ABSTRACT
Dengue has a major impact on global public health, and the use of dengue vaccine is very limited. In this study, we evaluated the immunogenicity and protective efficacy of a dengue vaccine made from a recombinant measles virus (MV) that expresses envelope protein domain III (ED3) of dengue-1 to 4. Following immunization with the MV-vectored dengue vaccine, mice developed specific interferon-gamma and antibody responses against dengue virus and MV. Neutralizing antibodies against MV and dengue viruses were also induced, and protective levels of FRNT50 ≥ 10 to 4 serotypes of dengue viruses were detected in the MV-vectored dengue vaccine-immunized mice. In addition, specific interferon-gamma and antibody responses to dengue viruses were still induced by the MV-vectored dengue vaccine in mice that were pre-infected with MV. This finding suggests that the pre-existing immunity to MV did not block the initiation of immune responses. By contrast, mice that were pre-infected with dengue-3 exhibited no effect in terms of their antibody responses to MV and dengue viruses, but a dominant dengue-3-specific T-cell response was observed. After injection with dengue-2, a detectable but significantly lower viremia and a higher titer of anti-dengue-2 neutralizing antibodies were observed in MV-vectored dengue vaccine-immunized mice versus the vector control, suggesting that an anamnestic antibody response that provided partial protection against dengue-2 was elicited. Our results with regard to T-cell responses and the effect of pre-immunity to MV or dengue viruses provide clues for the future applications of an MV-vectored dengue vaccine.
Introduction
As the leading cause of mosquito-borne viral disease, dengue results in approximately 400–500 million infections and 21,000 deaths annually, primarily affecting Southeast Asia and Latin American.Citation1 The disease burden has increased over recent decades due to global warming and an increase in international travel.Citation2 To date, there are 4 dengue virus serotypes (DENV-1 to 4) circulating in endemic regions and the treatments to reduce the risk of dengue infection are limited. DENV infections are usually asymptomatic or self-limited febrile illnesses and elicit long-lasting homotypic immunity to the infecting serotype and short-lived heterotypic immunity to the others.Citation3,4 However, a severe, life-threatening dengue hemorrhagic fever or dengue shock syndrome may occur in some individuals, especially those with a secondary infection with a different serotype or in infants with maternal antibodies.Citation5 Although the pathogenesis of severe dengue is still unclear, a non-protective heterotypic immune response has been reported to be associated with severe dengue.Citation6 For example, antibody-dependent enhancement (ADE) and the occurrence of original antigenic sin, as mediated by cross-reactive antibodies and T cells, contribute to the higher viremia and blood vessel damage observed in the pathogenesis of severe dengue diseases.Citation7-9 Therefore, it is believed that an ideal dengue vaccine would be able to induce a balanced immunity against all dengue serotypes.
Several dengue vaccine candidates, including live attenuated or inactivated virus, recombinant or chimeric viral vectors, subunit protein and DNA vaccines,Citation10-14 are under development, but none are currently licensed. Recently, a yellow fever virus-based chimeric tetravalent dengue vaccine (CYD) have shown promise in clinical trials for the prevention of dengue and was licensed in Mexico, Philippines and Brazil; however, its relatively weak efficacy against DENV-2 infection raises more concerns.Citation15,16 Similar to the other candidate vaccines, the chimeric CYD tetravalent dengue vaccine contains dengue membrane and envelope proteins that might be neutralized by pre-existing immunity against dengue or other flaviviruses. By contrast, DNA or viral vector-based dengue vaccines contain only the genes encoding dengue proteins, but not the proteins themselves, to avoid interference from pre-existing dengue-specific antibodies.
It is well known that neutralizing antibodies play an important role in blocking dengue virus infection. Dengue envelope protein domain III (ED3) is the major target for serotype-specific neutralizing antibodies.Citation17 In addition to neutralizing antibody, there is increasing evidence from human and animal studies to indicate that interferon (IFN)-γ-producing T cells contribute to protection against the dengue virus,Citation18-20 highlighting the importance of the T-cell responses that are induced by dengue vaccination. However, the ED3-specfic T-cell response is less understood, particularly for the responses elicited by tetravalent dengue vaccines. Therefore, a comprehensive study on the ED3-specific T-cell response is important for the development of ED3-based tetravalent dengue vaccines.
The current used live attenuated MV vaccine is capable of eliciting long-lasting immunity in infants without any severe adverse effects.Citation21 Recombinant virus technology allows the MV vaccine strain to become an efficient viral vector for vaccine delivery Citation22-24 and oncolytic virotherapy.Citation25 However, previous reports on MV-vectored dengue vaccines were focused on the antibody response, and they were tested in immunocompromised mice that lacked type-I interferon signaling,Citation23,26 which is important for activating dendritic cells and T-cell responses.Citation27 In this study, we extended the previous findings to analyze both the T-cell and antibody responses induced by the MV-vectored tetravalent dengue vaccine in immunocompetent C57BL/6 mice expressing MV receptor-human CD46 (hCD46 mice), and we evaluated the influence of pre-existing immunity to either MV or DENV on the immunogenicity and protection of the MV-vectored tetravalent dengue vaccine. Our data provide a further understanding of the application of the MV-vectored tetravalent dengue vaccine.
Results
Generation of a recombinant measles-vectored dengue vaccine
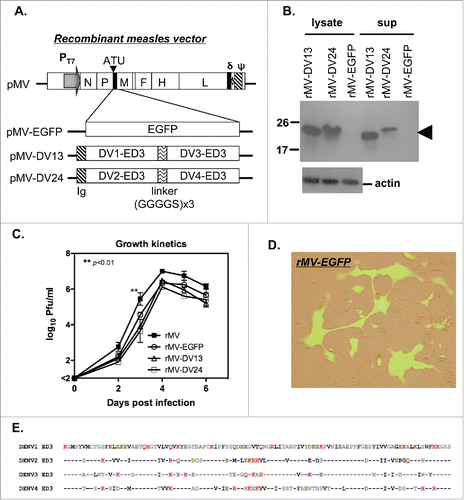
The construction of the full-length infectious clone of MV Moraten strain pMV was performed by overlapping RT-PCR. An additional transcription unit (ATU) was inserted into the intergenic boundary between the P and M genes for the expression of a foreign gene. To express tetravalent dengue ED3 protein, the genes encoding tandem repeats of bivalent ED3 proteins from DENV-1 and 3 or DENV-2 and 4 were subcloned into the ATU site of the MV infectious clone (). A gene encoding enhanced green fluorescent protein (EGFP) was also subcloned into the ATU site as a control. The amino acid sequence for 4 serotypes of ED3 was aligned, and it is shown in . To generate the recombinant viruses, an MV-susceptible 293-hSLAM cell line was used for MV rescue. Usually, recombinant viruses were obtained after 2–3 days and amplified on Vero cells. The purified recombinant viruses were harvested to analyze the expression of ED3 protein by Western blotting. As expected, ED3 proteins were detected in both the supernatant and cell lysates of Vero cells that were infected with rMV-DV13 and rMV-DV24, but not with the control rMV-EGFP (). This finding suggests that recombinant viruses successfully produce and secrete dengue ED3 proteins. To test the growth kinetics, we infected Vero cells with rMV, rMV-EGFP, rMV-DV13 or rMV-DV24 at a multiplicity of infection (MOI) of 0.02 and harvested the cells and lysates for the determination of virus titers by plaque assay. The titers of all recombinant viruses increased to a similar extent to those observed in the parental rMV, except that significantly higher titers were observed in rMV than in rMV-DV13 and rMV-DV24 at day 3 (p<0.01; n = 2; ). In addition, we also examined the expression of EGFP in rMV-EGFP-infected cells by infecting Vero cells with rMV-EGFP, and we observed the appearance of fluorescence. A typical image of MV-infected syncytial cells with green fluorescence is clearly shown in .
Figure 1. Preparation of the recombinant measles viral vector tetravalent dengue vaccine. (A) Schematic diagram of the infectious clone pMV containing the antigenomic cDNA of the Moraten MV strain is shown. The required elements including the T7 promoter (PT7), an additional transcription unit (ATU), the delta ribozyme (δ) and the T7 polymerase terminator (ψ) are also indicated. The infectious clones of the recombinant virus, which carried either an EGFP reporter gene (pMV-EGFP) or tandem repeats of ED3 from DENV-1 and 3 (pMV-DV13) and DENV-2 and 4 (pMV-DV24) with a secretory signal (Ig) and linker (GGGGS x3) are shown at the bottom. (B) The presence of dengue ED3 protein in the cell lysate and culture supernatant of Vero cells infected with recombinant viruses were detected by Western blotting with an anti-ED3 monoclonal antibody and indicated by the arrow; the signal for the actin protein in the cell lysate is also shown at the bottom. (C) Vero cells were infected with the different recombinant viruses and cell lysate and supernatant were harvested to determine the virus titers by plaque assay. The growth kinetics of recombinant viruses are presented with the mean and standard deviation (SD) from 2 experiments. (D) Vero cells were infected with rMV-EGFP, and the merged image of rMV-EGFP-infected syncytial cells from bright field and fluorescent microscopy is shown. (E) The consensus amino acid sequence from 4 serotypes of ED3 was aligned and listed.
The infection and replication of recombinant MV in hCD46 mice
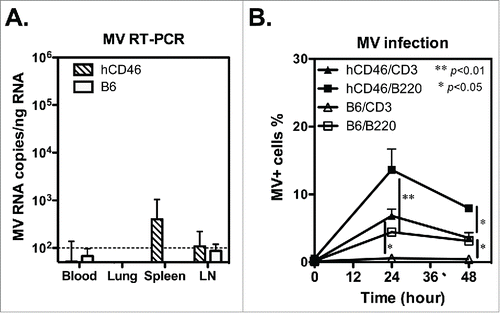
To determine the efficiency of recombinant virus replication in vivo, the plasma and tissues from parental rMV-infected hCD46 and C57BL/6 mice were collected at day 7, 9 and 15 for analysis because these times are comparable to the viremia peak and recovery stage in monkeys that were challenged with MV.Citation28 Although no MV plaque was detected in the plasma or tissues of the hCD46 or C57BL/6 mice, a low level of MV RNA was detected in the spleen and inguinal lymph nodes of rMV-infected hCD46 mice (401±643 and 109±114 copies/ng RNA, respectively) but not C57BL/6 mice 9 days after infection, and it was no longer detectable in all the samples 15 days after infection ( and Table S1). This finding implies that limited recombinant virus replication occurs in hCD46 mice. In addition, the hCD46-dependent infection by recombinant virus was also validated by flow cytometry. At 24 h post-infection, the percentage of rMV-infected CD3-gated T cells and B220-gated B cells for hCD46 mice were significantly higher than the percentages in C57BL6 mice (p<0.05; ), suggesting the susceptibility of hCD46 mice to recombinant MV infection.
Figure 2. The infection and replication of recombinant MV in CD46 transgenic mice. (A) Groups of human CD46 transgenic C57BL/6 mice (hCD46; n = 3) and wild-type C57BL/6 mice (B6; n = 2) were infected with 1 ×106 pfu of rMV by ip injection. The mouse tissue and blood cells were harvested 9 days after infection, and the MV gene copy number was determined by quantitative RT-PCR and presented as MV RNA copies per ng total RNA. (B) Spleen cells from hCD46 or B6 mice were infected with rMV (MOI = 3) in vitro, and the rMV infected cells were detected by flow cytometry with FITC-conjugated anti-MV nucleoprotein monoclonal antibody. The mean and standard deviation of MV-infected cells in the CD3+ and B220+ cell populations from 3 experiments are shown.
The induction of T-cell immune responses by a MV-vectored dengue vaccine
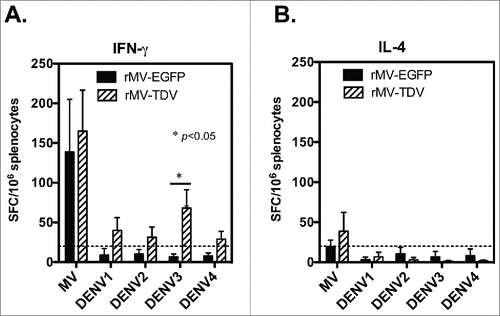
To understand the T-cell responses, we immunized hCD46 mice with either rMV-EGFP (2 × 106 pfu) or rMV-TDV (1 ×106 pfu of rMV-DV13 and 1 × 106 pfu of rMV-DV24) by ip injection. In a pilot study, the peak of specific responses appeared after the primary but not secondary rMV-TDV immunization (Fig. S1). Therefore, the spleens of immunized mice were removed 9 days after a single immunization for T-cell responses. The specific IFN-γ and interleukin (IL)-4 responses were quantitated by ELISPOT assay under the stimulation with dengue ED3 peptide mixtures or MV-infected cell lysates (Advanced Biotechnologies Inc., Columbia, MD). Comparable levels of MV-specific IFN-γ were detected in response to all the recombinant viruses (). The numbers of MV-specific IFN-γ-producing cells were 143 ± 133 and 165 ± 107 spot-forming cells (SFC) per million spleen cells for rMV-EGFP and rMV-TDV-infected hCD46 mice, respectively. In contrast to the MV-specific T-cell responses, only rMV-TDV- but not rMV-EGFP-infected hCD46 mice developed specific IFN-γ production against dengue ED3 and showed a significance of a higher DENV-3 specific IFN-γ responses compared to rMV-EGFP-infected mice (p < 0.05 by Mann-Whitney t-test; n=4). Among four serotypes of dengue viruses, the DENV-3 specific response (68 ± 46 SFC) was highest in comparison with DENV-1, 2 or 4 specific responses (40 ± 32, 31 ± 26 and 29±20 SFC, respectively; n = 4). Additionally, the low or undetectable ED3-specific IL-4 production observed in all immunized mice () for even the normal mitogenic IL-4 responses suggested that the recombinant MV-vectored dengue vaccine induced a Th1-biased response.
Figure 3. The induction of both MV- and DENV-specific T-cell responses by the MV-vectored dengue vaccine. Groups of hCD46 mice (n = 4) were immunized with 2 × 106 pfu of rMV-EGFP or rMV-TDV (1 × 106 pfu of rMV-DV13 and 1 × 106 pfu of rMV-DV24) by ip injection. Spleen cells were harvested 9 days after a single immunization for the detection of IFN-γ (A) and IL-4 (B) production specific to the MV or ED3 pooled peptides of each serotype by ELISPOT assay. The results are presented as the mean and SD of spot forming cells (SFC) per million splenocytes. Mann-Whitney t-tests were used for statistical analyses. The dashed line indicates the cutting-off of 2 times the background (medium alone).
Induction of antibody responses by the MV-vectored dengue vaccine
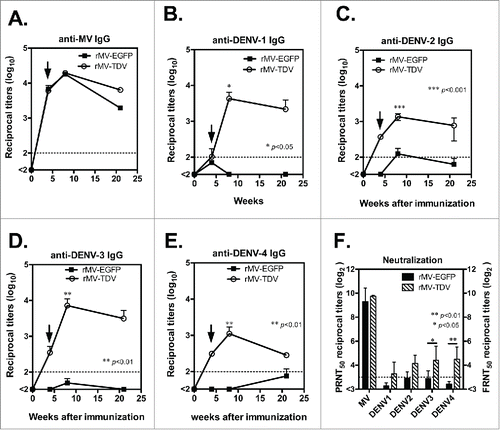
We further examined the specific IgG and neutralizing antibody responses that were elicited by the MV-vectored dengue vaccine. Groups of hCD46 mice (n = 4) were immunized ip with either rMV-EGFP or rMV-TDV and boosted 4 weeks later. IgG titers and neutralizing antibody titers for dengue viruses and MV were measured by ELISA and neutralization assay. A similar MV-specific IgG titer was observed in all immunized mice, regardless of the type of recombinant virus in use (). After a single immunization, MV-specific IgG was induced, reached to the peak after boosting and lasted for 20 weeks following immunization. By contrast, mice infected with rMV-TDV developed a significantly higher dengue-specific antibody response than rMV-EGFP-immunized mice 8 weeks after immunization (; p<0.05, p<0.001, p<0.01 and p<0.01 for DENV-1, 2, 3 and 4, respectively; n = 4), but the significance between rMV-TDV- and rMV-EGFP-immunized mice disappeared following the waning of IgG titers 21 weeks post immunization (n = 4). There was no difference between antibody titers against 4 serotypes of dengue virus in rMV-TDV-immunized hCD46 mice. In addition, neutralization titers were higher in rMV-TDV- than rMV-EGFP-immunized mice, with a significant difference in DENV-3 and DENV-4-specific neutralizing antibody responses (p<0.05 and p<0.001 for DENV-3 and 4, respectively; ; n = 4). The mean of FRNT50 for 4 serotypes of dengue virus in rMV-TDV-immunized hCD46 mice was ≥10 (10 ± 1.9, 18 ± 1.6, 21 ± 2.3 and 22 ± 2.1 for DENV-1, 2, 3 and 4, respectively). For the MV-specific neutralizing antibody, similar neutralizing titers were observed in both rMV-EGFP and rMV-TDV-infected mice.
Figure 4. The antibody responses elicited by the MV-vectored dengue vaccine. Groups of hCD46 mice (n = 4) were immunized with either 2 × 106 pfu of rMV-EGFP or rMV-TDV by ip injection and boosted 4 weeks later (indicated by arrow). The reciprocal titers of specific IgG to MV (A) and DENV-1 to 4 (B-E) were determined by ELISA. The results are presented as the mean and SD of specific IgG titers. (F) The neutralizing antibody titers against parental MV and the 4 serotypes of DENV were determined by plaque reduction and FRNT, respectively. The reciprocal titer leading to a ≥50% reduction (PRNT50 or FRNT50) is shown. The detection limits for the IgG ELISA or neutralization assay are indicated with a dashed line. Mann-Whitney t-tests were used for statistical analyses, and the significance compared with the rMV-EGFP control is shown.
The effect of pre-existing immunity on the immunogenicity of MV-vectored dengue vaccine
One challenge associated with the recombinant MV-vectored dengue vaccine is pre-existing immunity. Even in infants, maternal antibodies are still present, and they interfere with the efficacy of vaccinations.Citation29 Therefore, it is necessary to investigate the effect of pre-existing MV and DENV immunity. We chose DENV-3 as a model for evaluating pre-existing immunity to DENV because DENV-3 ED3 contains more T-cell epitopes than other serotypes in C57BL/6 genetic background (Fig S2). Groups of hCD46 mice were pre-immunized by ip injection with rMV-EGFP (1 × 106 pfu), DENV-3/H-087 (1 × 106 FFU) or PBS as a naïve control, prior to vaccination. After three weeks, all the mice were immunized with rMV-TDV by ip injection and boosted 4 weeks later. The T-cell responses to both MV and DENV ED3 were measured by ELISPOT. A comparably high level in the MV-specific IFN-γ response was observed in pre-immunized mice and naïve controls, suggesting that the MV-specific T-cell response was not affected by pre-existing immunity to MV (). In contrast to the similar T-cell responses to MV, a significantly higher IFN-γ response to DENV-3 was induced in mice that were pre-immunized with DENV-3 than in naïve mice (p<0.001; n = 4), but not to other serotypes.
Figure 5. The effect of pre-existing immunity on the immune responses induced by the MV-vectored dengue vaccine. The immunization schedule is shown at the top of the figure. Groups of hCD46 mice were pre-infected with 1 ×106 pfu of rMV-EGFP, 1 ×106 pfu of DENV-3 or a PBS-treated naïve control. After three weeks, all mice were immunized with rMV-TDV by ip injection and boosted 4 weeks later. (A) Specific T-cell responses to either MV or DENV were measured one week after a single immunization by ELISPOT assay and presented with the mean and SD of SFC per million spleen cells. The specific IgG titers to MV (B) or DENV-1 to 4 (C-F) were determined by ELISA. The detection limits for the ELISPOT assay or ELISA are indicated in a dashed line. Mann-Whitney t-tests were used for statistical analyses, and the significance compared with the naïve control is shown.
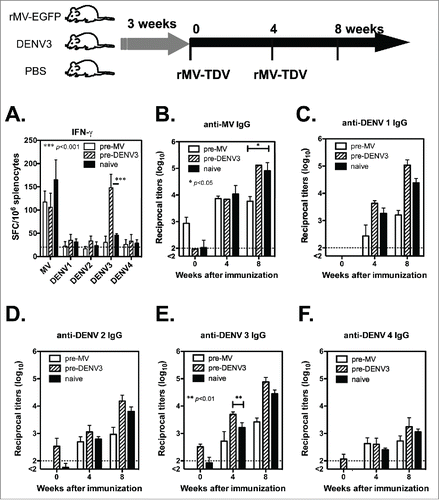
The pre-existing IgG titers were also determined prior to immunization; MV-specific IgG was only positive in mice that were pre-immunized with rMV-EGFP (), and DENV-specific IgG was also detectable in mice that were pre-immunized with DENV-3 (). After rMV-TDV vaccination, no difference was observed in the induction of MV-specific IgG responses between groups; however, mice that were pre-immunized with rMV-EGFP had significantly lower MV-specific IgG peak titers than those in the naïve control (p<0.05; n = 4). Similar results consisting of lower peak titers in mice that were pre-immunized with rMV-EGFP were also observed in DENV-specific IgG responses, although the difference was not statistically significant. Unlike the mice with pre-immunity to MV, there was no difference in the MV- and DENV-specific IgG titers between DENV-3 pre-immunized mice and the naïve control, except that significantly higher anti-DENV-3 IgG titers were observed in mice that had been pre-immunized with DENV-3 than in the naïve control (p<0.01; n = 4).
DENV-2 clearance in the MV-vectored dengue vaccine-immunized mice
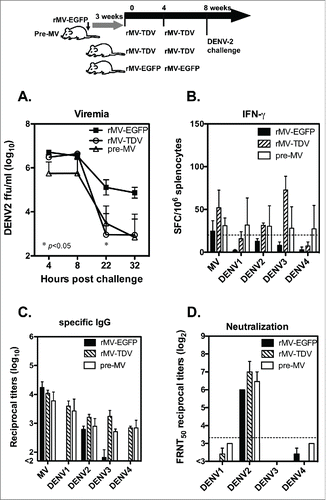
To test the potency of dengue vaccine, groups of hCD46 mice that were either pre-immunized with rMV-EGFP or not were vaccinated with rMV-TDV as described previously, and mice immunized with rMV-EGFP alone were used for the controls. All immunized mice were boosted 4 weeks later. Four weeks after the last immunization, the mice were introduced a viremia with an ip injection of 5 × 107 DENV-2-infected K562 cells because the infection efficiency is very low for non-mouse adapted strain of DENV-2. Their viremia was detected as being up to 106 focus-forming units (FFU)/ml at 4 h after the injection, and it gradually decreased (). A significantly lower viral load was observed in mice that were immunized with rMV-TDV 22 h post injection than that of the rMV-EGFP control (p<0.05; n=3). The comparable viremia observed in rMV-TDV-immunized mice that were either pre-immunized with MV or not immunized suggests that the presence of MV-specific immunity did not affect the protection conferred by the MV-vectored dengue vaccine. The post-injection T-cell and antibody responses were also measured, and the DENV-2-specific IFN-γ responses increased relative to those that occurred before the injection. However, the strongest IFN-γ responses were still targeted to DENV-3 in the rMV-TDV-immunized mice, similar to the pattern observed prior to the injection (). The IgG titers in response to MV and the 4 DENV serotypes post-injection were comparable to those that were observed before the injection, except for the increased DENV-2-specific IgG titers in rMV-EGFP-immunized mice (). The DENV-2 neutralizing antibody titers were significantly higher than other serotypes in all the groups after the DENV-2 injection (). Although higher neutralizing titers were detected in response to DENV-2 in rMV-TDV-immunized mice, no significant difference was observed relative to those of other groups.
Figure 6. The protective efficacy of the MV-vectored dengue vaccine was evaluated in terms of DENV-2 viremia in mice. The immunization and challenge schedule is shown at the top of the figure. Two groups of hCD46 mice (n = 3), with one pre-infected with 1 × 106 pfu of rMV-EGFP at 3 weeks prior to vaccination and one naïve group, were immunized with rMV-TDV by ip injection. As a control, hCD46 mice (n = 3) were immunized with 2 × 106 pfu of rMV-EGFP by ip injection. All the mice were boosted 4 weeks later with the same vaccine and introduced a viremia via an ip inoculation of 5 × 107 DENV-2/16681-infected K562 cells at week 8. (A) Plasma viremia titers from individual mice were determined by viremia assay and are represented as the mean and SD. (B) Spleen cells were harvested 1 month post-viremia for the detection of DENV ED3-specific IFN-γ production by ELISPOT assay. The sera collected at 1 month post-viremia were used to detect the ED3-specific IgG responses by ELISA (C) and the neutralizing titers to DENV-1 to 4 by FRNT (D).
Discussion
Advances in recombinant virus technology have caused the live attenuated MV vaccine to become a safe and immunogenic delivery vector for the prevention of MV and other infectious diseases. This 2-in-1 formulation and the advantages of the well-established manufacturing and transportation system make the MV-vectored vaccine more attractive for mass vaccination. An MV-vectored ED3-expressing tetravalent dengue vaccine was reported by Brandler et al, and it was evaluated in a mouse model.Citation26 The positive response of neutralizing titers against 4 serotypes of dengue viruses revealed excellent immunogenicity; however, the T-cell responses, including IFN-γ production, were not mentioned. Although T-cell responses as elicited by MV-vectored HIV or chikungunya virus vaccines have been reported,Citation22,30 the ED3-specific T-cell responses induced by the MV-vectored tetravalent dengue vaccine and the effect of pre-existing immunity in T-cell responses induced by tetravalent dengue vaccine are still unknown. Therefore, our results provide an extended understanding of the T-cell responses induced by the MV-vectored tetravalent dengue vaccine. Similar to findings in the MV-vectored HIV vaccine,Citation22 our data demonstrated that both MV- and DENV-specific T-cell responses were induced by the MV-vectored tetravalent dengue vaccine and biased toward IFN-γ responses, which is important for protection against dengue infection. In addition, MV-specific IFN-γ responses were higher than the DENV-specific IFN-γ responses, similar to the finding for the MV-vectored HIV vaccine.Citation22 Interestingly, we noticed that most DENV-specific T-cell responses were targeted to DENV-3, possibly because there are more T-cell epitopes in DENV-3 ED3 than in the other serotypes (Fig. S2).
One function of CD4+ T cells is to help stimulate B cells to produce antibodies. Correspondingly, immunization with the MV-vectored tetravalent dengue vaccine induced both MV- and DENV-specific IgG responses and lasted for over 4 months after the last vaccination (), suggesting that the MV-vectored dengue vaccine elicited long-lasting antibody responses similar to those of other MV-vectored vaccines.Citation31 Neutralizing antibody has been reported to play an important role in protecting against dengue infection. Even though ED3 is not the major target for human neutralizing antibodies after dengue infection, the high serotype specificity for ED3-specific neutralizing antibodies Citation32 also suggests ED3 as a good target for the induction of a balanced immune response by the tetravalent dengue vaccine. All four serotype-specific neutralizing antibody titers were induced by the MV-vectored tetravalent dengue vaccine, and the neutralizing titers for DENV-3 and 4 were significantly higher than those induced by the rMV-EGFP control. The lower response in neutralizing titers against DENV-1 differs from Brandler's results in which 83%, 70%, 58% and 47% of mice showed neutralizing titers of FRNT50 >10 for DENV-1, 2, 3 and 4, respectively.Citation26 This discrepancy could have occurred because the strain of DENV-1 virus (DENV-1/Hawaii) that we used is different from the strain (FGA/NA d1d) that they used.
The presence of pre-immunity is an important concern in the application of the MV-vectored dengue vaccine. A previous report demonstrated that pre-immunity to MV did not affect the antibody responses in MV-vectored HIV vaccine-immunized mice and monkeys.Citation22 Our data from the MV-vectored tetravalent dengue vaccine also confirmed that IgG production was successfully induced by all dengue virus serotypes; however, the peak titers were 10-fold lower in the MV-pre-immunized mice than in the naïve control. The boosting effect, which was observed in the naïve control but not in the pre-immunized mice, resulted in lower peak IgG titers in pre-immunized mice. This discrepancy may be caused by different mouse models used in our and others studies. Notably, type-I IFN deficiency is required to support MV efficient replication in hCD46 transgenic mice,Citation33 and a lower replication efficiency of recombinant MV was observed in our immunocompetent mouse model than in others type-I IFN deficient mouse model (∼103 compared with ∼105 copies of MV RNA/ng total RNA in the spleen; ).Citation31 We also need to notify that the virus used in was parental rMV but not MV-vectored dengue vaccine candidates and there might be different between these 2 strains of recombinant MV. By contrast, mice with pre-immunity to DENV-3 demonstrated a DENV-3 dominant T-cell response. However, the antibody responses to MV and DENV were comparable between the DENV-3 pre-immunized mice and the naïve control.
The major obstacle to dengue vaccine development is the lack of a reliable animal model for the evaluation of protective efficacy, and previous studies have not revealed the protection of MV-vectored dengue vaccine. In this study, we used an alternative artificial dengue viremia model via ip injection of DENV-infected K562 cells to induce a viremia, which is associated with the neutralizing antibody titers.Citation34,35 DENV-2 was used for the virus clearance test because of its clinical importance and for the lower efficacy observed in CYD dengue vaccine clinical trials. A significantly lower but detectable viremia observed in mice that were immunized with the rMV-TDV vs. the MV-EGFP control suggests that the MV-vectored dengue vaccine provides partial protection against DENV-2. The DENV-2-specific neutralizing titers in mice that were immunized with the MV-vectored dengue vaccine were boosted higher after the virus injection than those in the rMV-EGFP control, suggesting that a recalled antibody response was observed. This finding is consistent with those of other studies.Citation26,36 Surprisingly, the DENV-2 ED3-specific T-cell responses were still lower than the dominant DENV-3 specific T-cell responses after the challenge, suggesting that the DENV-2 ED3 region lacks a dominant T-cell epitope and highlighting the role of T cells that are targeted to other viral proteins such as NS3 Citation37 during the enhancement of DENV-2-specific neutralizing titers.
In conclusion, our studies have demonstrated the potential for MV-vectored dengue vaccine to induce both MV- and dengue-specific T-cell and antibody responses efficiently, even in individuals who received the MV vaccination or those who were pre-infected with dengue viruses. Despite the lower replicative efficiency of MV in mice, the immunity elicited by the MV-vectored dengue vaccine still provides partial protection against DENV-2 infection. This study contributes to an understanding of the MV-vectored dengue vaccine and the application of the MV vector in the future.
Materials and methods
Ethics statement
C57BL/6 mice were obtained from the National Laboratory Animal Center (Taipei, Taiwan), and human CD46 transgenic mice that were purchased from the Jackson Laboratory (Bar Harbor, Maine USA) were maintained in the animal facility of the National Health Research Institutes. The protocol was approved by the Animal Committee of the National Health Research Institutes (Protocol No: NHRI-IACUC-098097-A) and performed according to their guidelines. For anesthesia and euthanasia, the mice were subjected to 2–3% isoflurane and CO2 inhalation, respectively.
Cloning recombinant measles virus-vectored antigenomic cDNA
A Moraten strain of MV from Dr. Diane E. Griffin was used to clone infectious cDNA. In brief, the total RNA from MV-infected Vero cells was isolated and reverse-transcribed with a Superscript III kit (Life Sciences). Full-length MV antigenomic cDNA was obtained by PCR by using a panel of 26 forward and 23 reverse primer sets (Table S2). The mismatched nucleotides were corrected to the cDNA that was identical to the published sequence of the Moraten MV strain in GenBank (accession number: AF266287). To express the foreign gene, an additional transcriptional unit (ATU) with unique restriction enzyme sites was inserted into the intergenic region between the P and M genes. Delta virus ribozyme sequences and a T7 RNA polymerase terminator signal were added to the 3′-end of the MV antigenomic cDNA to allow the MV RNA transcript to be cleaved at a precise position. The recombinant MV antigenomic cDNA was inserted into the pVax-1 expression vector (pMV, as shown in ). A DNA fragment encoding the EGFP from a commercial plasmid (N3; Life Sciences) or tandem repeats of ED3 (please see for the amino acid sequence), which contain either one copy of DENV-1 and 3 ED3 (pMV-DV13) or one copy of DENV-2 and 4 ED3 (pMV-DV24) with the leading peptide of the immunoglobulin λ chain and a linker (GGGGS x3), were subcloned into the ATU site of pMV to form pMV-EGFP, pMV-DV13 and pMV-DV24 plasmids, respectively.
Generation of the recombinant measles viruses and the vectored dengue vaccine
For MV rescue, 293-hSLAM cells that stably expressed human CD150 (SLAM) were cultured in DMEM supplemented with 10% FBS in 6-well plates until they reached 90% confluence. These cells were infected with vTF7-3 (a gift from Dr. Moss) at an MOI of 0.5 for 1 h prior to transfection. A DNA mixture of infectious clones and 3 supporting plasmids (pCA7-N, pCA7-P and pCA7-9301B-L from Dr. Makoto Takeda) at ratios of 5: 1: 1.5: 1, respectively,Citation38 were co-transfected into vTF7-3-infected 293-hSLAM cells with Lipofectamine 2000 (Invitrogen). After 4 h, the medium was replaced with complete medium containing 100 μg/ml of 1-β-D-arabinofuranosyl cytosine (AraC; Sigma) to inhibit vTF7-3 replication. Typical MV syncytial cells were observed 2–3 days later, and recombinant MV (rMV, rMV-EGFP, rMV-DV13 and rMV-DV24 were rescued from the infectious clones of pMV vector, pMV-EGFP, pMV-DV13 and pMV-DV24, respectively) was continuously amplified in Vero cells and purified by plaque purification. For the preparation of the tetravalent MV-vectored dengue vaccine, Vero cells that were infected with rMV-DV13 or rMV-DV24 were harvested and lysed by freeze-thaw. After centrifugation, the supernatants of the lysed cells were collected and titrated for future use.
Western blot
Proteins produced from recombinant MV-vectored virus-infected Vero cells were analyzed by Western blotting. In brief, subconfluent Vero cells were infected with recombinant viruses at an M.O.I. of 0.02 for 1 h, and the medium was then replaced with complete DMEM-10% FCS for culture. After 72 h, the supernatant and cell lysate were collected separately and stored at −20°C. Samples containing 1 μg of total cellular protein or 30 μl of culture supernatant were loaded into the wells of a 4–20% gradient SDS-PAGE for electrophoresis. The proteins in the gel were transferred onto a nitrocellulose membrane and blotted with a monoclonal anti-dengue antibody with a strong specificity to DENV-1 ED3 and a weak cross-reactivity to DENV-2 ED3, but not to other serotypes (GeneTex), followed by a horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody (Pharmacia) and then developed by adding the substrate. In addition, a monoclonal antibody (Chemicon) that was specific to a housekeeping actin protein was used as a control.
Immunization and evaluation of the potency for dengue virus clearance
Groups of 6–8-week-old hCD46 or wild-type C57BL/6 mice were immunized ip with rMV-TDV, a mixture containing 1 × 106 pfu of both rMV-DV13 and rMV-DV24, or 2 × 106 pfu of rMV-EGFP for the control. The mice were boosted with the same recombinant viruses and dosed 4 weeks later. In the virus clearance experiments, the mice were introduced a viremia 4 weeks after the last immunization by intraperitoneal injections of 5 × 107 K562 cells that were infected with DENV-2/16681 (containing 5 × 107 FFU at inoculation time), as published elsewhere.Citation34
Intracellular staining of MV N protein
Fresh spleen cells (5 × 105) from hCD46 and C57BL/6 mice were cultured in 96-well round-bottomed plates at 37°C in a 5% CO2 incubator and infected with rMV (parental rMV with no insert) at an MOI of 3 or medium alone for 1 h, and the medium was then replaced with fresh RPMI complete medium for continuous culture. The cultured cells were harvested to analyze the MV-infected cells by flow cytometry. In brief, the cells were washed with staining buffer (PBS with 0.5% BSA) and blocked with anti-CD16 and anti-CD32 antibodies (BD Bioscience) for 20 min at 4°C. The surface markers were stained with anti-mouse CD3 and B220 conjugated with PE and APC (BD Bioscience), respectively, for 20 min at 4°C. After washing, the cells were fixed and permeabilized with Cytofix/Cytoperm buffer (BD Bioscience) and stained with FITC-conjugated anti-MV N protein monoclonal antibody (Chemicon) for 20 min at 4°C. Finally, the cells were resuspended in 0.5 ml of fixing solution (eBioscience) and assayed by FACSCaliber. The data were analyzed with FlowJo software (Treestar), and the CD3+ T-cell or B220+ B-cell populations were gated and presented.
Quantitative RT-PCR
Total RNA from the tissue homogenate and blood cells was isolated with an RNA isolation kit (Invitec), reverse-transcribed to cDNA by Superscript III (Invitrogen) and stored at −80°C until use. MV cDNA was detected by quantitative PCR as previously described.Citation28 In brief, the MV nucleoprotein (N) gene was amplified (Applied Biosystems Prism 7900) by using TaqMan primers and probes. The copy number was determined from the standard curve of 101 to 106 copies of a DNA plasmid encoding the MV N gene. The data were normalized to the amount of total RNA, and the results are expressed as follows: the number of copies of MV RNA per ng of total RNA.
ELISA
ED3-specific IgG titers were determined by ELISA as previously documented.Citation39 Four serotypes of rED3 were all expressed in E. coli system (BL21-DE3) and purified by affinity column. The endotoxin was removed by a polymyxin B agarose column and the level of endotoxin in the final product was below 3 EU/mg by Limulus amebocyte lysate assay. Purified recombinant ED3 was coated onto 96-well plates overnight and blocked with 2% bovine serum albumin (BSA) in PBS for 2 h at RT. The sera obtained by submandibular blood collection were diluted with 3-fold serial dilutions (starting at 1:100) and added to the wells. Bound IgG was detected with HRP-conjugated goat anti-mouse IgG antibody (GE Amersham). After the addition of 3,3′,5,5′-tetramethylbenzidine (TMB; KPL), the absorbance was measured with an ELISA reader at 450 nm. ELISA reciprocal titers were defined as the serum dilutions that gave optical density (OD) values that were 2-folds higher than the background. The serum dilution was obtained from the titration curve by interpolation. If the OD value was less than 2-folds of the background at the starting dilution, a titer of 33 was used for the calculations.
Neutralization tests and viremia assay
A traditional plaque reduction neutralization test (PRNT) was used for MV as previously described.Citation40 In brief, the 3-fold serially diluted sera (starting at 1:10) were pre-mixed with the Moraten strain of MV and added to a monolayer of Vero cells in 6-well plates in triplicate. After virus adsorption, an overlay medium containing 1% FBS and 0.8% methylcellulose in DMEM was added. After 4 days of infection, the cells were fixed for 15 min in 3.7% formaldehyde/PBS and stained with crystal violet. The plaques were counted, and the neutralizing antibody titer PRNT50 was calculated as the reciprocal titer that produced a 50% plaque reduction when compared with virus alone.
A modified focus reduction neutralization test (FRNT) was used for dengue viruses as described previously.Citation41 Sera were diluted with 2-fold serial dilutions (starting at 1:8), and the sera were heat-inactivated prior to testing. A monolayer of BHK-21 cells in 24-well plates was inoculated with virus (DENV-1/Hawaii, DENV-2/16681, DENV-3/H-087, and DENV-4/H241 amplified in Vero cells; gifts from Dr. Yi-Ling Lin) that had been pre-mixed at 4°C overnight with sera samples to a final volume of 0.5 ml. Viral adsorption was allowed to proceed for 3 h at 37°C. An overlay medium containing 2% FBS and 0.8% methylcellulose in DMEM was added after the adsorption. After 72 h of infection, the cells were fixed for 15 min in 3.7% formaldehyde/PBS, permeabilized with 0.1% Nonidet P40/PBS for 15 min and blocked with 3% BSA/PBS for 30 min. Infected cells were detected with a monoclonal anti-dengue antibody (2H2 for recognizing 4 serotypes of DENV), and they were detected with an HRP-conjugated secondary antibody (GE Amersham) and visualized using TMB (KPL). The FFUs were counted, and the neutralizing antibody titer FRNT50 was calculated as the reciprocal titer that produced a 50% reduction in FFUs when compared with the virus alone.
To assess viremia, blood was drawn from the mice after the injection and placed in tubes containing the anticoagulant EDTA. Plasma samples were diluted in 10-fold serial dilutions and added to monolayers of BHK-21 cells in 24-well plates to incubate for 3 h at 37°C for viral adsorption. Infected cells were detected as described above, and the FFUs were counted and represented as FFU/ml.
Enzyme-linked immunospot (ELISPOT) assay
The production of IFN-γ and IL-4 by mouse spleen cells was measured by ELISPOT assay, as described elsewhere.Citation28 In brief, multiscreen plates (Millipore) were coated with 2 μg/ml of anti-mouse IFN-γ or anti-mouse IL-4 antibody (all from BD PharMingen). After the plates were washed and blocked with culture medium, 5 × 105 or 1 × 105 fresh mouse splenocytes were added along with 2.5 μg/ml of ED3 peptide mixtures (a panel of 16 15-mer peptides with 9 overlapping amino acids; Table S3) for each serotype or 5 μg/ml concanavalin A (Sigma) as a positive control. After 40 h of incubation, the plates were washed and incubated with a biotinylated antibody against IFN-γ or IL-4 (2 μg/ml, BD Bioscience) for 2 h at 37°C. After the plates were washed, HRP-conjugated avidin (Research Laboratory Inc.) was added and incubated for 1 h at 37°C, and the assays were developed with AEC solution (Sigma). The reaction was stopped with tap water, and the plates were analyzed by using an ImmunoSpot reader with ImmunoSpot software, version 5.0.3 (CTL, Cleveland, OH). The data are presented as the number of spot-forming cells (SFCs)/106 splenocytes.
Statistical analyses
All statistical analyses were performed by using 2-way ANOVA with the Bonferroni post-test (GraphPad Prism), unless otherwise specified. Differences with a p value of less than 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Material.zip
Download Zip (294.7 KB)Acknowledgments
We are especially grateful to Dr. Diane E. Griffin, Dr. Makoto Takeda, Dr. Yi-Ling Lin and Dr. B. Moss for providing the viruses and plasmids used in this study.
Funding
This study was supported by grants 01A1-IVPP29 (C.H.P.) from the National Health Research Institutes and NSC99-2320-B-400-004-MY3 (C.H.P.) and MOST103-2320-B-400-MY3 (C.H.P.) from the Ministry of Sciences and Technology, Taiwan.
References
- Thomas SJ, Endy TP. Vaccines for the prevention of dengue: development update. Hum Vaccin 2011; 7:674-84; PMID:21508679; http://dx.doi.org/10.4161/hv.7.6.14985
- Ramasamy R, Surendran SN. Global climate change and its potential impact on disease transmission by salinity-tolerant mosquito vectors in coastal zones. Front Physiol 2012; 3:198; PMID:22723781; http://dx.doi.org/10.3389/fphys.2012.00198
- Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg 1952; 1:30-50; PMID:14903434
- Innis BL. Antibody responses to dengue virus infection. New York: CAB Int., 1997.
- Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science 1988; 239:476-81; PMID:3277268; http://dx.doi.org/10.1126/science.3277268
- Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol 2008; 62:71-92; PMID:18429680; http://dx.doi.org/10.1146/annurev.micro.62.081307.163005
- Wahala WM, Silva AM. The human antibody response to dengue virus infection. Viruses 2011; 3:2374-95; PMID:22355444; http://dx.doi.org/10.3390/v3122374
- Rothman AL. T lymphocyte responses to heterologous secondary dengue virus infections. Ann N Y Acad Sci 2009; 1171 Suppl 1:E36-41; PMID:19751400; http://dx.doi.org/10.1111/j.1749-6632.2009.05055.x
- Nguyen TH, Lei HY, Nguyen TL, Lin YS, Huang KJ, Le BL, Lin CF, Yeh TM, Do QH, Vu TQ, et al. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J Infect Dis 2004; 189:221-32; PMID:14722886; http://dx.doi.org/10.1086/380762
- Whitehead SS, Falgout B, Hanley KA, Blaney JE, Jr., Markoff L, Murphy BR. A live, attenuated dengue virus type 1 vaccine candidate with a 30-nucleotide deletion in the 3′ untranslated region is highly attenuated and immunogenic in monkeys. J Virol 2003; 77:1653-7; PMID:12502885; http://dx.doi.org/10.1128/JVI.77.2.1653-1657.2003
- Guirakhoo F, Weltzin R, Chambers TJ, Zhang ZX, Soike K, Ratterree M, Arroyo J, Georgakopoulos K, Catalan J, Monath TP. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J Virol 2000; 74:5477-85; PMID:10823852; http://dx.doi.org/10.1128/JVI.74.12.5477-5485.2000
- Putnak R, Fuller J, VanderZanden L, Innis BL, Vaughn DW. Vaccination of rhesus macaques against dengue-2 virus with a plasmid DNA vaccine encoding the viral pre-membrane and envelope genes. Am J Trop Med Hyg 2003; 68:469-76; PMID:12875299
- Hermida L, Bernardo L, Martin J, Alvarez M, Prado I, Lopez C, Sierra Bde L, Martinez R, Rodriguez R, Zulueta A, et al. A recombinant fusion protein containing the domain III of the dengue-2 envelope protein is immunogenic and protective in nonhuman primates. Vaccine 2006; 24:3165-71; PMID:16490289; http://dx.doi.org/10.1016/j.vaccine.2006.01.036
- Mota J, Acosta M, Argotte R, Figueroa R, Mendez A, Ramos C. Induction of protective antibodies against dengue virus by tetravalent DNA immunization of mice with domain III of the envelope protein. Vaccine 2005; 23:3469-76; PMID:15837370; http://dx.doi.org/10.1016/j.vaccine.2004.12.028
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 2012; 380:1559-67; PMID:22975340; http://dx.doi.org/10.1016/S0140-6736(12)61428-7
- Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384:1358-65; PMID:25018116; http://dx.doi.org/10.1016/S0140-6736(14)61060-6
- Modis Y, Ogata S, Clements D, Harrison SC. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol 2005; 79:1223-31; PMID:15613349; http://dx.doi.org/10.1128/JVI.79.2.1223-1231.2005
- Hatch S, Endy TP, Thomas S, Mathew A, Potts J, Pazoles P, Libraty DH, Gibbons R, Rothman AL. Intracellular cytokine production by dengue virus-specific T cells correlates with subclinical secondary infection. J Infect Dis 2011; 203:1282-91; PMID:21335561; http://dx.doi.org/10.1093/infdis/jir012
- Yauch LE, Prestwood TR, May MM, Morar MM, Zellweger RM, Peters B, Sette A, Shresta S. CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J Immunol 2010; 185:5405-16; PMID:20870934; http://dx.doi.org/10.4049/jimmunol.1001709
- Zellweger RM, Miller R, Eddy WE, White LJ, Johnston RE, Shresta S. Role of humoral versus cellular responses induced by a protective dengue vaccine candidate. PLoS Pathog 2013; 9:e1003723; PMID:24204271; http://dx.doi.org/10.1371/journal.ppat.1003723
- Moss WJ, Griffin DE. Global measles elimination. Nat Rev Microbiol 2006; 4:900-8; PMID:17088933; http://dx.doi.org/10.1038/nrmicro1550
- Lorin C, Mollet L, Delebecque F, Combredet C, Hurtrel B, Charneau P, Brahic M, Tangy F. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J Virol 2004; 78:146-57; PMID:14671096; http://dx.doi.org/10.1128/JVI.78.1.146-157.2004
- Brandler S, Lucas-Hourani M, Moris A, Frenkiel M-P, Combredet C, Février M, Bedouelle H, Schwartz O, Desprès P, Tangy F. Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus. PLoS Negl Trop Dis 2007; 1:e96; PMID:18160988; http://dx.doi.org/10.1371/journal.pntd.0000096
- del Valle JR, Devaux P, Hodge G, Wegner NJ, McChesney MB, Cattaneo R. A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against measles virus challenge. J Virol 2007; 81:10597-605; PMID:17634218; http://dx.doi.org/10.1128/JVI.00923-07
- Blechacz B, Russell SJ. Measles virus as an oncolytic vector platform. Curr Gene Ther 2008; 8:162-75; PMID:18537591; http://dx.doi.org/10.2174/156652308784746459
- Brandler S, Ruffie C, Najburg V, Frenkiel M-P, Bedouelle H, Desprès P, Tangy F. Pediatric measles vaccine expressing a dengue tetravalent antigen elicits neutralizing antibodies against all four dengue viruses. Vaccine 2010; 28:6730-9; PMID:20688034
- Pinto AK, Daffis S, Brien JD, Gainey MD, Yokoyama WM, Sheehan KC, Murphy KM, Schreiber RD, Diamond MS. A temporal role of type I interferon signaling in CD8+ T cell maturation during acute West Nile virus infection. PLoS Pathog 2011; 7:e1002407; PMID:22144897; http://dx.doi.org/10.1371/journal.ppat.1002407
- Pan CH, Greer CE, Hauer D, Legg HS, Lee EY, Bergen MJ, Lau B, Adams RJ, Polo JM, Griffin DE. A chimeric alphavirus replicon particle vaccine expressing the hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques from measles. J Virol 2010; 84:3798-807; PMID:20130066; http://dx.doi.org/10.1128/JVI.01566-09
- Borras E, Urbiztondo L, Costa J, Batalla J, Torner N, Plasencia A, Salleras L, Dominguez A, Working Group for the Study of Measles Immunity in C. Measles antibodies and response to vaccination in children aged less than 14 months: implications for age of vaccination. Epidemio Infect 2012; 140:1599-606; PMID:22074684; http://dx.doi.org/10.1017/S0950268811002184
- Brandler S, Ruffie C, Combredet C, Brault JB, Najburg V, Prevost MC, Habel A, Tauber E, Despres P, Tangy F. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine 2013; 31:3718-25; PMID:23742993; http://dx.doi.org/10.1016/j.vaccine.2013.05.086
- Zuniga A, Wang Z, Liniger M, Hangartner L, Caballero M, Pavlovic J, Wild P, Viret JF, Glueck R, Billeter MA, et al. Attenuated measles virus as a vaccine vector. Vaccine 2007; 25:2974-83; PMID:17303293; http://dx.doi.org/10.1016/j.vaccine.2007.01.064
- Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology 2009; 392:103-13; PMID:19631955; http://dx.doi.org/10.1016/j.virol.2009.06.037
- Mrkic B, Pavlovic J, Rulicke T, Volpe P, Buchholz CJ, Hourcade D, Atkinson JP, Aguzzi A, Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J Virol 1998; 72:7420-7; PMID:9696838
- Yamanaka A, Konishi E. A simple method for evaluating dengue vaccine effectiveness in mice based on levels of viremia caused by intraperitoneal injection of infected culture cells. Vaccine 2009; 27:3735-43; PMID:19464557; http://dx.doi.org/10.1016/j.vaccine.2009.03.083
- Chiang CY, Hsieh CH, Chen MY, Tsai JP, Liu HH, Liu SJ, Chong P, Leng CH, Chen HW. Recombinant lipidated dengue-4 envelope protein domain III elicits protective immunity. Vaccine 2014; 32:1346-53; PMID:24486311; http://dx.doi.org/10.1016/j.vaccine.2014.01.041
- Konishi E, Kosugi S, Imoto J. Dengue tetravalent DNA vaccine inducing neutralizing antibody and anamnestic responses to four serotypes in mice. Vaccine 2006; 24:2200-7; PMID:16316713; http://dx.doi.org/10.1016/j.vaccine.2005.11.002
- Weiskopf D, Yauch LE, Angelo MA, John DV, Greenbaum JA, Sidney J, Kolla RV, De Silva AD, de Silva AM, Grey H, et al. Insights into HLA-restricted T cell responses in a novel mouse model of dengue virus infection point toward new implications for vaccine design. J Immunol 2011; 187:4268-79; PMID:21918184; http://dx.doi.org/10.4049/jimmunol.1101970
- Takeda M, Ohno S, Seki F, Hashimoto K, Miyajima N, Takeuchi K, Yanagi Y. Efficient rescue of measles virus from cloned cDNA using SLAM-expressing Chinese hamster ovary cells. Virus Res 2005; 108:161-5; PMID:15681066; http://dx.doi.org/10.1016/j.virusres.2004.09.002
- Leng C-H, Liu S-J, Tsai J-P, Li Y-S, Chen M-Y, Liu H-H, Lien S-P, Yueh A, Hsiao K-N, Lai L-W, et al. A novel dengue vaccine candidate that induces cross-neutralizing antibodies and memory immunity. Microbes Infect 2009; 11:288-95; PMID:19114121; http://dx.doi.org/10.1016/j.micinf.2008.12.004
- Pan CH, Jimenez GS, Nair N, Wei Q, Adams RJ, Polack FP, Rolland A, Vilalta A, Griffin DE. Use of Vaxfectin adjuvant with DNA vaccine encoding the measles virus hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques against measles virus. Clin Vaccine Immunol 2008; 15:1214-21; PMID:18524884; http://dx.doi.org/10.1128/CVI.00120-08
- Chiang CY, Pan CH, Hsieh CH, Tsai JP, Chen MY, Liu HH, Liu SJ, Chong P, Leng CH, Chen HW. Lipidated dengue-2 envelope protein domain III independently stimulates long-lasting neutralizing antibodies and reduces the risk of antibody-dependent enhancement. PLoS Negl Trop Dis 2013; 7:e2432; PMID:24069487; http://dx.doi.org/10.1371/journal.pntd.0002432
