41-43 The numbers of hospitalized cases for HZ and varicella are derived from the German hospital statistics representing the main diagnosis (ICD-10; varicella B01 (subcodes 0/1/2/8/9); herpes zoster B02 (subcodes 0/1/2/3/7/8/9)) of all hospitalized cases in Germany from 2000 to 2011. Hospitalization probability and case fatality were estimated by dividing the age-specific number of hospitalized cases or cases of death by all cases. While there is clear evidence that varicella vaccination affects the number of hospitalized cases in individuals under 10 years (short-term effect)Citation46 , an effect in older age groups is so far not visible. Therefore, varicella estimates for individuals younger than 10 years are based on data from the pre-vaccination era (2000 to 2004) while estimates for older individuals are based on all available data (2000 to 2011).
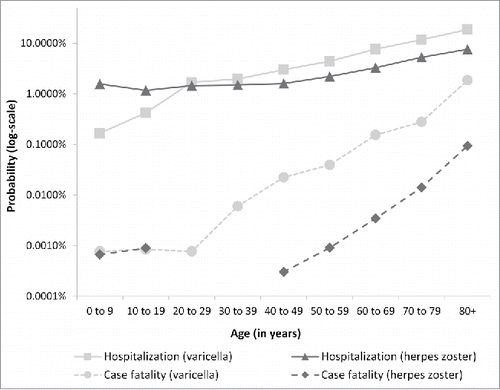
The annual number of deaths associated with varicella and HZ was derived from the official causes of death statistics for Germany. Incidence of hospitalization for HZ increased during the entire study period (2000 to 2011) in each age group except for children 0 to 4 years. However, mortality of HZ did not increase during the period according to cause of death statistics. For simplicity, consistency, and to get stable case fatality estimates, we used data from the entire time period 2000 to 2011 for HZ estimates. Estimated probability of hospitalization and case fatality rate by age are shown in ().
Modeling of varicella vaccine uptake
For the modeling of vaccination uptake, information on vaccination coverage at the age of 24 months for the birth cohorts 2004 to 2009 was used (; left panel). In Germany, the recommended age for vaccination is 11–14 months for the first dose and 15–24 months for the second dose. For simplicity, age at vaccination was set in the model to 12 months for the first and 24 months for the second dose. For example, vaccination coverage at 24 months for the 2005 birth cohort (66.4% /12.5%) is referring to first dose vaccination uptake in 2006 (when the children were 1 year old) and second dose vaccination uptake in 2007 (when the children were 2 years old). In addition, vaccination uptake for the first dose in 2004 was assumed to be half of the one in 2005 and for the second dose was set to zero for the years 2004 and 2005. Future vaccination uptake was assumed to continue at the level of 2011 (; right panel).
Figure 12. Varicella vaccination coverage (at the age of 24 months) as obtained from surveillance data for Germany (left) and vaccine uptake as used in the model (right).
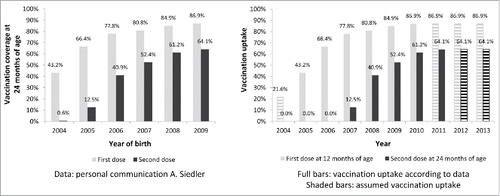
Regional vaccination coverage differed greatly for the birth years 2004 and 2005Citation47 due to different time points of implementation of the recommendation in the federal states of Germany. However, there are no big differences in later birth cohorts.Citation48 For simplification, homogenous coverage was assumed over the whole time period.
HZ Vaccination
HZ vaccination is implemented in the base case analysis at the age of 60 years with a vaccine efficacy of 64% as reported in the registration trial for the age group 60 to 69 years.Citation49 Average duration of vaccine-induced protection is assumed to be 12 years.Citation31,32 Vaccination coverage is assumed to be 20%, which corresponds to the observed pneumococcal vaccination coverage in Germany targeting a similar age group.Citation31
Model parameters
Model parameters and corresponding values used for the base case scenario as well as for the sensitivity analyses are displayed in .
Table 1. Parameter values used in this study (base case scenario and sensitivity analyses.
Disclosure of potential conflicts of interest
W. Greiner and O. Damm worked on projects sponsored by Sanofi Pasteur MSD and GlaxoSmithKline. H. Hengel is member of the German Standing Committee on Vaccination (STIKO). All other authors declare no conflict of interests.
Supplementary files
Download Zip (810.4 KB)References
- Fairley CK, Miller E. Varicella-zoster virus epidemiology–a changing scene? J Infect Dis 1996; 174 Suppl 3:S314-9; PMID:8896538; http://dx.doi.org/10.1093/infdis/174.Supplement_3.S314
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ open 2014; 4:e004833; PMID:24916088; http://dx.doi.org/10.1136/bmjopen-2014-004833
- Begründung der STIKO für eine allgemeine Varizellenimpfung. Epidemiologisches Bulletin 2004; 49:421.
- Dworkin RH, Portenoy RK. Pain and its persistence in herpes zoster. Pain 1996; 67:241-51; PMID:8951917; http://dx.doi.org/10.1016/0304-3959(96)03122-3
- Streng A, Grote V, Carr D, Hagemann C, Liese JG. Varicella routine vaccination and the effects on varicella epidemiology - results from the Bavarian Varicella Surveillance Project (BaVariPro), 2006–2011. BMC Infect Dis 2013; 13:303; PMID:23815523; http://dx.doi.org/10.1186/1471-2334-13-303
- Siedler A, Arndt U. Impact of the routine varicella vaccination programme on varicella epidemiology in Germany. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 2010; 15(13):pii=19530
- Hope-Simpson RE. The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc Royal Soc Med 1965; 58:9-20; PMID:14267505
- Brisson M, Gay NJ, Edmunds WJ, Andrews NJ. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine 2002; 20:2500-7; PMID:12057605; http://dx.doi.org/10.1016/S0264-410X(02)00180-9
- Ogunjimi B, Van Damme P, Beutels P. Herpes Zoster Risk Reduction through Exposure to Chickenpox Patients: A Systematic Multidisciplinary Review. PloS one 2013; 8:e66485; PMID:23805224; http://dx.doi.org/10.1371/journal.pone.0066485
- Brisson M, Edmunds WJ, Gay NJ, Law B, De Serres G. Modelling the impact of immunization on the epidemiology of varicella zoster virus. Epidemiol Infect 2000; 125:651-69; PMID:11218215; http://dx.doi.org/10.1017/S0950268800004714
- Gidding HF, Brisson M, Macintyre CR, Burgess MA. Modelling the impact of vaccination on the epidemiology of varicella zoster virus in Australia. Aust N Z J Public Health 2005; 29:544-51; PMID:16366065; http://dx.doi.org/10.1111/j.1467-842X.2005.tb00248.x
- Schuette MC, Hethcote HW. Modeling the effects of varicella vaccination programs on the incidence of chickenpox and shingles. Bull Math Biol 1999; 61:1031-64; PMID:17879870; http://dx.doi.org/10.1006/bulm.1999.0126
- Coudeville L, Brunot A, Szucs TD, Dervaux B. The economic value of childhood varicella vaccination in France and Germany. Value Health 2005; 8:209-22; PMID:15877593; http://dx.doi.org/10.1111/j.1524-4733.2005.04005.x
- Brisson M, Melkonyan G, Drolet M, De Serres G, Thibeault R, De Wals P. Modeling the impact of one- and two-dose varicella vaccination on the epidemiology of varicella and zoster. Vaccine 2010; 28:3385-97; PMID:20199763; http://dx.doi.org/10.1016/j.vaccine.2010.02.079
- van Hoek AJ, Melegaro A, Zagheni E, Edmunds WJ, Gay N. Modelling the impact of a combined varicella and zoster vaccination programme on the epidemiology of varicella zoster virus in England. Vaccine 2011; 29:2411-20; PMID:21277405; http://dx.doi.org/10.1016/j.vaccine.2011.01.037
- van Hoek AJ, Melegaro A, Gay N, Bilcke J, Edmunds WJ. The cost-effectiveness of varicella and combined varicella and herpes zoster vaccination programmes in the United Kingdom. Vaccine 2012; 30:1225-34; PMID:22119592; http://dx.doi.org/10.1016/j.vaccine.2011.11.026
- Bilcke J, Ogunjimi B, Hulstaert F, Van Damme P, Hens N, Beutels P. Estimating the age-specific duration of herpes zoster vaccine protection: a matter of model choice? Vaccine 2012; 30:2795-800; PMID:21964056; http://dx.doi.org/10.1016/j.vaccine.2011.09.079
- Ogunjimi B, Willem L, Beutels P, Hens N. Integrating between-host transmission and within-host immunity to analyze the impact of varicella vaccination on zoster. eLife 2015; 4:e07116; PMID:26259874; http://dx.doi.org/10.7554/eLife.07116
- Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS medicine 2008; 5:e74; PMID:18366252; http://dx.doi.org/10.1371/journal.pmed.0050074
- Karhunen M, Leino T, Salo H, Davidkin I, Kilpi T, Auranen K. Modelling the impact of varicella vaccination on varicella and zoster. Epidemiol Infect 2010; 138:469-81; PMID:19796447; http://dx.doi.org/10.1017/S0950268809990768
- Guzzetta G, Poletti P, Del Fava E, Ajelli M, Scalia Tomba GP, Merler S, et al. Hope-Simpson's progressive immunity hypothesis as a possible explanation for herpes zoster incidence data. Am J Epidemiol 2013; 177:1134-42; PMID:23548754; http://dx.doi.org/10.1093/aje/kws370
- Ultsch B, Koster I, Reinhold T, Siedler A, Krause G, Icks A, et al. Epidemiology and cost of herpes zoster and postherpetic neuralgia in Germany. Eur J Health Econ 2012; 14(6):1015-26; PMID:23271349
- Poletti P, Melegaro A, Ajelli M, Del Fava E, Guzzetta G, Faustini L, et al. Perspectives on the impact of varicella immunization on herpes zoster. A model-based evaluation from three European countries. PloS one 2013; 8:e60732; PMID:23613740; http://dx.doi.org/10.1371/journal.pone.0060732
- Weinmann S, Chun C, Schmid DS, Roberts M, Vandermeer M, Riedlinger K, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J Infect Dis 2013; 208:1859-68; PMID:23922376; http://dx.doi.org/10.1093/infdis/jit405
- Baxter R, Ray P, Tran TN, Black S, Shinefield HR, Coplan PM, et al. Long-term effectiveness of varicella vaccine: a 14-Year, prospective cohort study. Pediatrics 2013; 131:e1389-96; PMID:23545380; http://dx.doi.org/10.1542/peds.2012-3303
- Hardy I, Gershon AA, Steinberg SP, LaRussa P. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N Engl J Med 1991; 325:1545-50; PMID:1658650; http://dx.doi.org/10.1056/NEJM199111283252204
- Stein M, Cohen R, Bromberg M, Tasher D, Shohat T, Somekh E. Herpes zoster in a partially vaccinated pediatric population in central Israel. Pediatric Infect Dis J 2012; 31:906-9; PMID:22627868; http://dx.doi.org/10.1097/INF.0b013e31825d33f9
- Goldman GS. Cost-benefit analysis of universal varicella vaccination in the U.S. taking into account the closely related herpes-zoster epidemiology. Vaccine 2005; 23:3349-55; PMID:15837242; http://dx.doi.org/10.1016/j.vaccine.2003.10.042
- Civen R, Chaves SS, Jumaan A, Wu H, Mascola L, Gargiullo P, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatric Infect Dis J 2009; 28:954-9; PMID:19536039; http://dx.doi.org/10.1097/INF.0b013e3181a90b16
- van Hoek AJ, Gay N, Melegaro A, Opstelten W, Edmunds WJ. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine 2009; 27:1454-67; PMID:19135492; http://dx.doi.org/10.1016/j.vaccine.2008.12.024
- Ultsch B, Weidemann F, Reinhold T, Siedler A, Krause G, Wichmann O. Health economic evaluation of vaccination strategies for the prevention of herpes zoster and postherpetic neuralgia in Germany. BMC Health Services Res 2013; 13:359; PMID:24070414; http://dx.doi.org/10.1186/1472-6963-13-359
- de Boer PT, Pouwels KB, Cox JM, Hak E, Wilschut JC, Postma MJ. Cost-effectiveness of vaccination of the elderly against herpes zoster in The Netherlands. Vaccine 2013; 31:1276-83; PMID:23306360; http://dx.doi.org/10.1016/j.vaccine.2012.12.067
- Prymula R, Bergsaker MR, Esposito S, Gothefors L, Man S, Snegova N, et al. Protection against varicella with two doses of combined measles-mumps-rubella-varicella vaccine versus one dose of monovalent varicella vaccine: a multicentre, observer-blind, randomised, controlled trial. Lancet 2014; 383:1313-24; PMID:24485548; http://dx.doi.org/10.1016/S0140-6736(12)61461-5
- Shapiro ED, Vazquez M, Esposito D, Holabird N, Steinberg SP, Dziura J, et al. Effectiveness of 2 doses of varicella vaccine in children. J Infect Dis 2011; 203:312-5; PMID:21208922; http://dx.doi.org/10.1093/infdis/jiq052
- Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B, et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatric Infect Dis J 2004; 23:132-7; PMID:14872179; http://dx.doi.org/10.1097/01.inf.0000109287.97518.67
- Parker AA, Reynolds MA, Leung J, Anderson M, Rey A, Ortega-Sanchez IR, et al. Challenges to implementing second-dose varicella vaccination during an outbreak in the absence of a routine 2-dose vaccination requirement–Maine, 2006. J Infect Dis 2008; 197 Suppl 2:S101-7; PMID:18419381; http://dx.doi.org/10.1086/522134
- Tugwell BD, Lee LE, Gillette H, Lorber EM, Hedberg K, Cieslak PR. Chickenpox outbreak in a highly vaccinated school population. Pediatrics 2004; 113:455-9; PMID:14993534; http://dx.doi.org/10.1542/peds.113.3.455
- Marziano V, Poletti P, Guzzetta G, Ajelli M, Manfredi P, Merler S. The impact of demographic changes on the epidemiology of herpes zoster: Spain as a case study. Proc Biol Sci 2015; 282:20142509; PMID:25761709; http://dx.doi.org/10.1098/rspb.2014.2509
- Horn J, Damm O, Kretzschmar M, Siedler A, Wichmann O, Ultsch B, et al. Mathematische Modellierung der Auswirkungen der Varizellen-Impfung auf die Inzidenz von Herpes Zoster in Deutschland. Jahrestagung DGEpi 2013. Leipzig, 26. - 29. Sep 2013, 2013; 232:193.
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087-96; PMID:25916341; http://dx.doi.org/10.1056/NEJMoa1501184
- Wutzler P, Farber I, Wagenpfeil S, Bisanz H, Tischer A. Seroprevalence of varicella-zoster virus in the German population. Vaccine 2001; 20:121-4; PMID:11567755; http://dx.doi.org/10.1016/S0264-410X(01)00276-6
- Wutzler P, Neiss A, Banz K, Goertz A, Bisanz H. Can varicella be eliminated by vaccination? Potential clinical and economic effects of universal childhood varicella immunisation in Germany. Med Microbiol Immunol 2002; 191:89-96; PMID:12410347; http://dx.doi.org/10.1007/s00430-002-0123-4
- Zur Seroprävalenz gegen Varizella-Zoster-Virus und zur Verlässlichkeit anamnestischer Angaben. Epidemiologisches Bulletin 2003; 43:347
- Ultsch B, Siedler A, Rieck T, Reinhold T, Krause G, Wichmann O. Herpes zoster in Germany: quantifying the burden of disease. BMC Infect Dis 2011; 11:173; PMID:21679419; http://dx.doi.org/10.1186/1471-2334-11-173
- Schiffner-Rohe J, Jow S, Lilie HM, Koster I, Schubert I. [Herpes zoster in Germany. A retrospective analyse of SHL data]. MMW Fortschritte der Medizin 2010; 151 Suppl 4:193-7; PMID:21595148
- Statistisches Bundesamt. GENESIS Online-Datenbank. 2015.
- Reuss AM, Feig M, Kappelmayer L, Siedler A, Eckmanns T, Poggensee G. Varicella vaccination coverage of children under two years of age in Germany. BMC Public Health 2010; 10:502; PMID:20723217; http://dx.doi.org/10.1186/1471-2458-10-502
- Rieck T, Feig M, Eckmanns T, Benzler J, Siedler A, Wichmann O. Vaccination coverage among children in Germany estimated by analysis of health insurance claims data. Hum Vaccin Immunother 2014; 10:476-84; PMID:24192604; http://dx.doi.org/10.4161/hv.26986
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271-84; PMID:15930418; http://dx.doi.org/10.1056/NEJMoa051016
- Garnett GP, Grenfell BT. The epidemiology of varicella-zoster virus infections: the influence of varicella on the prevalence of herpes zoster. Epidemiol Infect 1992; 108:513-28; PMID:1318219; http://dx.doi.org/10.1017/S0950268800050019
- Bayer O, Heininger U, Heiligensetzer C, von Kries R. Metaanalysis of vaccine effectiveness in varicella outbreaks. Vaccine 2007; 25:6655-60; PMID:17706845; http://dx.doi.org/10.1016/j.vaccine.2007.07.010
- Seward JF, Marin M, Vazquez M. Varicella vaccine effectiveness in the US vaccination program: a review. J Infect Dis 2008; 197 Suppl 2:S82-9; PMID:18419415; http://dx.doi.org/10.1086/522145
- Izurieta HS, Strebel PM, Blake PA. Postlicensure effectiveness of varicella vaccine during an outbreak in a child care center. JAMA 1997; 278:1495-9; http://dx.doi.org/10.1001/jama.1997.03550180045035
- Oxman MN, Levin MJ, Shingles Prevention Study G. Vaccination against Herpes Zoster and Postherpetic Neuralgia. J Infect Dis 2008; 197 Suppl 2:S228-36; PMID:18419402; http://dx.doi.org/10.1086/522159
- Schmader KE, Levin MJ, Gnann JW, Jr., McNeil SA, Vesikari T, Betts RF, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis 2012; 54:922-8; PMID:22291101; http://dx.doi.org/10.1093/cid/cir970