ABSTRACT
It is estimated that more than 2.5 million individuals worldwide have multiple sclerosis (MS). MS is an autoimmune neurodegenerative disease resulting from the destruction of the myelin sheath that enwraps axons driven by an immune cell attack to the central nervous system. Current therapeutic programs for MS focus in immunosuppression and more recently in the use of immunomodulatory molecules. These therapeutic approaches provide significant improvements in the management of the disease, but are frequently associated with an increased susceptibility of opportunistic infection. In this commentary, we highlight the application of nano and micro-technologies as emerging and innovative solutions for MS therapy with the potential to restore immune homeostasis via antigen-specific interactions. Furthermore, we propose and discuss the usage of a minimally invasive approach, namely microneedle patches, as a new therapeutic route. Microneedle patches for the delivery of specific antigens to restore immunotolerance in the context of multiple sclerosis.
Multiple sclerosis: Etiology and current therapeutics
Multiple sclerosis (MS) is a chronic immune mediated demyelinating disease of the central nervous system (CNS) caused by a strong T cell attack directed toward proteins of the myelin sheath enwrapping CNS axons; this ultimately culminates in demyelination and neuronal degeneration.Citation1,2 In developed countries it is the second cause of neurological disability in young adults, with high burden for the patient, the family and the resources of the health system.Citation3 It is a complex disease and its underlying mechanisms are only partially understood. Most patients initially present with a clinically isolated syndrome (CIS). These CIS patients experience an acute episode, which typically affects one brain region, being the clinical symptoms variable depending on the involvement of motor, sensory, visual or autonomic systems.Citation4 Some CIS patients will evolve to definite MS disease, while others won't. Nowadays, the diagnosis of definite MS is based on recognized clinical criteria, with the support of magnetic resonance imaging (MRI) data and cerebrospinal fluid (CSF) analysis,Citation2 and can only be done when there is dissemination of neurologic dysfunction in space and time,Citation2,4,5 and after differential diagnosis is excluded.Citation5 Concerning the MRI findings, the presence of multifocal demyelinating lesions at different timepoints involving preferentially the periventricular white matter, the brain stem, the cerebellum and the spinal cord are indicative of MS.Citation2 Furthermore, the presence of oligoclonal bands or increased concentration of immunoglobulin (Ig)G in the patients' CSF are widely used to support MS diagnosis, but are not MS- specific.Citation2,6
Patients with definite MS can develop different profiles of the disease, being classified as relapse-remitting (RR)-MS, primary progressive (PP)-MS or secondary progressive (SP)-MS. RR-MS represents about 80–85% of MS cases Citation7 and is characterized by transient symptoms (relapse) that often improve within weeks (remission). However, the ability to fully recover from relapse episodes diminishes with time, and irreversible damage accumulates in the CNS, giving rise to SP-MS. The remaining 15–20% of patients has PP-MS, and does not show this relapse-remitting pattern; rather, their symptoms become gradually worst along the course of the disease.
MS is nowadays a treatable, although not curable, disease. The first proven MS treatments were approved in the nineties and consisted in different formulations of interferon-1 administered intramuscularly or subcutaneously. Although a major breakthrough at the time and still an important part of treatment options today, given their excellent safety, interferon-1 based treatments are only moderately efficacious, leading to full control of the disease in only a small percentage of patients.Citation8 Interferons have pleiotropic effects, including a reduced T-cell entry into the CNS.Citation9 Glatiramer acetate is a mixture of oligopeptides designed to mimic the aminoacid composition of myelin that induces a skew toward a regulatory response. Also administered subcutaneously, it has an efficacy similar to interferons and an excellent safety record that make it still an important player in MS treatment options.Citation8
Recently the portfolio of approved MS treatments was enriched by more options that encircle two major therapeutic approaches targeting either T-cells or B-cells. Concerning T-cells modulation a collection of drugs is currently being applied in the clinical setting, with moderate success, as next described. Teriflunomide is an oral medication that interferes with the fast expansion of recently activated lymphocytes, preserving the basal proliferation of memory cells. It has a moderate efficacy, similar to interferons, but some safety concerns, including teratogenic potential.Citation10 Dymethylfumarate is an oral treatment with a putative dual mechanism of action, including immunosuppression and neuroprotection. In clinical trials it demonstrated a good efficacy in controlling the disease but concerns regarding its long-term safety, particularly the profound lymphopenia and the risk of a serious opportunistic CNS infection, progressive multifocal leukoencephalopathy (PML), might limit its use.Citation11 Fingolimod, a functional antagonist of S1P receptors that blocks lymphocyte egress from lymph nodes,Citation12 has also a good efficacy but similar concerns over lymphopenia and PML.Citation8 Natalizumab is a highly efficacious monoclonal antibody that blocks lymphocyte entry into the CNS; however, it is associated with a high risk of PML in patients which have antibodies against the causing organism, JC virus, which almost limits its use to seronegative patients representing less than half of MS patients.Citation13
While all the above-mentioned drugs interfere with T-cell function, alternatively, ocrelizumab is a monoclonal antibody that destroys B-cells and, surprisingly, was shown to have a good efficacy in MS patients.Citation14 Although not yet approved, it has forced a major revision of MS pathogenesis to take into account the role of B-cells, which are not only seen as antibody producers but also as antigen presenting and cytokine releasing cells, able to activate Th1 and Th17 responses and induce pathology.Citation15 Although data are still preliminary, ocrelizumab may increase the risk of serious infections, including PML, which might limit its use. Targeting both B and T cells, alemtuzumab is a monoclonal antibody that induces a severe depletion of circulating lymphocytes and is said to “reset” the immune system.Citation16 Upon immune reconstitution, alemtuzumab-treated patients are able to stay disease free for periods of up to 5 y (longer follow-ups are still scarce), in what represents a first step toward an effective cure.Citation16 However, such efficacy comes at the cost of an increased risk of serious infections in the first weeks, requiring antibiotic profilaxis and precautions, and a significantly higher risk of autoimmune disturbances in the following years.Citation16
As can be gleaned from the above, MS treatments are in an exciting era, with several new options being approved, many more in the pipeline, and new drug targets and modes of action being available. More importantly, some treatments have been shown to allow a reset of the immune system, which might make a cure even more reachable than before. However, we weren't able, so far, of breaking the close ties between efficacy and risk. These severely limit the use of more efficacious medications in all patients and the extension of their benefits to all patients. Thus, the finding of a highly efficacious and safe therapeutic is still an unmet medical need. This paves the way for nanotechnology-based approaches.
Nanoengineered systems for MS therapeutics
The use of nanotechnology and in particular of nanoparticles has been actively investigated for the development of new therapies for MS. Taking advantage of their size, nanoparticles are easily internalized by the cells, being suitable carriers for drugs, immunomodulatory molecules or antigens. The use of materials at the nanoscale is expected to provide unique opportunities to improve drug solubility and bioavailability, allowing targeted delivery, controlled release and consequently more effective routes of administration and lower toxicity.Citation17 Interestingly, not only nanoparticles can serve as carriers of relevant molecules as they can also trigger an immunomodulatory effect. In fact, variations in the chemical composition, size and shape of nanoparticles differentially impact the immune response,Citation18,19 which might be even more significant in the context of autoimmune diseases.Citation20
In MS, the use of nanoparticles has been investigated within 2 major applications: 1) as drug delivery systems, and 2) as vectors for antigen-specific immunomodulation. Nanoparticles can bring new solutions for the delivery of drugs that specifically target the immune or the neurodegenerative aspects of MS. Recent reports describe the encapsulation in liposomal formulations of immunomodulatory drugs currently applied in MS therapeutics such as methylprednisolone Citation21 or fingolimod.Citation22 These nano-sized formulations showed a higher efficiency in a MS animal model due to improved pharmacokinetics and biodistribution when compared to the free drugs. Focusing in reducing neurodegeneration, polymeric nanoparticles targeted to oligodendrocyte precursor cells were applied to deliver leukemia inhibitory factor (LIF) and to successfully promote remyelinization.Citation23
Importantly, nanoparticles have also been explored as vectors for antigen-specific immunomodulation. The delivery of autoantigens related with the autoimmune response in MS is expected to allow the specific blockade of the damaging effects of self-reactive immune-cell function while maintaining the ability of the immune system to clear non-self antigens, thus restoring immunotolerance. This “tolerant approach” was firstly tested by the administration of soluble autoantigens, but the massive amounts of antigen required Citation24 along with some reported cases of anaphylactic response Citation25 prompt the need for new and safer solutions. The administration of peptides crosslinked to splenic leucocytes demonstrated very promising results, inducing a robust antigen-specific tolerance.Citation26,27 Nonetheless, in order to circumvent the use of cellular components and the drawbacks associated, like cost and manipulation, researchers have been focused in the development of alternative strategies based on nanoparticulate systems. The premise is that nanoparticles crosslinked with disease-associated antigens and their epitopes can target antigen-presenting cells (APCs) capable to regulate T-cell function and simultaneously support the induction and/or expansion of regulatory T-cells (Tregs), restoring immunological tolerance.
The intravenous administration of poly(D,L-lactide-co-glycolide) (PLGA) microparticles crosslinked with proteolipid protein (PLP)139–151 peptide (the immunodominant T cell myelin epitope in SJL mice from the myelin most abundant protein - PLP) showed remarkable results, being able not only to reduce the clinical score if administrated prophylactically, but also to treat ongoing disease.Citation28 Interestingly, PLP139–151 administrated in the form of colloidal hydrogel demonstrated to be effective only if administrated before the disease onset.Citation29 The use of poly(ethylene-co-maleic acid) (PEMA) as surfactant for the preparation of PLGA nanoparticles provided a reliable platform for different antigen crosslinking, as demonstrated by the relevant results in the induction of immunological tolerance both in the context of experimental autoimmune encephalomyelitis (EAE), the most frequently used animal model for MSCitation30 and in a transplantation model.Citation31
Alternatively to antigen crosslinking, nanoparticles can be loaded with the antigen of interest. This concept can be extended to the development of multifunctional systems that combine the delivery of antigens with other molecules/drugs as a mean to turn the immune response more specific and/or more effective. Loading gold nanoparticles with the T-cell epitope from myelin oligodendrocyte glycoprotein (MOG35–55) and ITE (2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester - a tolerogenic molecule) showed to induce functional regulatory T-cells in an EAE animal model more efficiently than MOG-loaded particles.Citation32 PLGA nanoparticles containing MOG35–55 and interleukin-10 (IL-10) mediate a sustained release of the moleculesCitation33 and, although the results in terms of regulatory T-cells expansion were not as impressive as the obtained for crosslinked nanoparticles, it was shown that the severity of the disease can be reduced via subcutaneous administration of the particles. In the PLP-associated EAE model, the use of rapamycin, an immunosuppressant molecule, loaded in PLGA nanoparticles along with PLP139–151 peptide promoted complete inhibition of disease relapses after both intravenous and subcutaneous administration.Citation34
The results achieved so far using nanoparticles to induce immunotolerance are promising and should soon initiate clinical trials. A further step toward the use of nanoparticles to induce immunotolerance might be achieved when combined with alternative and minimally invasive routes of administration, which will next be explored.
A microneedle-based immunotolerance approach for MS
Microneedles have been extensively investigated in the recent years as mean to mediate the delivery of drugs and/or antigens to the epidermal and/or intradermal space, overcoming the skin stratum corneum barrier. These devices hold the potential of allowing self-administration and painless application. Moreover, microneedle devices can be designed to dissolve in the skin, eliminating the issue of microneedle remaining and removal from the skin and allowing a safe disposal without biohazardous waste.Citation35
Although microneedles show great promise for the delivery of drugsCitation36 and also as sensing devices,Citation37 it is in the research area of vaccines that they showed, so far, more advances. It was demonstrated that vaccination using microneedles triggers stronger immune responses comparing to conventional injection procedures, allowing sparing of antigens.Citation38 Indeed, the use of microneedle-based devices for influenza vaccination is currently under clinical trials.
The improved antigen immunogenicity using microneedle devices is considered to be related to the delivery of antigens at the epidermal and intradermal layer of the skin. The skin is highly rich in immunologically active APCs, which deliver antigens to the proximal lymph nodes where T- and B-cells are activated, triggering the immune response.Citation39 Also, in the skin (particularly at the epidermis and the epithelium from the hair follicles) monocytes and Langerhans cells are abundant. Langerhans cells display intrinsic tolerogenic properties in vivo.Citation40 Moreover, a small trial enrolling 14 MS patients showed that the passive diffusion of myelin-related peptides through the skin induces some improvements in the disease, namely reducing the incidence of relapses and the area of lesion (assessed by MRI).Citation41 These findings highlight the potential of this route of administration in immunotolerance-based therapies (CitationFigure 1).
Figure 1. Microneedle patches for the delivery of specific antigens to restore immunotolerance in the context of multiple sclerosis.
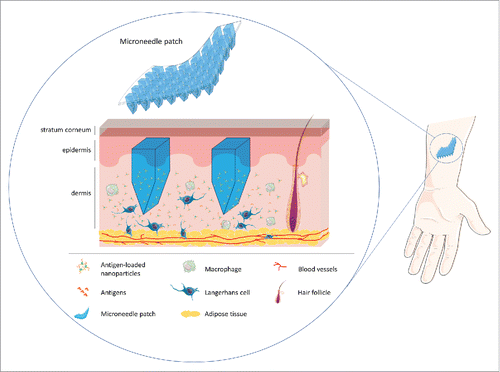
Overall, it is clear that therapeutics based on immunotolerance will take remarkable benefit from the application of micro and nano- technological knowledge. Nanoparticle design can assure antigen-specific response, targeted delivery and controlled dosing; whereas microneedle devices, as we propose, open the unique opportunity for sustained intradermal delivery, further contributing to an antigen-specific response and a tolerogenic effect in a minimally invasive therapeutic approach for MS.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This project has received funding from the European Union's Seventh Framework Program for research, technological development and demonstration under grant agreement no 600375. João Cerqueira received a grant from 2CA - Braga to the project “The impact of modafinil in cognition in multiple sclerosis.”
References
- Lassmann H, van Horssen J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett 2011; 585:3715-23; PMID:21854776; http://dx.doi.org/10.1016/j.febslet.2011.08.004
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Eng J Med 2000; 343:938-52; http://dx.doi.org/10.1056/NEJM200009283431307
- Borreani C, Bianchi E, Pietrolongo E, Rossi I, Cilia S, Giuntoli M, Giordano A, Confalonieri P, Lugaresi A, Patti F, et al. Unmet needs of people with severe multiple sclerosis and their carers: qualitative findings for a home-based intervention. PLoS One 2014; 9:e109679; PMID:25286321; http://dx.doi.org/10.1371/journal.pone.0109679
- Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372:1502-17; PMID:18970977; http://dx.doi.org/10.1016/S0140-6736(08)61620-7
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdová E, Hutchinson M, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69:292-302; PMID:21387374; http://dx.doi.org/10.1002/ana.22366
- Fossey SC, Vnencak-Jones CL, Olsen NJ, Sriram S, Garrison G, Deng X, Crooke PS, Aune TM. Identification of molecular biomarkers for multiple sclerosis. J Mol Diagn 2007; 9:197-204; PMID:17384211; http://dx.doi.org/10.2353/jmoldx.2007.060147
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol 2005; 23:683-747; PMID:15771584; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115707
- Oh J, O'Connor PW. Established disease-modifying treatments in relapsing-remitting multiple sclerosis. Curr Opin Neurol 2015; 28:220-29; PMID:25923124; http://dx.doi.org/10.1097/WCO.0000-000000000202
- Kieseier BC. The mechanism of action of interferon-β in relapsing multiple sclerosis. CNS Drugs 2011; 25:491-502; PMID:21649449; http://dx.doi.org/10.2165/11591110-000000000-00000
- Miller AE. Teriflunomide: A Once-daily Oral Medication for the Treatment of Relapsing Forms of Multiple Sclerosis. Clin Ther 2015; 37:2366-80; PMID:26365096; http://dx.doi.org/10.1016/j.clinthera.2015.08.003
- Bomprezzi R. Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: an overview. Ther Adv Neurol Disord 2015; 8:20-30; PMID:25584071; http://dx.doi.org/10.1177/1756285614564152
- Subei AM, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis. CNS Drugs 2015; 29:565-75; PMID:26239599; http://dx.doi.org/10.1007/s40263-015-0261-z
- Derfuss T, Kuhle J, Lindberg R, Kappos L. Natalizumab therapy for multiple sclerosis. Semin Neurol 2013; 33:26-36; PMID:23709210; http://dx.doi.org/10.1055/s-0033-1343793
- Kappos L, Li D, Calabresi PA, O'Connor P, Bar-Or A. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. The Lancet 2011; 78:1779-87; http://dx.doi.org/10.1016/S0140-6736(11)61649-8
- Hauser SL. The Charcot Lecture | beating MS: a story of B cells, with twists and turns. Mult Scler 2015; 21:8-21; PMID:25480864; http://dx.doi.org/10.1177/1352458514561911
- Hartung HP, Aktas O, Boyko AN. Alemtuzumab: a new therapy for active relapsing-remitting multiple sclerosis. Mult Scler 2015; 21:22-34; PMID:25344374; http://dx.doi.org/10.1177/1352458514549398
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: Therapeutic applications and developments. Clin Pharmacol Ther 2008; 83:761-69; PMID:17957183; http://dx.doi.org/10.1038/sj.clpt.6100400
- Cruz LJ, Tacken PJ, Fokkink R, Joosten B, Stuart MC, Albericio F, Torensma R, Figdor CG. Targeted PLGA nano- but not microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. J Control Release 2010; 144:118-26; PMID:20156497; http://dx.doi.org/10.1016/j.jconrel.2010.02.013
- Beletskii A, Galloway A, Rele S, Stone M, Malinoski F. Engineered PRINT (R) nanoparticles for controlled delivery of antigens and immunostimulants. Hum Vaccin Immunother 2014; 10:1908-13; PMID:25424798; http://dx.doi.org/10.4161/hv.28817
- Roberts RA, Eitas TK, Byrne JD, Johnson BM, Short PJ, McKinnon KP, Reisdorf S, Luft JC, DeSimone JM, Ting JP. Towards programming immune tolerance through geometric manipulation of phosphatidylserine. Biomaterials 2015; 72:1-10; PMID:26325217; http://dx.doi.org/10.1016/j.biomaterials.2015.08.040
- Turjeman K, Bavli Y, Kizelsztein P, Schilt Y, Allon N, Katzir TB, Sasson E, Raviv U, Ovadia H, Barenholz Y. Nano-Drugs Based on Nano Sterically Stabilized Liposomes for the Treatment of Inflammatory Neurodegenerative Diseases. Plos One 2015; 10:e0130442; PMID:26147975; http://dx.doi.org/10.1371/journal.pone.0130442
- Mao Y, Wang J, Zhao Y, Wu Y, Kwak KJ, Chen CS, Byrd JC, Lee RJ, Phelps MA, Lee LJ, et al. A novel liposomal formulation of FTY720 (Fingolimod) for promising enhanced targeted delivery. Nano Med 2014; 10:393-400; PMID:23969101; http://dx.doi.org/10.1016/j.nano.2013.08.001
- Rittchen S, Boyd A, Burns A, Park J, Fahmy TM, Metcalfe S, Williams A. Myelin repair in vivo is increased by targeting oligodendrocyte precursor cells with nanoparticles encapsulating leukaemia inhibitory factor (LIF). Biomaterials 2015; 56:78-85; PMID:25934281; http://dx.doi.org/10.1016/j.biomaterials.2015.03.044
- Karpus WJ, Kennedy KJ, Smith WS, Miller SD. Inhibition of relapsing experimental autoimmune encephalomyelitis in SJL mice by feeding the immunodominant PLP139-151 peptide. J Neurosci Res 1996; 45:410-23; PMID:8872901; http://dx.doi.org/10.1002/(SICI)1097-4547(19960815)45:4<410::AID-JNR10>3.0.CO;2-4
- Smith CE, Eagar TN, Strominger JL, Miller SD. Differential induction of IgE-mediated anaphylaxis after soluble vs. cell-bound tolerogenic peptide therapy of autoimmune encephalomyelitis. Proc Natl Acad Sci USA 2005; 102:9595-600; PMID:15983366; http://dx.doi.org/10.1073/pnas.0504131102
- Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, Getts MT, Martin AJ, Luo X, Terry RL, et al. Tolerance Induced by Apoptotic Antigen-Coupled Leukocytes Is Induced by PD-L1(+) and IL-10-Producing Splenic Macrophages and Maintained by T Regulatory Cells. J Immunol 2011; 187:2405-17; PMID:21821796; http://dx.doi.org/10.4049/jimmunol.1004175
- Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Gettss DR, Xia G, He J, Zhang X, Kaufman DB, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci USA 2008; 105:14527-32; PMID:18796615; http://dx.doi.org/10.1073/pnas.0805204105
- Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo X, King NJC, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotech 2012; 30:1217-24; http://dx.doi.org/10.1038/nbt.2434
- Bueyuektimkin B, Wang Q, Kiptoo P, Stewart JM, Berkland C, Siahaan TJ. Vaccine-like Controlled-Release Delivery of an Immunomodulating Peptide To Treat Experimental Autoimmune Encephalomyelitis. Mol Pharm 2012; 9:979-85; PMID:22375937; http://dx.doi.org/10.1021/mp200614q
- Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, Miller SD. A Biodegradable Nanoparticle Platform for the Induction of Antigen-Specific Immune Tolerance for Treatment of Autoimmune Disease. ACS Nano 2014; 8:2148-60; PMID:24559284; http://dx.doi.org/10.1021/nn405033r
- Bryant J, Hlavaty KA, Zhang X, Yap W-T, Zhang L, Shea LD, Luo X. Nanoparticle delivery of donor antigens for transplant tolerance in allogeneic islet transplantation. Biomaterials 2014; 35:8887-94; PMID:25066477; http://dx.doi.org/10.1016/j.biomaterials.2014.06.044
- Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 2012; 109:11270-75; PMID:22745170; http://dx.doi.org/10.1073/pnas.1120611109
- Cappellano G, Woldetsadik AD, Orilieri E, Shivakumar Y, Rizzi M, Carniato F, Gigliotti CL, Boggio E, Clemente N, Comi C, et al. Subcutaneous inverse vaccination with PLGA particles loaded with a MOG peptide and IL-10 decreases the severity of experimental autoimmune encephalomyelitis. Vaccine 2014; 32:5681-89; PMID:25149432; http://dx.doi.org/10.1016/j.vaccine.2014.08.016
- Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O'Neil C, Altreuter DH, Browning E, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci USA 2015; 112:E156-E65; PMID:25548186; http://dx.doi.org/10.1073/pnas.1408686111
- Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008; 29:2113-24; PMID:18261792; http://dx.doi.org/10.1016/j.biomaterials.2007.12.048
- Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev 2004; 56:581-87; PMID:15019747; http://dx.doi.org/10.1016/j.addr.2003.10.023
- Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci USA 2015; 112:8260-65; PMID:26100900; http://dx.doi.org/10.1073/pnas.1505405112
- Koutsonanos DG, Vassilieva EV, Stavropoulou A, Zarnitsyn VG, Esser ES, Taherbhai MT, Prausnitz MR, Compans RW, Skountzou I. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep 2012; 2:357; PMID:22500210; http://dx.doi.org/10.1038/srep00357
- Koutsonanos DG, Martin MdP, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, Skountzou I. Transdermal Influenza Immunization with Vaccine-Coated Microneedle Arrays. Plos One 2009; 4:e4773; PMID:19274084; http://dx.doi.org/10.1371/journal.pone.0004773
- Shklovskaya E, O'Sullivan BJ, Lai Guan N, Roediger B, Thomas R, Weninger W, de St Groth BF. Langerhans cells are precommitted to immune tolerance induction. Proc Natl Acad Sci USA 2011; 108:18049-54; PMID:22006331; http://dx.doi.org/10.1073/pnas.1110076108
- Walczak A, Siger M, Ciach A, Szczepanik M, Selmaj K. Transdermal Application of Myelin Peptides in Multiple Sclerosis Treatment. JAMA Neurol 2013; 70:1105-09; PMID:23817921; http://dx.doi.org/10.1001/jamaneurol.2013.3022
