ABSTRACT
In Korea, 2 inactivated Japanese encephalitis vaccines from Nakayama-NIH and Beijing-1 strain have been utilized to date. The 1st national standard for lot release testing of the JE vaccine was established in 2002. The 2nd national standard, established in 2007, is currently in use for JE vaccine (Nakayama-NIH strain) potency testing. However, the supply of this standard is expected to be exhausted by 2015, necessitating the establishment of a new national standard with quality equivalent to that of the existing standard. Quality control tests were performed to verify that the new standard candidate material was equivalent to that of the 2nd national standard, proving its appropriateness for potency testing of JE vaccine. In addition, based on the results of a collaborative study conducted among 4 institutions including Ministry of Food and Drug Safety, the potency of the new national standard material was determined to be 2.69 neutralizing-antibody titer (log10) per vial. Therefore, the newly established national standard material is expected to be used for the Japanese encephalitis vaccine lot release in Korea.
The Japanese encephalitis virus (JEV), which belongs to the genus Flavivirus of the family Flaviviridae, causes viral encephalitis, in the Asia-Pacific region.Citation1,2 Approximately 50,000 cases of viral encephalitis are reported every year, of which 10,000 result in death, of those who recover, up to 15,000 are left with severe complications such as permanent brain damage.Citation3,4 Three types of Japanese encephalitis (JE) vaccines for human use are currently in use: a mouse brain-derived and inactivated vaccine, a cell culture-derived inactivated vaccine, and a cell culture-derived live attenuated JE vaccine.Citation2 The mouse brain-derived inactivated JE vaccine is produced in several Asian countries, and also widely used within the Korean JE control program.Citation2,5 In 2013, the Korean Ministry of Food and Drug Safety (MFDS) commissioned the production of the 3rd national standard candidate material to a Korean JE vaccine manufacturer in order to replace the 2nd national standard to encourage national lot-release potency testing of the Nakayama-NIH strain–derived JE vaccine. The manufacturing method used in this exercise was same as that used during establishment of the 1st and the 2nd national standard material.Citation5 Following the manufacturing process, 3 thousand lyophilized vials were delivered to MFDS and some of them were used to qualify the material. The quality control (QC) items were selected on the basis of the testing criteria for the JE vaccine per the Minimum Requirements for Biological Products (MBP) of Korea as well as the World Health Organization requirements for JE vaccines (inactivated) for human use.Citation2,6 Although the potency testing was performed in accordance with the test method in the MBP, other assays except potency testing were accomplished by Korean Pharmacopoeia (KP).Citation7
shows the results of QC assessment during the manufacture of the candidate material. As shown, residual protein was detected at a concentration of 37.8 μg/mL in the final product, which was lower than the minimum criterion (80 μg/mL). The pH was 7.2 and moisture content was 0.8%, which fulfilled the specifications of the JE vaccine standard. In terms of sterility testing, no bacterial or fungal growth was observed in the final product, confirming that the candidate material was manufactured under sterile conditions. In addition, Foreign Insoluble Matter Test and Insoluble Particulate Matter Test for Injections satisfied to the respective specifications. Using storage conditions identical to those used for JE vaccine standard material, testing at −70 ± 10°C for up to 24 months resulted in no major changes in the potency of the candidate material. Additionally, the moisture content of the candidate material was similar to that of the 2nd standard material. (data not shown) The long-term stability of the standard material will continue to be monitored under the assurance program for national standards by MFDS.
Table 1. QC test results for the 3rd national JE vaccine standard candidate material.
This study was conducted over a-2 year period (approximately), which included manufacture and QC testing of the candidate material. During the 2nd year, a collaborative study was performed to confirm the potency of the candidate material. Four institutions, including MFDS and Korean JE vaccine manufacturers; Boryung Biopharma, Co., Green Cross, Corp., and Korea Vaccine, Co., participated in this collaborative study. As shown in , each of the institutions, randomly designated A to D, performed 10 iterations of plaque reduction neutralization (PRN) assays to compare the potency of the 2nd standard and the 3rd standard candidate material. Plaque reduction neutralization assays currently used for lot release testing of inactivated JE vaccine products measure the extent of plaque reduction in chicken embryo cells previously inoculated with serum from mice immunized with vaccine products or national standard, followed by exposure to a suspension of challenge virus.Citation6 The mean values of potency were 2.67 and 2.69 neutralizing-antibody (NT-Ab) titers (log10) for the 2nd and 3rd standard materials, respectively, while the intra-assay coefficient of variation (CV) values for the various institutions were 6.69 and 7.92 for the 2nd and 3rd standard materials, respectively. The proficiency of the 4 test institutions in terms of Z-scores were −0.05, 0.19, −0.25, and −0.15 for the 2nd national standard and 0.46, 0.60, −0.74, and −0.32 for the 3rd national standard candidate material, for institutions A, B, C, and D, respectively. As all values were within the range of ± 2, the proficiency between the institutions was determined to be satisfactory. As shown in , based on the results from the collaborative study between the 4 institutions, the assigned potency of the 3rd national standard candidate material was determined to be 2.69 NT-Ab titer (log10) per vial. The upper and lower limits of the 95% confidence interval of the mean were 2.76 and 2.62 for the 3rd standard candidate material and 2.72 and 2.61 for the 2nd standard material. Additionally, as the p-value was 0.53 (which exceeded 0.05) it was concluded that there was no significant difference between the 2nd national standard and the 3rd standard candidate material.
Figure 1. The assigned potency of the 3rd national JE vaccine standard candidate material The potency of the candidate material was determined to be 2.69 NT-Ab titer (log10) via statistical analysis of results of 40 PRN assays performed by 4 institutions. Geometric mean titers ± SDs were 2.67 ± 0.18 and 2.69 ± 0.21 for the 2nd and the 3rd standard material, respectively.
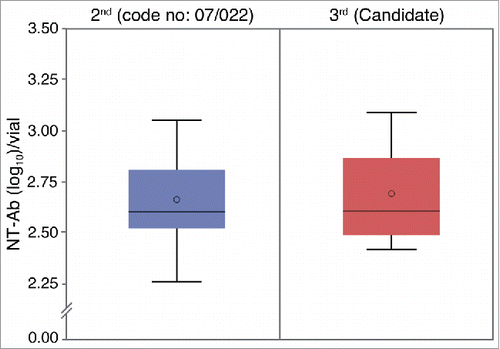
Table 2. Results of the collaborative study comparing the potency of the JE vaccine standard material.
QC tests of the 3rd national standard candidate material for the Nakayama-NIH strain-based JE vaccine revealed that the candidate material fulfilled the criteria described by the current official compendium of Korea. The collaborative study found that the potency of the 3rd national standard material (2.69 NT-Ab titer (log10) per vial) was equivalent to that of the 2nd national standard. Therefore, the tested candidate material was established as the national standard for potency testing of the JE vaccine following the 2nd national standard in Korea. However, there is a recent, active, international movement to transition from in vivo potency testing to more sensitive and reproducible in vitro tests.Citation8,9,10 Accordingly, the application of in vitro methods for lot release testing will require additional studies to estimate the in vitro potency values for the newly established 3rd national JE vaccine standard.
Abbreviations
| JEV | = | Japanese encephalitis virus |
| MFDS | = | Ministry of Food and Drug Safety |
| JE | = | Japanese encephalitis vaccine |
| MBP | = | Minimum Requirements for Biological products |
| PRN | = | plaque reduction neutralization |
| NT-Ab | = | neutralizing-antibody titer |
| QC | = | quality control |
| CV | = | coefficient of variation |
| KP | = | Korean Pharmacopoeia. |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Boryung Biopharma Co., Green Cross Corp. and Korea Vaccine Co. for their participation in the collaborative study and assistance with experiments.
Funding
This study was supported by a grant from the scientific research program (13171Bio309) at the National Institute of Food and Drug Safety Evaluation of the Ministry of Food and Drug Safety, Republic of Korea.
References
- Misra UK, Kalita J. Overview: Japanese encephalitis. Prog Neurobiol 2010; 91:108-12; PMID:20132860; http://dx.doi.org/10.1016/j.pneurobio.2010.01.008
- World Health Organization. Requirements for Japanese Encephalitis Vaccine (inactivated) for human use. In: Technical Report Series No 771. 1988; http://www.who.int/biologicals/publications/trs/areas/vaccines/jap_encephalitis/WHO_TRS_771_(part2)_A6.pdf?ua=1
- World Health Organization. Guidelines for the production and control of Japanese encephalitis vaccine (live) for human use. In: Technical Report Series No 910. 2002; http://www.who.int/biologicals/publications/trs/areas/vaccines/jap_encephalitis/WHO_TRS_910_A3.pdf?ua=1
- Kurane I, Takasaki T. Immunogenicity and protective efficacy of the current inactivated Japanese encephalitis vaccine against different Japanese encephalitis virus stains M. Vaccine 2000; 18:33-5; PMID:10821971; http://dx.doi.org/10.1016/S0264-410X(00)00041-4
- Kim JO, Shin JH, Baek SY, Min KI, Min BS, Ryu SR, Kim BK, Kim DK, Ahn MJ, Park M, et al. A collaborative study on Korean standard JE vaccine for potency assay. J Microbiol Biotechnol 2004; 14:745-50.
- Ministry of Food and Drug Safety. Minimum requirement for Biological Products. In: MFDS 2011-74. 2011; http://law.go.kr/admRulSc.do?menuId=1&subMenu=1&query=#liBgcolor0
- Ministry of Food and Drug Safety. Korean Pharmacopoeia (X). In: MFDS 2012-9. 2012; http://www.mfds.go.kr/eng/index.do?nMenuCode=50
- Kim DK, Kim HY, Kim JY, Park KB, Han E, Kim J, Ban S, Han S, Park YK, Nam JH. Development of an in vitro antigen-detection test as an alternative method to the in vivo plaque reduction neutralization test for the quality control of Japanese encephalitis virus vaccine. Microbiol Immunol 2012; 56:463-71; PMID:22486472; http://dx.doi.org/10.1111/j.1348-0421.2012.00462.x
- Butrapet S, Kimura J, Zhou S, Yasui K. Neutralizing mechanism of a monoclonal antibody against Japanese encephalitis virus glycoprotein E. Am J Trop Med Hyg 1998; 58:389-98; PMID:9574781
- Srivastava K, Aira Y, Mori C, Kobayashi Y, Igarashi A. Antigenicity of Japanese encephalitis virus envelope glycoprotein V3 (E) and its cyanogens bromide cleaved fragment examined by monoclonal antibodies and Western blotting. Arch Virol 1998; 96:97-107; http://dx.doi.org/10.1007/BF01310993
