ABSTRACT
Most adverse events (AEs) during the immunization of rabies vaccine were slight, there was little information about the allergic reaction induced by rabies vaccines and had to stop or change the immunization program. Here, we reported a case that a 4-year-old boy had category II exposure to rabies and showed severe allergic reaction after being immunized with lyophilized purified vero cell rabies vaccine (PVRV). After the anti-allergy therapy with hormone, allergy testing indicated medium allergy to egg and milk, and implied the allergic reaction most likely associated with animal-sourced gelatin in lyophilized PVRV. Therefore, a new immunization program with liquid PVRV without stabilizers under the Zegrab regimen (2-1-1) was enrolled at day 7 post-exposure. Although lower than the levels of normal <5 -year population at day 14 and 45, the neutralizing antibody (RVNA) titers of this boy showed adequate protective antibody (≥ 0.5 IU/ml), even after 365 d post-immunization. This study not only highlighted the importance of several types of rabies vaccines co-existing in the market, but also implied the necessary for doctors to fully understand the allergies history of patients prior to immunize rabies vaccine.
Introduction
Because human rabies is practically a 100% fatal disease,Citation1 it is mandatory to immunize with rabies vaccines as soon as post-exposure of rabies. Timely and proper use of modern rabies vaccines and immunoglobulins is a crucial method to prevent rabies virus infection post-exposure. Although rabies vaccines in the market had been carefully evaluated, both local adverse events (AEs) and systemic AEs were normally reported during the immunization process Citation2,3 and mild systemic reactions had been reported in up to 40% of recipients.Citation4 However, severe adverse events that had to stop or change the immunization program were rarely reported. In this study, we reported a case of severe allergic reaction to the rabies vaccine.
Patient presentation
A 4-year-old boy was hospitalized for urticarial drug eruption at June 21, 2014. His parents reported an immunization history of lyophilized purified vero cell rabies vaccine (PVRV, 1.0ml/dose) on June 20 because of category II exposure to highly suspected dog's bite. On the admission, he presented severe allergic reaction according to the “Preventive vaccine clinical, averse events grading guidelines” issued by the China Food and Drug Administration. He had fever (38.9°C), listlessness, weakness, headache within 8 hours after the immunization with lyophilized PVRV. Many red wheals can be found all around his body, especially on the face. Laboratory test found a significant high level of high-sensitivity C-reactive protein (HS-CRP, 20.7 mg/L), which implied the allergy might be possibly induced by an exogenous stimulation. His parents also proved that he had allergy history. Thus, drug allergy to rabies vaccine was primary concluded, and he was injected with 2 mg Dexamethasone Sodium Phosphate for 2 days, plus 2.5 mg oral Cetirizine hydrochloride tables for 2 times in first day and 3 mg for one time per day in the following 2 d.
After getting out of hospital, allergy testing was suggested by the doctors in Wuhan Centers for Disease Prevention & Control (Wuhan CDC) and the results tested with UniCAP systems (Pharmacia Biotech, Uppsala, Sweden) by Tongji Hospital, Tongji Medical College of Huazhong University of Science & Technology were shown in , of which medium allergy reactions to egg and milk were flagged. As described above, his parents reported no changes on the living conditions, and also denied eating egg or milk, drug allergy to rabies vaccine was the most reasonable explanation. Because the first injection of rabies vaccine at June 20 was lyophilized PVRV, the stabilizer used in vaccine was gelatin with a component of bull bone, thus most likely induced severe allergic reaction.Citation5 Based on the allergy testing results, we excluded purified chick embryo cell vaccine (PCECV) or PVRV that had components of bull bone or bovine serum. Finally, a liquid PVRV (0.5 ml/dose) without stabilizers was enrolled for the new anti-rabies immunization program. In addition, rabies virus neutralizing antibody (RVNA) titers in the serum were measured by rapid fluorescent focus inhibition test (RFFIT) in the virology laboratory of Wuhan Centers for Disease Prevention and Control (Wuhan CDC) as we described before,Citation3 and the result at June 24 indicated lower level of antibodies (). In order to achieve adequate immune response as soon as possible, Zagreb regimen (2-1-1, consisting of 4 doses in 3 visits on days 0, 7 and 21, 2-site intradermal injections on day 0) was used for the new immunization program. Following up reported no further allergy happening, and immunogenicity analysis indicated successful protection (RVNA titer ≥ 0.5 IU/ml, an indicator of an adequate adaptive immune response used by the World Health Organization [WHO] Citation6) was deduced by liquid PVRV, RVNA of this case at day 14 (2.11 vs 7.03 IU/ml) and day 45 (6.25 vs 23.31 IU/ml), however, remained significant lower than that of normal < 5-year children in our previous study ().Citation3
Figure 1. The rabies virus neutralizing antibody (RVNA) titers of a 4-year-old boy after being immunized with a liquid PVRV under Zagreb regimen, who showed severe allergic reaction to lyophilized PVRV because of the component of bull bone 7 d ago (−7), and thus had been treated with 3 days' anti-allergy therapy. RVNA titers in serum were measured under masked conditions using a rapid fluorescent focus inhibition test (RFFIT).Citation2,3 Reference data was adapted from the mean RVNA titers of local < 5-year-old children under Zagreb regimen in our previous studyCitation3
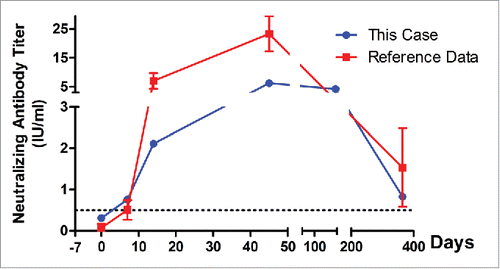
Table 1. The results of allergy testing.
The Institutional Review Board of Wuhan CDC approved this study, and written informed consent was obtained from his parents.
Discussion
Although mild systemic reactions, such as headache, nausea, abdominal pain, myalgia, had been reported in up to 31% of PCECV vaccine recipients Citation6 and about 1% of PVRV vaccines,Citation7,8 severe allergic reaction, like this reported case, was rare. It was very difficult to define the allergic reaction associated to the rabies vaccine, in particular for the reaction to only one component that was not noted. In fact, most occurrences of anaphylaxis to live virus vaccines reported were caused by animal-sourced gelatin.Citation5 Therefore, it was challenge for the patients with allergy history to choose suitable rabies vaccines. However, as pointed out by Prof. Grill, it is far outweigh any risks for administering rabies vaccine and/or immune globulin to individuals with suspected exposure.Citation4 It was necessary to continue the administration with a selected rabies vaccine for this suspected child, despite of a medium allergy to egg and milk.
According to the neutralizing antibody titers showed in , no adequate immune response was achieved post first injection immediately after the exposure to rabies virus. Based on our previous study that Zagreb can achieve more quick response to rabies vaccine than Essen (1-1-1-1-1, consisting of 5 doses in 5 visits on days 0, 3, 7, 14 and 28),Citation2 the parent chose Zagreb regimen for the new immunization program. However, safety was more important than immunogenicity for the clinical vaccination, especially for this child with allergy history and under Zagreb regimen, as we previously found that most adverse events (AEs) occurred in <5-year children and Zagreb showed more AEs than Essen after first dose of vaccination.Citation2 More importantly, we can't absolutely certain the allergic reaction was caused solely by animal-sourced gelatin. Thus, it should be very careful to monitor the safety during the immunization. In addition, the type and volume of primary immunized vaccine should be also taken into account for the new immunization program. Luckily, the child showed no further allergic reaction to the new PVRV.
The neutralization antibody titers analysis () indicated that RVNA titers of this boy remained lower than that of normal <5-year children we reported before,Citation3 which might be effected by the hormone during the anti-allergy therapy, as previously we also observed negative effect of the hormone on RVNA titers in a case with acute disseminated encephalomyelitis.Citation9 Although China pharmacopoeia (2010) ruled that the using of PVRV should not company with corticosteroids, RVNA results in this study indicated protective antibody titers (≥ 0.5 IU/ml) induced by the new PVRV, even at day 365 post-immunization. Given that there was 4-day gap between the use of hormone and immunized PVRV, a question had been raised that, when the patient was being treated with corticosteroids but had to be immunized with PVRV at the same time, what should we do?
In summary, the treatment of this case with severe allergic reaction to PVRV was very difficult and complex. It is lucky that, unlike many countries else in the world, China has multiple types of rabies vaccines (ex. PCECV, lyophilized or liquid PVRV,Citation2 primary hamster kidney cell (PHKC) rabies vaccine,Citation10 and human diploid cell rabies vaccine (HDCV) Citation9) available for being chose. This case report highlighted the importance of multiple types of vaccines co-existing in the market. In addition, in order to choose suitable rabies vaccines and immunization program, it was necessary for clinical doctors to fully understand the past medical history of patients, especially for allergies history.
Abbreviations
| AEs | = | adverse events |
| PVRV | = | purified vero cell rabies vaccine |
| RVNA | = | rabies virus neutralizing antibody |
| PCECV | = | purified chick embryo cell vaccine |
| RFFIT | = | rapid fluorescent focus inhibition test. |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Abela-Ridder B. Rabies: 100 per cent fatal, 100 per cent preventable. Vet Rec 2015; 177:148-9; PMID:26251539; http://dx.doi.org/10.1136/vr.h4196
- Hu Q, Liu MQ, Zhu ZG, Zhu ZR, Lu S. Comparison of safety and immunogenicity of purified chick embryo cell vaccine using Zagreb and Essen regimens in patients with category II exposure in China. Hum Vaccin Immunother 2014; 10:1645-9; PMID:24632727; http://dx.doi.org/10.4161/hv.28420
- Fang Y, Chen L, Liu MQ, Zhu ZG, Zhu ZR, Hu Q. Comparison of safety and immunogenicity of PVRV and PCECV immunized in patients with WHO category II animal exposure: a study based on different age groups. PLoS Negl Trop Dis 2014; 8:e3412; PMID:25522244; http://dx.doi.org/10.1371/journal.pntd.0003412
- Sabella O. Side effects and risks of rabies vaccine. Can Fam Physician. 2009; 55:470; author reply; PMID:19439692
- Sakaguchi M, Nakayama T, Fujita H, Toda M, Inouye S. Minimum estimated incidence in Japan of anaphylaxis to live virus vaccines including gelatin. Vaccine 2000; 19:431-6; PMID:11027805; http://dx.doi.org/10.1016/S0264-410X(00)00206-1
- Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B, Guerra M, Meltzer MI, Dhankhar P, Vaidya SA, Jenkins SR, et al. Human rabies prevention–United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57:1-28; PMID:18496505
- Yu P, Huang Y, Zhang Y, Tang Q, Liang G. Production and evaluation of a chromatographically purified Vero cell rabies vaccine (PVRV) in China using microcarrier technology. Hum Vaccin Immunother 2012; 8:1230-5; PMID:22894963; http://dx.doi.org/10.4161/hv.20985
- Sudarshan MK, Narayana DH, Madhusudana SN, Holla R, Ashwin BY, Gangaboraiah B, Ravish HS. Evaluation of a one week intradermal regimen for rabies post-exposure prophylaxis: results of a randomized, open label, active-controlled trial in healthy adult volunteers in India. Hum Vaccin Immunother 2012; 8:1077-81; PMID:22699446; http://dx.doi.org/10.4161/hv.20471
- Peng J, Chen L, Zhu ZG, Zhu ZR, Hu Q, Fang Y. Effect of Corticosteroids on RVNA production of a patient with acute disseminated encephalomyelitis following rabies vaccination as well as administration of HRIG. Hum Vaccin Immunother 2014; 10:3622-6; PMID:25668669; http://dx.doi.org/10.4161/21645515.2014.979621
- Wu T, Lu Y, Ge X. [A study on efficacy of rabies vaccine prepared by primary hamster kidney cells in Guangxi]. Zhonghua Yu Fang Yi Xue Za Zhi 1998; 32:211-3; PMID:10322757
