ABSTRACT
Introduction: The objective of this study was to assess the incremental costs and benefits of the 9-valent HPV vaccine (9vHPV) compared with the quadrivalent HPV vaccine (4vHPV). Like 4vHPV, 9vHPV protects against HPV types 6, 11, 16, and 18. 9vHPV also protects against 5 additional HPV types 31, 33, 45, 52, and 58. Methods: We adapted a previously published model of the impact and cost-effectiveness of 4vHPV to include the 5 additional HPV types in 9vHPV. The vaccine strategies we examined were (1) 4vHPV for males and females; (2) 9vHPV for females and 4vHPV for males; and (3) 9vHPV for males and females. In the base case, 9vHPV cost $13 more per dose than 4vHPV, based on available vaccine price information. Results: Providing 9vHPV to females compared with 4vHPV for females (assuming 4vHPV for males in both scenarios) was cost-saving regardless of whether or not cross-protection for 4vHPV was assumed. The cost per quality-adjusted life year (QALY) gained by 9vHPV for both sexes (compared with 4vHPV for both sexes) was < $0 (cost-saving) when assuming no cross-protection for 4vHPV and $8,600 when assuming cross-protection for 4vHPV. Conclusions: Compared with a vaccination program of 4vHPV for both sexes, a vaccination program of 9vHPV for both sexes can improve health outcomes and can be cost-saving.
Introduction
Numerous models have shown favorable cost-effectiveness assessments of human papillomavirus (HPV) vaccination in the United States (US) and other developed countries, particularly for vaccination of 12-year-old females.Citation1-10 Consistent with health benefits estimated by these models, evaluations of vaccination programs have provided a growing body of evidence that documents the initial effects of HPV vaccination on the prevalence of HPV vaccine types, genital warts, and cervical precancers in the US and around the world.Citation11-18 In the future, more substantial reductions in these outcomes are expected, along with reductions in cervical cancer and other HPV-associated cancers.Citation5-10,13,19
The first 2 HPV vaccines licensed in the US were the bivalent vaccine (2vHPV, which targets HPV types 16 and 18) and the quadrivalent vaccine (4vHPV, which targets HPV types 6, 11, 16, and 18). HPV types 16 and 18 account for 66% of cervical cancer, the majority of anal, oropharyngeal, and vaginal cancers (79%, 60%, and 55%, respectively), and a substantial portion of penile and vulvar cancers (48% and 49%, respectively) in the US.Citation20 HPV types 6 and 11 account for more than 90% of genital warts and recurrent respiratory papillomatosis (RRP) in the US.Citation21 From 2006 to 2014, 4vHPV accounted for almost all HPV vaccine distributed in the US.Citation22 In December 2014, the US Food and Drug Administration approved a nonavalent HPV vaccine (9vHPV), which targets the same 4 types as 4vHPV as well as 5 additional cancer-causing HPV types (31, 33, 45, 52, and 58).Citation23 In the US, these additional 5 HPV types account for about 25% of cervical precancers, 15% of cervical cancers, and 4% to 18% of vaginal, vulvar, penile, anal, and oropharyngeal cancers.Citation20,24 Overall, about 14% of HPV-associated cancer in females and 4% in males are attributable to HPV 31, 33, 45, 52, and 58.Citation20,23
In this study, we used a simplified model of HPV transmission to estimate the additional health benefits and cost-effectiveness of 9vHPV compared with 4vHPV in the US. Although several complex models of HPV have been developed to assess the relative costs and benefits of various HPV vaccination strategies and these complex models are vital in informing recommendations for HPV vaccination, decision makers are often reassured when the results of highly complex models are consistent with simpler, more basic models that require less data and fewer assumptions. The estimates from our simplified model can therefore complement those of more complex models of 9vHPV in the USCitation25,26 and can help to inform 9vHPV vaccine recommendations.
Results
Health impact of vaccination
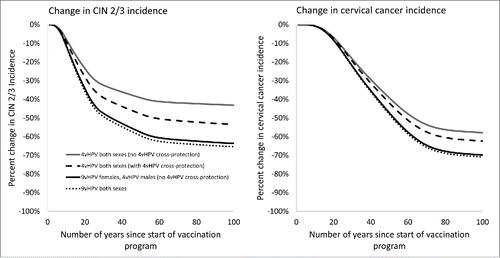
Under base case coverage assumptions, the long-term (100 y after start of the vaccination program) reduction in cervical intraepithelial neoplasia (CIN) 2/3 was 43.0% with 4vHPV for both sexes under the assumption of no cross-protection; 53.4% with 4vHPV for both sexes when assuming cross-protection, 63.5% with 9vHPV for females and 4vHPV for males (no cross-protection for 4vHPV), and 65.3% with 9vHPV for both sexes (). The projected long-term decreases in cervical cancer were more pronounced than for CIN 2/3, but were predicted to take longer to achieve. Of note, the reductions in incidence shown in are for the entire female population; the model predicts that reductions in incidence will occur earlier in younger age groups than in older age groups (e.g., ages 21 to 30 y compared with ages 60 y and older, results not shown).
Figure 1. Estimated reduction in cervical intraepithelial neoplasia (CIN) 2/3 and cervical cancer over 100 y for various HPV vaccination strategies. This figure shows the estimated reduction in CIN 2/3 and cervical cancer over 100 y for various HPV vaccination strategies, under the base case coverage scenario in which coverage in the 13- to 17-year age group reaches 45.5% for females and 28.6% for males in the 8th year of the vaccine program.
QALYs gained and costs averted by vaccination
A strategy of 9vHPV for both sexes (compared with 4vHPV for both sexes) saved about $386 million dollars (above and beyond the additional costs of 9vHPV vaccination) and resulted in a gain of 147,000 quality-adjusted life years (QALYs) (). Most of these gains were due to providing 9vHPV to females, as a strategy of 9vHPV for females compared with 4vHPV for females (assuming 4vHPV for males in both scenarios) increased the number of QALYs gained by 129,000. As expected, the number of QALYs gained and costs averted by providing 9vHPV instead of 4vHPV were smaller when assuming that 4vHPV offered some cross-protection against the additional HPV types included in 9vHPV than when assuming no cross-protection for 4vHPV.
Table 1. Base case results: Discounted vaccination costs, averted medical costs, quality-adjusted life-years (QALYs) gained, incremental change in net costs, incremental gain in QALYs, and incremental cost per QALY gained by HPV vaccination strategies, with and without cross-protection for quadrivalent HPV vaccine (4vHPV).
Cost-effectiveness of vaccination
Providing 9vHPV to females compared with 4vHPV for females (assuming 4vHPV for males in both scenarios) was cost-saving regardless of whether or not cross-protection for 4vHPV was assumed (). The cost per QALY gained by 9vHPV for both sexes (compared with 4vHPV for both sexes) was <$0 (cost-saving) when assuming no cross-protection for 4vHPV and $8,600 when assuming cross-protection for 4vHPV.
Sensitivity analyses
In the one-way sensitivity analyses, providing 9vHPV to both sexes (compared with 4vHPV for both sexes) cost < $10,000 per QALY in all but 3 scenarios (). The three exceptions were a cost per QALY of $50,400 when applying a time horizon of 25 years, a cost per QALY of $16,700 when applying the upper bound cost per 9vHPV series, and a cost per QALY of $10,900 when applying the lower bound percentage of disease attributable to the vaccine types. Providing 9vHPV to females compared with 4vHPV for females (assuming 4vHPV for males in both scenarios) was cost-saving in all but 4 scenarios. The four exceptions were a cost per QALY of $22,600 when applying a time horizon of 25 years, a cost per QALY of $4,100 when applying the upper bound cost per 9vHPV series, a cost per QALY of $200 when applying the lower bound percentage of disease attributable to the vaccine types, and a cost per QALY of $400 when assuming lower medical costs for each HPV-associated health outcome.
Figure 2. Tornado diagram showing the cost per quality-adjusted life year (QALY) gained by 9-valent HPV vaccination (compared with 4-valent HPV vaccination) in the one-way sensitivity analyses. Cost per QALY estimates <$0 (cost-saving) are entered in the chart as $0. “Both sexes” compared the strategy of 9-valent HPV vaccine (9vHPV) for both sexes to the strategy of 4-valent HPV vaccine (4vHPV) for both sexes. “Females only” compared 9vHPV for females with 4vHPV for females, assuming that males would receive 4vHPV in both scenarios (i.e., the strategy of “9vHPV for females, 4vHPV for males” was compared with “4vHPV for both sexes”). The higher cost per QALY gained was obtained in the following scenarios: lower bound value of time horizon (25 years), upper bound value of cost per 9vHPV series ($513), lower bound values of the percent of disease attributable the HPV vaccine types, lower bound values of the medical cost per health outcome, lower bound values of incidence of the health outcomes, and the higher vaccine coverage scenario. See the technical appendix for the ranges applied for the percent of disease attributable to the HPV vaccine types, the medical costs per health outcome, and the incidence rates of the health outcomes. Ranges for vaccine coverage are listed in . For “Both sexes” and “Females only,” the cost per QALY remained < $0 (cost-saving) when varying vaccine efficacy or the number of QALYs lost per health outcome (not shown in graph).
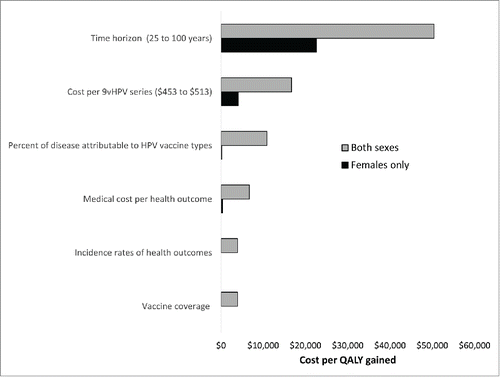
Table 2. HPV vaccine characteristics and coverage assumptions.
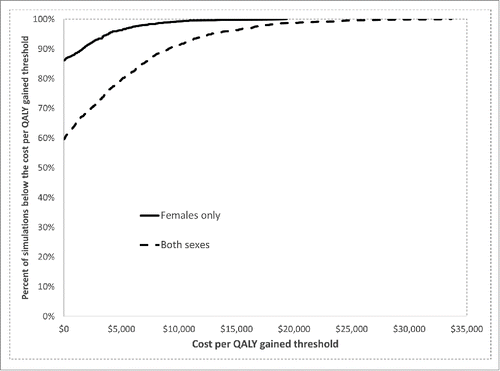
In the multiway sensitivity analyses (), estimates of the cost per QALY gained (based on the 5th and 95th percentiles of the simulations) ranged from < $0 (cost-saving) to $3,900 for providing 9vHPV to females (compared with 4vHPV for females) and <$0 (cost-saving) to $12,800 for providing 9vHPV to both sexes (compared with 4vHPV for both sexes).
Figure 3. Cost-effectiveness acceptability curves: percentage of simulations in the probabilistic sensitivity analyses in which the incremental cost per quality-adjusted life year (QALY) gained by 9-valent HPV vaccination was at or below a given cost per QALY threshold. “Females only” compared 9-valent HPV vaccine (9vHPV) for females with 4-valent HPV vaccine (4vHPV) for females, assuming that males would receive 4vHPV in both scenarios (i.e., the strategy of “9vHPV for females, 4vHPV for males” was compared with “4vHPV for both sexes”). “Both sexes” compared the strategy of 9vHPV for both sexes to the strategy of 4vHPV for both sexes.
Discussion
We found favorable cost-effectiveness ratios for 9vHPV (compared with 4vHPV) across a wide range of scenarios. Providing 9vHPV to females accounted for most of the medical costs averted and QALYs gained by a strategy of 9vHPV for both sexes. However, a strategy of 9vHPV for females and 4vHPV for males is likely not be a feasible policy alternative in the US due to the need for providers to stock 2 different HPV vaccines and, more importantly, the eventual lack of availability of 4vHPV in the US.Citation27 Accordingly, the comparison of 9vHPV for both sexes compared with 4vHPV for both sexes is likely the most relevant tradeoff to consider.
Our findings support current HPV vaccine recommendations in the US, as the Advisory Committee on Immunization Practices (ACIP) includes 9vHPV as one of the HPV vaccines that can be used for routine vaccination.Citation23 Most HPV vaccine administered in the US through 2014 was 4vHPV; transition from 4vHPV to 9vHPV is expected over time.Citation22,23 Our analysis suggests that the use of 9vHPV rather than 4vHPV can be cost-saving, given the current difference of $13 in the price per dose of the 2 vaccines.
The favorable cost-effectiveness ratios for 9vHPV we found using our simplified transmission model are quite consistent with results from 2 other, more complex models. Brisson and colleagues recently used a multifaceted, individual-based transmission dynamic model to examine the cost-effectiveness of 9vHPV in the US.Citation26 They estimated that in the absence of cross-protection for 4vHPV, 9vHPV was cost-saving compared with 4vHPV when focusing on females only or both sexes. Weiss and colleagues used a compartmental model of HPV to assess the impact and cost-effectiveness of 9vHPV in the US.Citation25 Preliminary results from the Weiss model indicated that in the absence of cross-protection for 4vHPV, 9vHPV was cost-saving compared with 4vHPV when considering 9vHPV for females only or both sexes when assuming 9vHPV cost $13 more per dose than 4vHPV.
Our analysis is subject to limitations. Briefly, our model incorporates many simplifying features and is far less complex than other published HPV models. The first main simplifying feature in our model is the use of discrete, 1-year time steps (rather than a continuous approach as in more complex models), which might cause an underestimation of the speed and degree to which HPV vaccination affects HPV transmission dynamics in the population. However, the indirect effects (or “herd effects”) predicted by our simple model over the long term were generally consistent with predictions of more complex models of HPV transmission in developed settings.Citation28 The second main simplifying feature of our model is that it does not explicitly account for cervical cancer screening and therefore cannot examine the impact of future changes in cervical cancer screening. Instead, our model approximates the long-term costs and benefits of HPV vaccination under the assumption that the probability of detection through screening remains constant over the duration of the HPV vaccine program and that the number of cervical cancer cases would remain constant over time in the absence of vaccination. A third main simplifying feature of the model is that it does not explicitly account for the progression from HPV infection to disease. Instead reductions in HPV-attributable outcomes are approximated based on the reduction in the acquisition of the HPV vaccine types over time. In addition to the simplifications in our model structure, we made a number of simplifying assumptions in our application of the model. For example, we assumed that all those who initiated the vaccine series would receive all 3 doses and that vaccine duration of protection would be lifelong. Finally, our analysis did not include potential adverse effects of vaccination (e.g., temporary pain at injection site) or the potential for HPV type replacement. Our model might underestimate the benefits of 9vHPV if there is type replacement after introduction of 4vHPV, although current data do not indicate the occurrence of type replacement.Citation29 These and other limitations of our modeling approach are discussed in more detail elsewhere.Citation10,19,30
The simplified structure of our model offers several important benefits. Most importantly, our model requires less data and fewer assumptions than more complex models. For example, because we apply annual probabilities of acquiring HPV rather than modeling sexual behavior directly, our model does not require data on factors such as the number of new sex partners per year, condom use, and the per-act or per-partnership probability of HPV transmission. Similarly, because we estimate reductions in HPV-attributable health outcomes as a function of the reduction in cumulative lifetime acquisition of HPV, our model does not require natural history data on the progression from HPV infection to disease.
Despite limitations, our simple model serves as a useful supplement to the more complex HPV models currently available. Our estimates of the cost-effectiveness of 9vHPV compared with 4vHPV in the US are generally consistent with these more complex models,Citation25,26 even though these other models more precisely account for factors such as the dynamics of sexual acquisition and transmission of HPV, the impact of cervical cancer screening, and the progression from HPV infection to disease. The consistency of the cost-effectiveness estimates for 9vHPV across distinct models with vast differences in structure and varying degrees of complexity will likely be reassuring to decision makers.
Methods
Study questions addressed
We examined vaccination of females aged 12 to 26 y and males aged 12 to 21 y in the US, based on current recommendations for routine HPV vaccination.Citation21 We addressed the following 2 study questions:
What is the cost-effectiveness of vaccinating females with 9vHPV instead of 4vHPV? Specifically, what is the cost-effectiveness of a vaccination program of 9vHPV for females and 4vHPV for males, compared with a program of 4vHPV for both sexes?
What is the cost-effectiveness of vaccinating both sexes with 9vHPV instead of 4vHPV? Specifically, what is the cost-effectiveness of a vaccination program of 9vHPV for both sexes, compared with a program of 4vHPV for both sexes?
For each of these 2 study questions, we assumed that the observed rates of cervical cancer applied in the model (those that have occurred in the context of cervical cancer screening practices in the US) would remain constant over time in the absence of HPV vaccination. Because we do not explicitly incorporate cervical cancer screening in our model, we cannot assess the impact of changing cervical cancer screening strategies.
Cost-effectiveness ratios
In addressing the 2 study questions, we calculated the incremental cost per QALY gained by each HPV vaccination strategy. The incremental cost per QALY was calculated as the change in net costs (vaccination costs minus medical costs averted by vaccination) due to the vaccination strategy divided by the number of QALYs gained by the vaccination strategy (compared with the comparator strategy). All costs were updated to 2013 US dollars using the Personal Consumption Expenditures price index (http://www.bea.gov/iTable/iTable.cfm?ReqID=12&step=1&acrdn=2).
Perspective, scope, time frame, and analytic horizon
We used a societal perspective and included costs and benefits of vaccination without regard to who incurs the costs or who receives the benefits. The costs and benefits we included were limited to the direct costs of vaccination, the direct medical costs averted by vaccination, and the QALYs saved by vaccination. Medical costs averted and QALYs saved were accrued by prevention of the following HPV-associated health outcomes: cervical intraepithelial neoplasia (CIN); genital warts; juvenile-onset RRP; and cervical, vaginal, vulvar, anal, oropharyngeal, and penile cancers. We used a 100-year time horizon, and all future costs and QALYs were discounted by 3% annually. For ease of comparison of vaccination strategies, each vaccination scenario we examined (4vHPV for both sexes, 9vHPV for females and 4vHPV for males, and 9vHPV for both sexes) was assumed to last for 100 y. For example, we did not consider a scenario in which a 4vHPV vaccination program was in place for 10 y and then replaced by a 9vHPV program for the remaining 90 y. We assessed the lifetime costs averted and lifetime health benefits of the HPV-attributable health outcomes that were prevented over the 100-year period.
Model overview
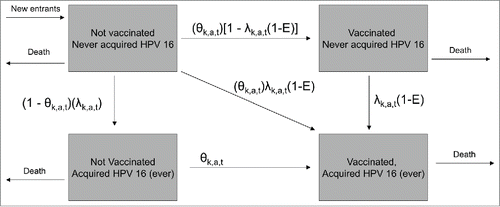
We used a deterministic, dynamic, population-based model (), adapted from a previously-published model of the potential impact and cost-effectiveness of 4vHPV vaccination in the US.Citation10 The model was expanded to include the 5 additional HPV types contained in 9vHPV. This section contains a brief description of the HPV 16 component of the model. The technical appendix contains a full description of the model.
Figure 4. Diagram of simplified model. A cohort of susceptible 8-year-olds enters the population each year. In each year of the vaccine program (from year 1 to 100), there is a probability of death from all causes, a probability of acquiring HPV 16 (λ) for those never infected with HPV 16 previously, and a probability of receiving HPV vaccination (θ). Vaccine efficacy against infection with HPV 16 is given by E. The subscripts k, a, and t denote sex, age, and year of vaccine program, respectively. The age- and sex-specific probabilities of HPV 16 infection (λ) were adjusted for each year t to reflect changes in HPV prevalence in sex partners as a result of HPV vaccination. The reduction in HPV 16-associated health outcomes due to vaccination for a given age cohort in a given year was assumed to be proportional to the reduction in cumulative HPV 16 infection in that age cohort attributable to vaccination. Reductions in health outcomes attributable to other HPV types were estimated in an analogous manner.
The model tracks ages 8 to 99 years; each year a new cohort of 8 y olds enters the model and the 99-year-old cohort exits the model. In order to estimate the population-level impact of HPV vaccination over a 100-year period, our model assesses the costs and benefits of HPV vaccination for 191 birth cohorts, including the 92 birth cohorts aged 8 to 99 y at the onset of the vaccine program and the subsequent 99 incoming birth cohorts of 8-year-olds in years 2–100 of the vaccine program. In order to incorporate HPV transmission dynamics, the age-specific annual probabilities of acquiring HPV in the model are adjusted in each year of the vaccine program to account for reductions in HPV in the population as a result of vaccination, as summarized below and described in detail in the technical appendix.
Model assessment of vaccine impact on cumulative HPV 16 acquisition
Vaccination can occur as early as age 12 y and as late as age 21 y for males and age 26 y for females (). There are 4 possible states within each age cohort based on vaccination status and cumulative HPV 16 acquisition status: (1) Not vaccinated; never acquired HPV 16; (2) Vaccinated, never acquired HPV 16; (3) Not vaccinated, acquired HPV 16 (ever); and (4) Vaccinated, acquired HPV 16 (ever). Movement occurs between the states according to age-specific probabilities of acquiring HPV 16 and probabilities of being vaccinated. Vaccination reduces the probability of acquiring HPV 16 according to the vaccine efficacy assumptions.
For each year t of the 100 y of the vaccination program, the model tracks cumulative HPV 16 infection through year t for each age cohort (8 to 99 years) by sex. In each year, cumulative HPV 16 infection for each age cohort in the scenario of HPV vaccination is compared with what it would have been in the absence of a vaccination program. Age-specific HPV 16 acquisition probabilities in year t+1 are adjusted proportionately according to reductions in cumulative HPV 16 infection in year t in the opposite sex, to reflect changes in HPV prevalence in sex partners as a result of HPV vaccination.
Model calculations of reductions in HPV 16-attributable health outcomes
One of the main simplifications of our approach is that we do not explicitly model the progression from HPV infection to HPV-attributable health outcomes. Instead, health outcomes attributable to HPV 16 were assumed to be reduced in proportion to the reduction in the acquisition of HPV 16. Specifically, HPV-16 attributable health outcomes for each age cohort (8 to 99 years) in year t were assumed to be reduced by the same proportion as the age cohort's reduction in cumulative HPV 16 acquisition, subject to a lag to establish a minimum time between vaccination and the prevention of a given health outcome. For example, using a 5-year lag for cervical cancer, the percentage reduction in HPV-16 attributable cervical cancer among females aged 45 in year 2065 was calculated as the percentage reduction in cumulative HPV 16 acquisition among females aged 40 y in 2060.
For each year of the 100 y of the vaccination program, the model assesses HPV 16-attributable outcomes averted for each age cohort (8 to 99 years) by sex. The number of discounted, averted outcomes is multiplied by the discounted lifetime medical cost and the discounted lifetime number of QALYs lost per health outcome to estimate the averted medical costs and QALYs saved. For example, for the cervical cancer costs averted by vaccination, we multiplied the number of cervical cancer cases averted in year t by the estimate of the discounted lifetime medical cost per case of cervical cancer. This yielded the lifetime medical costs saved by the cervical cancer outcomes averted in year t, discounted to year t. In order to discount these averted medical costs to the onset of the vaccination program, we discounted these averted medical costs by an additional t- 1 y. These calculations were conducted for cervical cancer cases averted in each of the 100 y of the vaccine program.
Modeling other HPV types: 6, 11, 18, 31, 33, 45, 52, and 58
The reduction in the number of cases of health outcomes attributable to other HPV types was estimated in a manner analogous to that of HPV-16 associated health outcomes. To clarify, we estimated 8 versions of the model described above, in order to estimate reductions in health outcomes attributable to (1) HPV 16, (2) HPV 18, (3) HPV 31, (4) HPV 33, (5) HPV 45, (6) HPV 52, (7) HPV 58, and (8) HPV 6/11, according to the percentage of each health outcome attributable to each HPV type as described in the technical appendix. These eight reductions in health outcomes were combined to estimate the impact and cost-effectiveness of HPV vaccination, according to the efficacy assumptions against each HPV type for the given HPV vaccine.
Vaccine characteristics
Assumptions regarding vaccine efficacy, cost, and coverage are summarized in . For both vaccines, we assumed 95% vaccine efficacy against the HPV vaccine types, based on vaccine trial data showing high vaccine efficacy against persistent infection.Citation31-34 Vaccine duration of protection was assumed to be lifelong. In scenarios in which we considered potential cross-protection against non-vaccine HPV types for 4vHPV, we assumed vaccine efficacies of 46.2% against HPV 31, 28.7% against HPV 33, 7.8% against HPV 45, 18.4% against HPV 52, and 5.5% against HPV 58.Citation35 The base-case vaccine cost per 3-dose series (including $15 per dose for administration costs) was set to $435 for 4vHPV and $474 (range: $453 to $513) for 9vHPV.Citation25 Under these assumptions, 9vHPV cost about $13 more per dose than 4vHPV, consistent with available vaccine price information for the public sector (http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html). The age- and sex- specific annual probabilities of vaccination we applied were calculated based on reported HPV coverage rates in the US, and over time these vaccination probabilities yield slightly higher coverage rates than currently reported as described in the technical appendix.Citation36,37
We assumed that everyone vaccinated would complete the full 3-dose vaccine series. In reality, the HPV 3-dose series completion rate among ages 13 to 17 in the US in 2013 was about 70% for females and 50% for males.Citation37 Accounting for those who do not complete the vaccine series would necessitate assumptions regarding vaccine efficacy and duration of protection for those who receive only one or 2 doses.Citation38-40 More complex models are better suited than ours for examining issues related to vaccine protection with less than 3 doses.Citation41 Instead, we used the simplifying assumption of 100% series completion not only to facilitate the use of our simplified model but also to allow for an easier interpretation of our results regarding the cost-effectiveness of 9vHPV compared with 4vHPV.
Other parameter values
Other key parameter values in our model included disease incidence rates in the absence of vaccination, the percent of health outcomes attributable to the HPV vaccine types, and the direct medical costs and number of QALYs lost per HPV-associated outcome. Selected parameter values are provided in for illustration purposes. The technical appendix contains a complete listing of all model parameter values and ranges for all age groups, as well as a description of the assumptions and sources behind these estimates.
Table 3. Selected values used in model: incidence, cost per case, and QALYs lost per case of CIN, genital warts, cervical and other cancers, and RRP.
Sensitivity analyses
We examined how the incremental cost-effectiveness of 9vHPV (compared with 4vHPV) varied under different assumptions about the additional cost of 9vHPV (compared with 4vHPV). We also examined how the cost-effectiveness results would change when examining a shorter time horizon of the vaccination program (25 y instead of 100 years). We then conducted additional one-way sensitivity analyses to see how the cost-effectiveness of 9vHPV vaccination strategies would change when varying one of the follow 5 sets of parameter values at a time: efficacy of the vaccine types, the cost per case of each health outcome, the number of QALYs lost per case of each health outcome, the incidence rates of the health outcomes in the absence of vaccination, and the percentages of the health outcomes attributable to the HPV vaccine types. We also examined how the cost-effectiveness of 9vHPV vaccination strategies would change when numerous parameter values (treatment costs and QALYs lost per case of each health outcome, the incidence rates of the health outcomes in the absence of vaccination, and the percentages of the health outcomes attributable to the HPV vaccine types) and the predicted health impact of the model were varied simultaneously in a probabilistic sensitivity analyses consisting of 2,000 model simulations (see technical appendix for details).
Disclosure of potential conflicts of interest
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Supplemental_Material.pdf
Download PDF (993.8 KB)Acknowledgments
We thank Meg Watson, Jessica King, and Trevor Thompson for providing data.
References
- Goldie SJ, Kohli M, Grima D, Weinstein MC, Wright TC, Bosch FX, Franco E. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004; 96: 604–615; PMID:15100338; http://dx.doi.org/10.1093/jnci/djh104
- Sanders GD, Taira AV. Cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003; 9:37–48; PMID:12533280; http://dx.doi.org/10.3201/eid0901.020168
- Kulasingam SL, Myers ER. Potential health and economic impact of adding a human papillomavirus vaccine to screening programs. JAMA 2003; 290:781–9; PMID:12915431; http://dx.doi.org/10.1001/jama.290.6.781
- Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004; 10:1915–23; PMID:15550200; http://dx.doi.org/10.3201/eid1011.040222
- Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis 2007; 13:28–41; PMID:17370513; http://dx.doi.org/10.3201/eid1301.060438
- Chesson HW, Ekwueme DU, Saraiya M, Markowitz LE. Cost-effectiveness of human papillomavirus vaccination in the United States. Emerg Infect Dis 2008; 14:244–51; PMID:18258117; http://dx.doi.org/10.3201/eid1402.070499
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359:821–32; PMID:18716299; http://dx.doi.org/10.1056/NEJMsa0707052
- Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. BMJ 2009; 339:b3884; PMID:19815582; http://dx.doi.org/10.1136/bmj.b3884
- Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine 2010; 28:6858–67; PMID:20713101; http://dx.doi.org/10.1016/j.vaccine.2010.08.030
- Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine 2011; 29:8443–50; PMID:21816193; http://dx.doi.org/10.1016/j.vaccine.2011.07.096
- Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003–2010: Potential impact of HPV vaccination. Am J Public Health 2013; 103:1428–35; PMID:23763409; http://dx.doi.org/10.2105/AJPH.2012.301182
- Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, Unger ER. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis 2013; 208:385–93; PMID:23785124; http://dx.doi.org/10.1093/infdis/jit192
- Hariri S, Markowitz LE, Dunne EF, Unger ER. Population impact of HPV vaccines: summary of early evidence. J Adolesc Health 2013; 53:679–82; PMID:24263069; http://dx.doi.org/10.1016/j.jadohealth.2013.09.018
- Bauer HM, Wright G, Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: an analysis of California public family planning administrative claims data, 2007–2010. Am J Public Health 2012; 102:833–5; PMID:22420808; http://dx.doi.org/10.2105/AJPH.2011.300465
- Pollock KG, Kavanagh K, Potts A, Love J, Cuschieri K, Cubie H, Robertson C, Cruickshank M, Palmer TJ, Nicoll S et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer 2014; 111:1824–30; PMID:25180766; http://dx.doi.org/10.1038/bjc.2014.479
- Baldur-Felskov B, Dehlendorff C, Junge J, Munk C, Kjaer SK. Incidence of cervical lesions in Danish women before and after implementation of a national HPV vaccination program. Cancer Causes Control 2014; 25:915–22; PMID:24797870; http://dx.doi.org/10.1007/s10552-014-0392-4
- Powell SE, Hariri S, Steinau M, Bauer HM, Bennett NM, Bloch KC, Niccolai LM, Schafer S, Unger ER, Markowitz LE. Impact of human papillomavirus (HPV) vaccination on HPV 16/18-related prevalence in precancerous cervical lesions. Vaccine 2012; 31:109–13; PMID:23137842; http://dx.doi.org/10.1016/j.vaccine.2012.10.092
- Hariri S, Bennett NM, Niccolai LM, Schafer S, Park IU, Bloch KC, Unger ER, Whitney E, Julian P, Scahill MW et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States - 2008–2012. Vaccine 2015; 33:1608–13; PMID:25681664; http://dx.doi.org/10.1016/j.vaccine.2015.01.084
- Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. Estimates of the timing of reductions in genital warts and high grade cervical intraepithelial neoplasia after onset of human papillomavirus (HPV) vaccination in the United States. Vaccine 2013; 31:3899–905; PMID:23820080; http://dx.doi.org/10.1016/j.vaccine.2013.06.050
- Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, Steinau M, Watson M, Wilkinson EJ, Hopenhayn C et al. US assessment of HPV types in cancers: Implications for current and 9-valent HPV Vaccines. J Natl Cancer Inst 2015; 107:djv086; PMID:25925419; http://dx.doi.org/10.1093/jnci/djv086
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JA, Jr., Unger ER. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63: 1–30; PMID:25167164.
- Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, Curtis RC, Markowitz L. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014. MMWR Morb Mortal Wkly Rep 2014; 63:620–4; PMID:25055185
- Petrosky E, Bocchini JA, Jr., Hariri S, Chesson H, Curtis CR, Saraiya M, Unger ER, Markowitz LE. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015; 64:300–4; PMID:25811679.
- Hariri S, Unger ER, Schafer S, Niccolai LM, Park IU, Bloch KC, Bennett NM, Steinau M, Johnson ML, Markowitz LE. HPV type attribution in high-grade cervical lesions: assessing the potential benefits of vaccines in a population-based evaluation in the United States. Cancer Epidemiol Biomarkers Prev 2015; 24:393–9; PMID:25416715; http://dx.doi.org/10.1158/1055-9965.EPI-14-0649
- Weiss TW, Pillsbury MT, Dasbach EJ. Potential health and economic impact of the investigational 9-valent HPV vaccine in the United States. 29th International Papillomavirus Conference and Clinical Workshop, Seattle, Washington, August 21-25, 2014.
- Brisson M, Laprise J-F, Chesson HW, Drolet M, Malagón T, Boily MC, Markowitz LE. Health and economic impact of switching from a 4-valent to a 9-valent HPV vaccination program in the United States. J Natl Cancer Inst 2015; 108:djv282; PMID:26438574
- Markowitz L. Proposed HPV vaccination recommendations. 2015. Presentation to the Advisory Committee on Immunization Practices, Atlanta, Georgia, February 26, 2015.
- Brisson M, Bénard E, Drolet M, Baussano I, Berkhof J, Boily MC, Canfell K, Chesson H, Jit M, Vänskä S, et al. Population-level impact, herd immunity and elimination after HPV vaccination: a systematic review and meta-analysis of predictions of 17 transmission dynamic models. Abstract HPV15–0413. 30th International Papillomavirus Conference and Clinical Workshop. September 17-21, 2015. Lisbon, Portugal.
- Drolet M, Bénard E, Boily MC, Ali H, Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JM, Cummings T et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:565-80; PMID:25744474; http://dx.doi.org/10.1016/S1473-3099(14)71073-4
- Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The estimated impact of human papillomavirus vaccine coverage on the lifetime cervical cancer burden among girls currently aged 12 years and younger in the United States. Sex Transm Dis 2014; 41:656-9; PMID:25299411; http://dx.doi.org/10.1097/OLQ.0000000000000199
- Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915-27; PMID:17494925; http://dx.doi.org/10.1056/NEJMoa061741
- Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928-43; PMID:17494926; http://dx.doi.org/10.1056/NEJMoa061760
- Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6:271-8; PMID:15863374; http://dx.doi.org/10.1016/S1470-2045(05)70101-7
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED, Jr., Ngan Y, Petersen LK, Lazcano-Ponce E et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372:711-23; PMID:25693011; http://dx.doi.org/10.1056/NEJMoa1405044
- Malagón T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, Brisson M. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:781-9; PMID:Can't; http://dx.doi.org/10.1016/S1473-3099(12)70187-1
- Dorell C, Stokley S, Yankey D, Jeyarajah J, MacNeil J, Markowitz L. National and state vaccination coverage among adolescents aged 13 through 17 years–United States, 2011. MMWR Morb Mortal Wkly Rep 2012; 61:671-7; PMID:22932301.
- Elam-Evans LD, Yankey D, Jeyarajah J, Singleton JA, Curtis RC, MacNeil J, Hariri S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years–United States, 2013. MMWR Morb Mortal Wkly Rep 2014; 63:625-33; PMID:25055186.
- Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, Langley JM et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309:1793-802; PMID:23632723; http://dx.doi.org/10.1001/jama.2013.1625
- Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Behre U et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum Vaccin Immunother 2014; 10:1155-65; PMID:24576907; http://dx.doi.org/10.4161/hv.28022
- Lazcano-Ponce E, Stanley M, Muñoz N, Torres L, Cruz-Valdez A, Salmerón J, Rojas R, Herrero R, Hernández-Ávila M. Overcoming barriers to HPV vaccination: non-inferiority of antibody response to human papillomavirus 16/18 vaccine in adolescents vaccinated with a two-dose compared with a three-dose schedule at 21 months. Vaccine 2014; 32:725-32; PMID:24355090; http://dx.doi.org/10.1016/j.vaccine.2013.11.059
- Jit M, Choi YH, Laprise JF, Boily MC, Drolet M, Brisson M. Two-dose strategies for human papillomavirus vaccination: how well do they need to protect? Vaccine 2014; 32:3237-3242; PMID:24726246; http://dx.doi.org/10.1016/j.vaccine.2014.03.098
- Henk HJ, Insinga RP, Singhal PK, Darkow T. Incidence and costs of cervical intraepithelial neoplasia in a US commercially insured population. J Low Genit Tract Dis 2010; 14:29-36; PMID:20040833; http://dx.doi.org/10.1097/LGT.0b013e3181ac05e9
- Insinga RP, Glass AG, Rush BB. Diagnoses and outcomes in cervical cancer screening: A population-based study. Am J Obstet Gynecol 2004; 191:105-13; PMID:15295350; http://dx.doi.org/10.1016/j.ajog.2004.01.043
- Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol 2004; 191:114-20; PMID:15295351; http://dx.doi.org/10.1016/j.ajog.2004.01.042
- Drolet M, Brisson M, Maunsell E, Franco EL, Coutlee F, Ferenczy A, Fisher W, Mansi JA. The psychosocial impact of an abnormal cervical smear result. Psychooncology 2012; 21:1071-81; PMID:21695747; http://dx.doi.org/10.1002/pon.2003
- Hoy T, Singhal PK, Willey VJ, Insinga RP. Assessing incidence and economic burden of genital warts with data from a US commercially insured population. Curr Med Res Opin 2009; 25:2343-51; PMID:19650749; http://dx.doi.org/10.1185/03007990903136378
- Chesson HW, Ekwueme DU, Saraiya M, Watson M, Lowy DR, Markowitz LE. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine 2012; 30:6016-9; PMID:22867718; http://dx.doi.org/10.1016/j.vaccine.2012.07.056
- Drolet M, Brisson M, Maunsell E, Franco EL, Coutlee F, Ferenczy A, Ratnam S, Fisher W, Mansi JA. The impact of anogenital warts on health-related quality of life: a 6-month prospective study. Sex Transm Dis 2011; 38:949-56; PMID:21934571; http://dx.doi.org/10.1097/OLQ.0b013e3182215512
- Woodhall SC, Jit M, Soldan K, Kinghorn G, Gilson R, Nathan M, Ross JD, Lacey CJ. The impact of genital warts: loss of quality of life and cost of treatment in eight sexual health clinics in the UK. Sex Transm Infect 2011; 87:458-63; PMID:21636616; http://dx.doi.org/10.1136/sextrans-2011-050073
- Jit M, Chapman R, Hughes O, Choi YH. Comparing bivalent and quadrivalent human papillomavirus vaccines: economic evaluation based on transmission model. BMJ 2011; 343:d5775; PMID:21951758; http://dx.doi.org/10.1136/bmj.d5775
- Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care 1998; 36:778-92; PMID:9630120; http://dx.doi.org/10.1097/00005650-199806000-00002
- Armstrong LR, Preston EJ, Reichert M, Phillips DL, Nisenbaum R, Todd NW, Jacobs IN, Inglis AF, Manning SC, Reeves WC. Incidence and prevalence of recurrent respiratory papillomatosis among children in Atlanta and Seattle. Clin Infect Dis 2000; 31:107-9; PMID:10913405; http://dx.doi.org/10.1086/313914
- Chesson HW, Forhan SE, Gottlieb SL, Markowitz LE. The potential health and economic benefits of preventing recurrent respiratory papillomatosis through quadrivalent human papillomavirus vaccination. Vaccine 2008; 26:4513-18; PMID:18598734; http://dx.doi.org/10.1016/j.vaccine.2008.06.045
- Marsico M, Mehta V, Chastek B, Liaw KL, Derkay C. Estimating the incidence and prevalence of juvenile-onset recurrent respiratory papillomatosis in publicly and privately insured claims databases in the United States. Sex Transm Dis 2014; 41:300-5; PMID:24722383; http://dx.doi.org/10.1097/OLQ.0000000000000115
